Using past and present habitat to predict the current distribution and abundance of a rare cryptic lizard, Delma impar (Pygopodidae)
Abstract
We present logistic regression models predicting the distribution and abundance of a threatened cryptic lizard, Delma impar (Pygopodidae), in the Australian Capital Territory (ACT). The models incorporate current habitat and historical land use and habitat change (woodland clearance, ploughing, grazing, fertilizer application). Information on historical land use was acquired from land survey maps, aerial photographs and from floristic indices of land management. Floristic indices were developed from a survey of local agronomists who scored individual plant species, responses to grazing, ploughing and fertilizer application. Floristic indicies proved to be more informative than floristic ordination analyses. It emerged that historical factors were of key importance for predicting the distribution and abundance of D. impar. Since European settlement, D. impar has apparently spread from primary (naturally treeless grasslands) into secondary grasslands (grassland formerly with an overstorey of trees) and has been locally excluded by some farming activities such as ploughing. We conclude that a combination of current habitat and past changes in habitat may be necessary to understand the current distributions of plant and animal species that have limited dispersal ability and that are susceptible to local temporary habitat destruction. Active conservation strategies involving, for example, assisted dispersal, may be important for these species.
INTRODUCTION
Human-induced land cover changes and habitat fragmentation are a major cause of changes in the distribution and abundance of plant and animal populations ( Wilcox & Murphy 1985; Groombridge 1992; Andren 1994). Species response to these landscape changes depend on whether or not the new habitat is favourable, and their dispersal ability. A high dispersal ability may connect patches of favourable habitat even when the landscape is fragmented; however, a low dispersal ability may result in population fragmentation and limit spread through favourable habitat. Thus species with poor dispersal ability are much more threatened by landscape change. For these species the response to land use change is likely to be the ongoing outcome of historical patterns of land use, and current habitat may provide a poor indication of species distribution or abundance. Recognition of suitable habitat for such species may be confounded by the historical patterns. These species may not occupy all suitable habitats and where habitat is patchy do not have a stable metapopulation structure ( Husband & Barrett 1996). Direct human assistance in their dispersal or invasion may be necessary to ensure their long-term survival.
Over the past 200 years, much woodland habitat in temperate Australia has been fragmented and degraded by human land use to form discrete patches among a mosaic of pastoral or cropping land ( Hobbs & Hopkins 1990). In contrast, the impacts of clearing and pastoralism on the structure of grasslands and grass-dominated understorey vegetation are less obvious because these typically remain as herbaceous pasture or crop communities. Typically, the distribution of grass-dominated vegetation has increased following clearance of woodland, although floristic composition and structure has been altered through grazing, fertilizers, cultivation, changes in fire frequency, introduction of exotic plant species and loss of native species ( Moore 1970; McIntyre & Lavorel 1994; Lunt 1997). McIntyre & Barret (1992) have argued that grass-dominated ecosystems typically undergo habitat variegation rather than habitat fragmentation. Such varied and varying habitats, coupled with dispersal limitation, have led to some species of plants and animals having relatively unpredictable habitat associations, best explained by incorporating historical ecological information.
In this paper we examine the distribution of the Striped Legless Lizard, Delma impar, in relation to current habitat, past habitat and known and inferred historical human land uses. Delma impar is a small (90 mm snout to vent), cryptic, burrowing pygopodid restricted to grassland habitats in temperate southeastern Australia where it has a limited, patchy distribution and is regarded as threatened ( Fig. 1a)( Webster et al. 1992 ; Osborne et al. 1993 ; ANZECC 1995; IUCN 1996). It feeds on a variety of invertebrates, foraging in the grass and litter layer, occasionally basking in the upper grass canopy and sheltering in burrows, soil crevices of cracking clays or beneath rocks ( Coulson 1990; Hadden 1995) (Distribution, population habitat estimates and habitat requirements of the striped legless lizard Delma impar (Kluge). Unpublished report to the Australian Nature Conservation Agency).
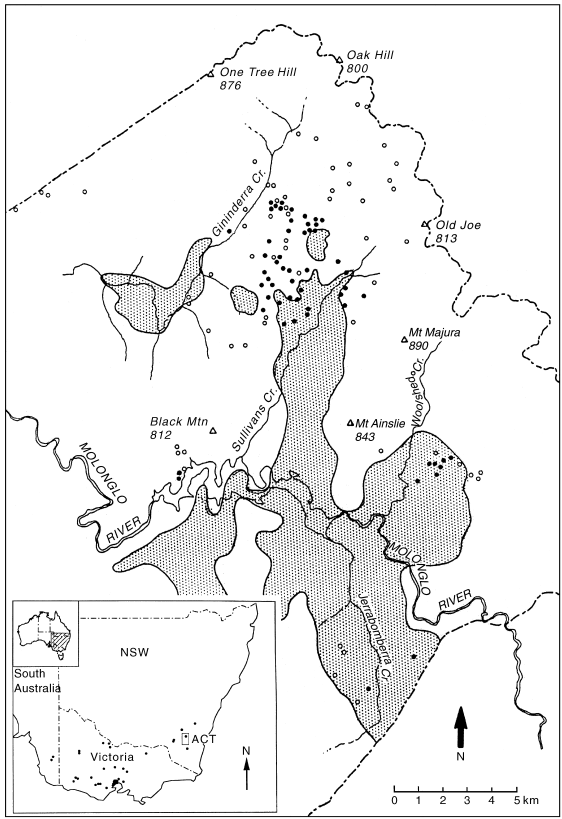
Distribution of Delma impar in southeastern Australia (inset). The distribution of treeless grasslands (shaded areas) in the ACT and the known distribution of D. impar from trap sites (●). Trap sites where D. impar has not been detected (○).
By 1995 less than 1000 individuals had been recorded from 90 sites Australia-wide, despite intensive surveys at more than 150 apparently favourable locations and numerous surveys of less favourable habitat ( Williams & Kukolic 1991; Kukolic 1993; Kukolic et al. 1994 ; Rauhala et al. 1995 ; P. Robertson, personal communication, 1997). Because the species is hard to detect, emphasis has been placed on the use of habitat to predict its distribution.
It has been suggested that D. impar is restricted to relatively undisturbed primary native grasslands (naturally treeless grasslands) dominated by a dense cover of perennial tussock-forming grasses, particularly Themeda triandra ( Williams & Kukolic 1991; Kukolic & Osborne 1992; Cogger et al. 1993 ; Kukolic 1993; Osborne et al. 1993 ; Kukolic et al. 1994 ). However, D. impar has also been recorded in secondary grasslands (formerly with an overstorey of trees) dominated by native plant species and agriculturally modified land dominated by exotic pasture species. Nevertheless, D. impar is absent from many primary, secondary and exotic-dominated grassland sites, suggesting that a more complex historical explanation of the distribution may be required. All grass-dominated habitats have been modified by various and changing agricultural practices and, near Canberra, fragmented by suburban development ( Cogger et al. 1993 ; Osborne et al. 1993 ; Benson 1994).
This paper focusses on three related issues: (1) Are measures of current habitat sufficient to explain the current distribution of D. impar? (2) Do measures of historical habitat change improve predictions of the distribution of the species and can reliable reconstructions of ecological history be made? (3) How can this information contribute to the future management of the species?
METHODS
Study area and D. impar survey
Grasslands near Canberra were selected for study as they support the largest known population of D. impar and a substantial trapping programme had already been undertaken by the Australian Capital Territory Parks and Conservation Service (ACTPCS). This trapping programme forms the core of this study. The study area (35°7–23′S, 140°3–15′E) encompasses all ACT sites where D. impar has been recorded ( Fig. 1). Most of the area consists of broad valleys with gently undulating terrain and deep clay-rich soils at 550–650 m altitude, intersected by higher ridges with skeletal soils. The lowest lying areas were described as treeless grassy plains when Europeans arrived in the early 1820s ( Cambage 1919). In summer, exposed soil surfaces may reach 55°C while in winter, cold air draining onto the plains results in nocturnal foliage temperatures as low as –15°C (Dorrough & Ash, unpublished data, 1994). Around these grassy frost hollows there was an inverted treeline characterized by Eucalyptus pauciflora or Eucalyptus rubida, while the surrounding slopes supported woodland of Eucalyptus melliodora and Eucalyptus blakelyi passing into Eucalyptus rossii and Eucalyptus macrorhyncha on ridges ( Pryor 1939; Prior 1954). During 170 years of European land use, the woodlands on lower slopes have been largely cleared, leaving a few patches of open grassy woodlands and creating extensive secondary grasslands. The better valley soils were cultivated, initially for wheat production ( King 1946). Later, sheep grazing became the dominant activity and recently some areas have been encroached upon by suburban development.
Habitat data from 100 sites trapped for D. impar were used in this study. Ninety of these sites were trapped by the ACTPCS between 1991 and 1994 ( Williams & Kukolic 1991; Kukolic 1993; Kukolic et al. 1994 ; Rauhala et al. 1995 ). Selection of these sites was determined by the need to make rapid responses to urban planning proposals and to detect populations, rather than to examine the effect of different habitats or to make a predictive model of D. impar distribution. Locally, sites were selected to maximize capture rates based on current knowledge of the habitat requirements of D. impar. Despite these constraints, the surveys included a range of habitats and localities. The additional 10 sites trapped in 1994–5 were located to complement the previous surveys and were selected to test hypotheses about habitat and dispersal, and therefore included remote and marginal habitat.
Pitfall trapping was used in the survey following methodology developed by ACTPCS (e.g. Kukolic 1993). At each site, 20 pit traps were laid out in a cross with 25-m-long arms and pits spaced every 5 m along a 0.3-m-high shade-cloth drift fence. Pits were lined with 0.4-m-deep, 0.25-m-diameter, metal cans set flush with ground level, and grass litter was placed in the base for shelter. Where necessary, a wire strand fence was erected around each site to exclude stock. Sites were trapped for a minimum of four consecutive weeks during November–December, when catch rates are highest (Kukolic, personal communication, 1994; Osmond 1994).
Present-day habitat: floristics, vegetation structure and soils
Ten 1-m2 quadrats were located at 5-m intervals along a transect running north–south across each trap site. The vascular plant species present in each quadrat were recorded and the number of quadrats in which a species was recorded was used as the measure of its frequency. Data for 69 sites, collected in 1992 and 1994, were provided by ACTPCS. Data for a further 31 sites were recorded by the authors in 1994–95. All sites were surveyed in early to mid-summer.
The vertical structure of the vegetation was characterized using a rising plate meter of 10 × 10 cm, giving a compression of 0.6 g cm–2. The height at which the plate was supported above ground level was well correlated with above-ground biomass measured as oven-dry weight (r2 = 0.94; F = 122; P < 0.001; n = 30). One measurement was made at 1-m intervals along the 50 m floristic transect at each site. The mean and variance at each site were calculated. Variance provided an estimate of the ‘tussockness’ at a site, with low variance indicating a uniform sward. Bare ground was estimated as the percentage of zero recordings at each site; this does not necessarily indicate bare soil but rather a very low plant cover.
Species recorded in the floristic survey were classified as annuals or perennials and whether they form tufts or tussocks. Three summary statistics were calculated that indicate the potential vegetation structure based on the ungrazed growth forms of the species present: the number of perennial species at the site, the mean frequency of perennial tussock-forming species and the mean frequency of perennial tussock- and tuft-forming species.
Indirect soil measures were applied to assess soil suitability for burrowing and the tendency for cracking. Soil shear strength at 0–15 cm depth was measured using a torsional shear vane ( Serota & Jangle 1972). Four measurements were made at each site and gravimetric soil moisture content was determined to allow standardization of the values. A high clay content was used as an indicator of soil cracking. Four soil samples were collected at each site and the clay content was estimated by using the soil texture key of Northcote (1971).
Historical vegetation survey and land use records
Early land surveys provide the most reliable and precise record of primary native grassland distribution at the time of European settlement and have great value for historical reconstructions ( Watson 1988; Lunt 1994). The first European settlers arrived in the Canberra region 5–10 years before the Government Surveyors and the first detailed land surveys were completed in the late 1820s and early 1830s ( Boden 1971; Moore 1978; Gillespie 1992). However, the majority of pertinent land surveys date from 1861 to 1885. Land portion maps and surveyors’ notebooks provide brief descriptions of vegetation cover and current or potential land use. Clear boundaries between timbered land and open plains were often indicated and portion corners were shown with direction and distance (measured in chains) to the nearest tree. Fortuitously, these maps provide ideal information for accurately delineating woodland and primary grassland boundaries. Additional information was gained from the Federal Territory Feature Map ( Department of Home Affairs 1913) and Pryor (1939).
At various periods, most of the deeper soils of the ACT have been cultivated for crop production and pasture improvement ( King 1946). Information on cultivation and associated ploughing was obtained from the Federal Territory Feature Map ( Department of Home Affairs 1913), aerial photography dating from 1955 to the present (source of photographs: Australian Surveying and Land Information Group, National Library of Australia), L. Tong (ACTPCS) and personal field observations. Ploughed areas were mapped and the date each site was last ploughed was recorded.
Life history and physiological characteristics of plant species can result in markedly different responses to agricultural land uses and it was considered that the abundance of certain species could be used to construct indices of past agricultural land use. However, many changes in species composition have not been well documented ( Tremont & McIntyre 1994), although they may be recognized by farmers, agronomists and field botanists. A survey was conducted of five experts with extensive knowledge of local grasslands. They were asked to score the change in abundance of plant species recorded at trapping sites in response to stock grazing, application of fertilizers and ploughing. The scale ranged from –2 for a strong negative response to +2 for a strong positive response. If there were at least two responses given and the scores for a species were similar (± 1), it was given the median score and retained as a past management indicator, otherwise it was excluded. For each site, indices (SI) for past grazing, fertilizer addition and ploughing were separately calculated using a weighting for the frequency of each individual indicator species (fi) recorded at the site and the species management score (Ii) of the form:
where n is the number of indicator species present at the site.
Statistical analyses
Logistic regression models ( McCullagh & Nelder 1983) were used to examine both the abundance (number of D. impar caught per trap-day) and presence of D. impar. A scale factor, the mean residual deviance, was used to counter over-dispersion in the abundance model. Variables were added and subtracted from the models in turn until no significant change in deviance was observed. The relative importance of each variable in the final model was assessed by removing that variable and noting the change in deviance. A plot of predicted values against measured abundance indicated the fit of abundance models. The fit for the binary model was assessed by grouping the predictions in probability classes and comparing this with the observed proportion of sites containing D. impar.
Floristic data were included in the models in two ways: as the frequency of individual species and as axis scores for sites derived using hybrid multidimensional scaling (HMDS; Belbin 1990) on a Bray–Curtis index of site floristic similarity. A four-dimensional HMDS was required to reduce stress to <0.2 (0.16 exactly) with the exception of axis 1, no axis, or combination of axes were significantly related to D. impar abundance; thus no secondary axes or site groups were defined.
Sites were clustered within some areas of the study region, so an analysis was performed to determine how this affected the models. Sites were grouped into 10 spatial clusters using an unweighted pair-group mean-average algorithm calculated from a matrix of intersite distances. The resulting spatial groups were 1–10 km apart and were included in models as categorical groups.
RESULTS
Factors affecting the abundance and distribution of D. impar
Delma impar was captured at 50 of the 100 trap sites. The mean number of animals caught was 0.01 ± 0.005 per trap-day (a trap-day being equal to one pit trapped over a 24-h period) at occupied sites. For sites where D. impar was recorded, the number of captures approximated a zero-truncated geometric distribution ( Johnson & Kotz 1969) with a mean of 4.2 and variance of 26.5. The expected number of zeros was estimated, suggesting an 82% sampling efficiency in detecting the species and that D. impar might have been missed in about nine sites by chance. Our predictions of D. impar distribution may therefore be slightly underestimated.
All sites yielding D. impar were characterized as grassland. At some sites mature eucalypt trees, relicts of former woodland, were present; however, they were widely scattered with a grassy understorey. There was no evidence to suggest that woodland could provide habitat for D. impar so further analysis focussed on grass-dominated vegetation (primary, secondary and exotic grassland). Early survey records indicate that the pre-European distribution of grassland was restricted to low-lying topography ( Fig. 1b). Delma impar now occupies some sites that were originally woodland and which are up to 2 km from the original grassland– woodland boundary.
Four variables were significant for predicting abundance of D. impar ( Table 1). In declining order of deviance explained in the models, these were: distance from primary grasslands, years since ploughing, the fertilizer index and HMDS axis 1 of the floristic data. Delma impar abundance was higher in sites close to primary grasslands which had not been ploughed recently, had a low fertilizer index and low values for HMDS axis 1. No measured environmental or land use variables explained variation along HMDS axis 1, so it is difficult to interpret the effect on D. impar. Examination of floristic composition did not reveal any clear trends along HMDS axis 1. None of the individual plant species, vegetation structure parameters, soil measures or physical landscape features (e.g. altitude) were significant in the final abundance model.
| Variable | Change in deviance | d.f. | Estimate | Standard error | P |
|---|---|---|---|---|---|
| Constant | NA | NA | –4.707 | 0.3934 | |
| Distance from primary grassland (km) | 14.01 | 1 | –0.3798 | 0.1229 | <0.001 |
| Ploughing (years since) | 13.06 | 1 | 0.0109 | 0.0031 | <0.001 |
| Fertilizer index | 9.231 | 1 | –1.088 | 0.3515 | <0.01 |
| HMDS axis 1 | 6.526 | 1 | –0.6346 | 0.2537 | <0.05 |
- Abundance incorporates a scale estimate of 5.38. Changes in deviance are from the full model (residual deviance 93.54 with 91 d.f.). The null model has a deviance of 142.6 with 95 d.f. Significance levels refer to change in deviance from the full model, which approximate a χ2 distribution. NA, not applicable.
Logistic regression identified five significant variables for predicting presence/absence of D. impar ( Table 2). Assuming probability values derived from the model of >0.5 indicate suitability for D. impar, the model correctly predicts D. impar to be present in 44 of the 50 sites occupied ( Fig. 2). The probability of D. impar occurrence declined with the frequency of the small sedge, Schoenus apogon, distance from primary grassland, bare ground and increasing fertilizer index, and increased with time since ploughing. Thus, distance from primary grassland, ploughing and fertilizer effects are common to both models, while the floristic HMDS axis 1, bare ground and S. apogon are restricted to particular models.
| Variable | Change in deviance | d.f. | Estimate | Standard error | P |
|---|---|---|---|---|---|
| Constant | NA | NA | 2.873 | 1.417 | |
| Schoenus apogon | 16.75 | 1 | –1.582 | 0.5621 | <0.001 |
| Distance from primary grassland (km) | 12.80 | 1 | –0.9068 | 0.3026 | <0.001 |
| Bare ground (%) | 5.131 | 1 | –0.078 | 0.004 | <0.05 |
| Ploughing (years since) | 4.171 | 1 | 0.018 | 0.0095 | <0.05 |
| Fertilizer index | 4.054 | 1 | –2.835 | 1.512 | <0.05 |
- Changes in deviance are from the full model (residual deviance 83.01 with 90 d.f.). The null model has a deviance of 132.9 with 95 d.f. Significance levels refer to change in deviance from the full model, which approximate a χ2 distribution. NA, not applicable.
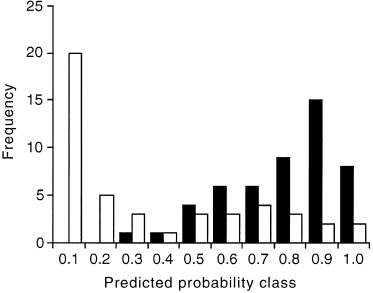
Goodness of fit for the binary logistic regression model of Delma impar presence and absence. Model predictions are grouped into 0.1 probability classes and differentiated according to whether D. impar is actually present (shaded) or absent (unshaded).
Soil strength and clay content were not significant predictors in either models, possibly because availability of burrows is not a limiting factor. All sites were clay-rich and appeared to have numerous invertebrate burrows that might be utilized by D. impar.
To assess the role of distance from primary grasslands in predicting D. impar distribution it is necessary to consider if the distance effect correlates with other habitat factors. The only significant relationships between distance and the predictive variables were that fertilizer index values declined slightly (r2 = 0.07; F = 7.4; P < 0.05) and the abundance of S. apogon increased (r2 = 0.14; F = 15.63; P < 0.001) with increasing distance from primary grassland. Our models suggest that declining fertilizer index values should favour D. impar and thus may counter the distance effect. The trend in S. apogon abundance was generated by one distant site with very high abundance. The furthest D. impar record from the primary grassland boundary was approximately 2 km. By refitting the predictive models (abundance and presence/absence) without the distance term a large proportion of sites outside the 2 km limit were predicted to have high probabilities of supporting D. impar ( 3, 4). Thus, there is little evidence to suggest that the predictive variables are confounded by distance, and further expansion into suitable habitat may be possible.
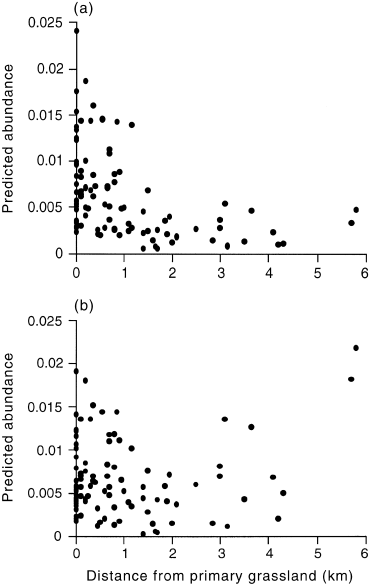
Predicted abundance of Delma impar at increasing distance from primary grassland. (a) Predictions derived from the logistic regression model of D. impar abundance, which includes distance as a variable. (b) Predictions derived from a logistic regression model of D. impar abundance refitted to incorporate all significant terms except distance. No relationship with distance is observed (r2 = 0.012; F = 1.1863; P > 0.05).
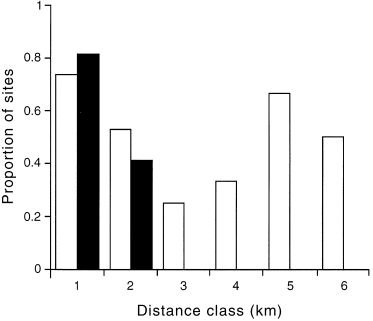
Proportion of sites with a predicted probability >0.5 for each 1-km distance class for presence/absence models of Delma impar fitted with and without the variable ‘distance from primary grassland’. The number of sites for each distance class are: 1 km, n = 64; 2 km, n = 17; 3 km, n = 4; 4 km, n = 6; 5 km, n = 3; 6 km, n = 2. Shaded columns are the proportion of sites for which D. impar was predicted to be present (>0.5) when distance was included as a predictive variable.
Interpretation of analyses incorporating spatial groups of sites is complex due to correlations with localized features including distance from primary grassland, altitude, soil type and land use. Spatial group was the best single predictor of abundance, while ploughing (years since ploughed), the floristic HMDS axis 1 and the fertilizer index remained significant in addition to bare ground ( Table 3). Distance from primary grassland was confounded with spatial group and was not significant. The three variables common between both abundance models account for less deviance in the spatial clustering model, suggesting that they may also be correlated with spatial groups.
| Variable | Change in deviance | d.f. | P |
|---|---|---|---|
| Spatial group | 111.3 | 9 | <0.001 |
| Ploughing (years since) | 24.42 | 1 | <0.001 |
| HMDS axis 1 | 15.06 | 1 | <0.001 |
| Fertilizer index | 8.977 | 1 | <0.01 |
| Bare ground percentage | 8.199 | 1 | <0.01 |
- Residual deviance 87.38 with 82 d.f. Abundance measured as number caught per trap-day. The model incorporates spatial group as a fixed effect and has a scale estimate of 2.725. The null model has a residual deviance of 281.5 with 95 d.f. Significance levels refer to the change in deviance from the full model, which approximate a χ2 distribution.
Spatial group was also the major predictor in the presence/absence model for D. impar ( Table 4). In addition, bare ground, the floristic HMDS axis 1 and distance from primary grassland were significant and had similar effects to the site models outlined above. Ploughing and the fertilizer index were no longer significant. This model correctly predicts (>0.5) D. impar to be present in 45 of 50 occupied sites ( Fig. 5).
| Variable | Change in | d.f. | P |
|---|---|---|---|
| deviance | |||
| Spatial group | 48.92 | 9 | <0.001 |
| Bare ground (%) | 9.582 | 1 | <0.01 |
| HMDS axis 1 | 6.452 | 1 | <0.05 |
| Distance from primary | 5.341 | 1 | <0.05 |
| grassland (km) |
- Residual deviance of 54.08 with 83 d.f. The model incorporates spatial groups as a fixed effect. Significance levels refer to change in deviance from the full model, which approximate a χ2 distribution. The null model has a residual deviance of 132.9 with 95 d.f.
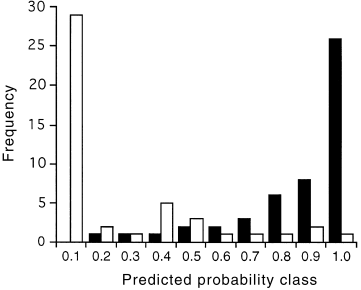
Goodness of fit for the binary model of Delma impar presence and absence. The model incorporates 10 spatial groups. Model predictions are grouped into 0.1 probability classes and differentiated according to whether D. impar is present (shaded) or absent (unshaded).
Past land use
Of the 162 herbaceous plant species for which information was sought, 29 individual species and an additional nine genera, whose species were all assessed to respond in similar fashion, were selected as reliable indicators of past land use ( Table 5). Thirty-three of the selected plant indicators were included in all three indices ( Table 5). Rare species are poorly known and are probably under-represented in the responses.
| Species | Fertilizer | Grazing | Ploughing |
|---|---|---|---|
| Agrostis spp. | +1 | –1 | +2 |
| Aira spp. | 0 | +1 | +2 |
| Alternanthera aff. nana | +1 | ||
| Aristida spp. | –1 | +1 | –2 |
| Austrostipa bigeniculata | +1 | 0 | –1 |
| Avena fatua | +2 | –1 | +2 |
| Bothriochloa macra | 0 | –1 | |
| Briza minor | +1 | +1 | |
| Bromus diandrus | +1 | +1 | |
| Bromus hordeaceus | +1 | +1 | |
| Bulbine bulbosa | –1 | ||
| Carthamus lanatus | +2 | +1 | +1 |
| Cheilanthes tenuifolia | –1 | –2 | |
| Chenopodium pumilo | +1 | –1 | +1 |
| Chloris truncata | 0 | –1 | |
| Chondrilla juncea | +1 | +1 | +2 |
| Cirsium vulgare | +1 | +1 | +1 |
| Craspedia variabilis | –1 | –2 | |
| Critesion leporinum | +2 | +2 | |
| Cynodon dactylon | +2 | 0 | +2 |
| Dactylis glomeratum | +2 | +1 | +1 |
| Dichelachne spp. | –1 | –1 | –2 |
| Elymus scaber | +1 | –1 | –1 |
| Enneapogon nigricans | –1 | ||
| Eragrostis spp. | +1 | 0 | +1 |
| Glycine clandestina | –2 | ||
| Lolium spp. | +2 | 0 | –1 |
| Lolium rigidum | +2 | –1 | +2 |
| Marrubium vulgare | +2 | ||
| Microleana stipoides | +1 | +1 | +1 |
| Onopordum acanthium | +2 | 0 | +2 |
| Panicum effusum | +1 | 0 | +1 |
| Paspalum dilatum | +2 | 0 | +2 |
| Phalaris aquatica | +2 | 0 | |
| Poa spp. | +1 | 0 | –1 |
| Themeda triandra | –1 | –1 | –2 |
| Vulpia spp. | +2 | +2 |
- Responses to land use are on a scale of –2 to +2, where –2 is a strong negative response and +2 a strong positive response.
All grassland sites have been utilized for stock grazing, many for 170 years, but grazing index responses were not very strong and even sites that have had stock excluded for several decades were not very distinct.
There was appreciable variation in site index values for fertilizer response. However, records of past fertilizer application were generally not available so it was not possible to test the reliability of the fertilizer index. In contrast, it was possible to test the ploughing index with actual ploughing records. A logistic regression model fitted with ploughed/unploughed as the binary response indicated that ploughed sites have significantly higher ploughing indices. (Residual deviance: null model = 137.6, 99 d.f.; full model = 111.1, 98 d.f. Constant: estimate = –0.27, SE = 0.26. Ploughing index: change in deviance from null model = 26.5, 1 d.f.; estimate = 4.52, SE = 1.09; P < 0.001). An improved fit was not achieved by modelling ploughing index with time since ploughing.
DISCUSSION
Habitat requirements
All records of D. impar for which habitat data are available are from grasslands. Some early accounts specified woodland and forest as habitat for this species ( Jenkins & Bartell 1980; Cogger 1992); however, these appear to be based on a single specimen from a grassland/open forest boundary (W. Osborne, personal communication, 1995). Where D. impar occurs near trees it is always in an adjacent grassy area.
Recent publications suggest that D. impar is associated with native grasslands dominated by dense and relatively undisturbed, perennial, tussock-forming species ( Williams & Kukolic 1991; Kukolic & Osborne 1992; Cogger et al. 1993 ; Kukolic 1993; Kukolic et al. 1994 ). In the ACT, the population density of D. impar is highest in sites where Stipa bigeniculata (Spear Grass) or Themeda triandra (Kangaroo Grass) form a dense sward or thatch ( Kukolic & Osborne 1992). Our study indicates that D. impar can utilize primary, secondary and exotic-dominated grasslands and is not dependent on any particular grassland plant species or communities. Though several significant predictors of D. impar were based on combined floristic measures, these are treated as indices of past management rather than of direct floristic effects. However, D. impar was generally absent in communities where the sedge S. apogon was abundant. These are low-lying areas likely to become waterlogged in winter ( Burbidge & Gray 1970). For a species occupying underground burrows, well drained soil could be essential and poorly drained soil might only provide suitable habitat during dry conditions. Treeless waterlogged drainage lines may be unfavourable habitat despite often being uncultivated and dominated by native plant species.
The potential vegetation structure and the mean (~ biomass) or variance (~ tussockness) of grassland structure at a site were not good predictors of utilization by D. impar. However, very short grass or bare ground resulted in a significant decline in the probability of D. impar being present. This may have been influenced by a drought in 1994, although examination of individual sites suggested that the vegetation structure in most cases was unaffected as the trapping areas were fenced off from grazing. A few sites heavily grazed in 1994, for which data were available over consecutive years and at which D. impar was known to be present, had low or zero capture rates. Subsequent trapping and observations revealed that D. impar was still present at these sites (Rauhala, personal communication, 1996), suggesting bare ground may have been limiting movement. Preliminary studies indicate that sand beds are avoided by D. impar, and further suggest that bare ground may be avoided (Dorrough & Ash, unpublished data, 1995). Osmond (1994) suggested that suitable cover is required to maintain body temperature and moisture requirements, so animals may vary their activity and microhabitat according to prevailing microclimatic conditions. Avoidance of predators, such as raptors, may influence habitat selection, while D. impar activity may also be a response to invertebrate prey abundance, which is likely to decline due to heavy grazing and low vegetation structure ( King & Hutchinson 1983; Greenslade 1992). Avoidance of bare ground by D. impar could severely limit dispersal when pastures are overgrazed and might prevent crossing of roads or paths.
Effect of past land use
Floristic indices such as those developed here for grazing, ploughing and application of fertilizers make direct and explicit a priori links between floristics and processes that may affect grassland community composition. In particular, they can detect effects that cannot be so readily detected from simple floristic analysis, including multivariate procedures. For example, different sites may have different combinations of species but these may indicate a similar management effect. A posteriori procedures, such as ordination, are less sensitive for detecting processes that may be important, such as ploughing, and when they do detect an effect, such as the role of ‘floristic HMDS axis 1’ in this study, it may not be readily interpreted and might be a chance correlation.
The survey of experts used for deriving these indices has both strengths and limitations. The strength is that the knowledge represents an accumulation of expert observations over many sites and seasons; equivalent to about 100 field-seasons of observation in this study. The limitation lies in the poorly defined accuracy and precision of the assessment, though by examining the consistency of responses between observers the overall agreement may be assessed and inconsistent plant species ignored. The best test is to examine the indices against known management histories. This was only possible for ploughing and in this case gave a significant agreement between the index and the known history. This suggests that at least the ploughing index may be used with some confidence and the other indices may be as reliable. Evidently, more effort should be directed to synthesis of current knowledge of plant species, which could generate useful indices, rather than undertaking new surveys as though there was no prior information.
Lower probabilities of presence and lower abundances of D. impar were associated with recent ploughing events and a high index for fertilizer application. The negative response of D. impar to the fertilizer index is possibly the result of higher levels of grazing and changes in site floristic composition and invertebrate populations rather than a negative response to the fertilizer itself. Ploughing, however, is likely to have direct effects on D. impar populations. Animals may be killed outright, exposed to unfavourable conditions or predators and may subsequently lack grass habitat and prey. Recent ploughing was more significant than past ploughing for predicting occupation by D. impar, suggesting either that D. impar populations may respond positively to subsequent successional changes in habitat or that D. impar population recovery is dependent on the time for dispersal from adjacent habitats. Floristic composition also responds to ploughing but the recovery appears to be slower. It may only be necessary for an appropriate vegetation cover and associated invertebrate prey to develop before D. impar can invade from adjacent sites. The limited occurrence of D. impar in exotic-dominated habitats is, perhaps, a consequence of more frequent ploughing and disturbance, rather than of the inherent unsuitability of the grassland species.
There was no significant relationship between D. impar and the index for grazing. Because all grasslands within the ACT have been grazed by stock for much of the duration of European settlement, determination of grazing history based on floristic elements may be difficult. However, grazing does result in short-term changes in the structure of grassland. High grazing intensity, typically associated with drought, can produce bare ground, which appears to be unfavourable to D. impar. Under such conditions populations might fail to rear young and may suffer losses of adults. In wetter seasons, with greater biomass, the impact of grazing on D. impar may be slight.
At the time of European settlement, grasslands in the ACT were largely continuous throughout the valley floors ( Pryor 1939) and the distribution of D. impar may also have been continuous through these areas. High estimated levels of gene flow between ACT populations of D. impar, as indicated by allozyme electrophoresis, may reflect this continuity ( Osmond 1994). European farming has had a mixture of effects, expanding grasslands through woodland clearance but also locally degrading the grassland by ploughing etc. so that it temporarily no longer supports D. impar. Primary grasslands occupying the deeper soils of low-lying valleys have been the focus of cultivation, so the less disturbed secondary grasslands have apparently become major population refuges. In recent decades the expansion of Canberra suburbs has encroached on the grassland habitat and poses novel problems including habitat destruction, fragmentation by roads and predation by cats and dogs. These effects may not be readily apparent from examining site floristics or habitat structure.
Implications for conservation management
It seems likely that there are undiscovered populations of this cryptic lizard. By examining essential features of the species habitat and habitat history these populations may be more readily discovered: by applying these principles, an outlying population of D. impar has been discovered in unploughed grassland near Cooma ( Dorrough et al. 1996 ). As D. impar appears to be dependent on continuity of grassland habitat, knowledge of the changing distribution of grasslands during the last glacial cycle may also provide a broader framework for understanding the current patchy distribution of the species.
This research suggests that the dependence of D. impar on primary grassland is less than has formerly been proposed. However, such grasslands, if undisturbed, do appear to be the preferred habitat. There is a strong case for jointly conserving these grasslands and D. impar where possible. As most primary grassland is already heavily disturbed ( Benson 1994), secondary grasslands play a key role in conservation both of D. impar and of other native grassland fauna and flora.
Though individuals may roam further, the spread of D. impar populations is apparently relatively slow, estimated to be <12 m year–1 (based on a maximum rate of 2 km/170 years), and appears to be a major constraint on their occupation of potential habitat. The effective connectivity of favourable habitat patches becomes a major concern. Ploughed and bare ground, such as roads or paths, might represent a barrier to dispersal and possibly fragment habitat. Both these factors suggest that translocation of breeding individuals to favourable habitat might be a desirable conservation strategy. An additional benefit of translocation would be to provide a test of predictive variables identified in this research.
As this study has indicated, it is necessary to include current habitat and past changes in habitat to explain the distribution of D. impar. Failure to consider both could result in the development of inappropriate population models and management strategies. Similar conclusions are likely to apply to other flora and fauna that share a limited dispersal ability and susceptibility to local temporary habitat destruction. In these respects the population dynamics of D. impar are likely to be similar to many grassland plants and small, strictly terrestrial animals. A combination of mapping of past habitat and inference of past disturbance may be necessary and this study has indicated that a combination of historical records and use of indicator species can be successful.
Acknowledgements
Funding assistance and a student scholarship were kindly provided by the ACTPCS.
We would like to thank the following people for their assistance and provision of information: Kruno Kukolic, Frank Ingwersen, Sarah Sharp, Marjo Rauhala and Dr David Shorthouse of the ACTPCS, and Dr Will Osborne, Dr Peter Robertson and Dr Paul Cooper. Thanks to Denys Garden, David Eddy, Richard Groves, David Mallinson and Frank Ingwersen for providing the information used to construct the floristic indices. Very special thanks to Laurie Tong (ACTPCS) for providing otherwise inaccessible information on land use history in the ACT. Trapping and floristic data were made available by the ACTPCS. We greatly appreciate the comments of two anonymous referees.




