The social organization of a sexually dimorphic honeyeater: the Crescent Honeyeater Phylidonyris pyrrhoptera, at Wilsons Promontory, Victoria
Abstract
The social organization of the sexually dimorphic and dichromatic Crescent Honeyeater Phylidonyris pyrrhoptera (Latham 1801) was studied at Wilsons Promontory National Park between January 1994 and January 1997. All breeding attempts at one site were followed during the spring and summer of 1996. Most pairs were multibrooded, with pair-bonds maintained throughout the study. Breeding males held well-defined territories. Females, although generally restricting their activities to within their mate’s territory, visited other males’ territories more frequently than did their mates and did so close to the time of egg laying. Males aggressively chased intruding males, but appeared to permit intrusions by non-mate females. Circumstantial evidence suggests some males may kill neighbour’s nestlings. Female Crescent Honeyeaters are solely responsible for nest construction and incubation. While both sexes attended nestlings, males contributed to parental care significantly less than females. Males also provided significantly less parental care than has been reported for monomorphic species of honeyeater. Single syllable calls are given throughout the year. Peaks in the rate of double and multisyllable calls corresponded with peaks in breeding activity. Only males were observed uttering loud multisyllable calls. In contrast to predictions arising from sexual selection theory and observations of the mating system of sexually dimorphic hummingbirds, the population of Crescent Honeyeaters studied exhibited a socially monogamous mating system. However, the very limited male role in parental care and non-observance of male territory boundaries by females suggest the genetic mating system may not be one of monogamy.
INTRODUCTION
Honeyeaters (Family Meliphagidae) are the most diverse and widely distributed family of Australian passerines. Most are sexually monochromatic and monomorphic, with only six of the 67 Australian species (9%) exhibiting marked sexual dichromatism ( Slater et al. 1989 ). Although McFarland & Ford (1991) suggested that Australian honeyeaters are monogamous (with the only known exception being the promiscuous Noisy Miner Manorina melanocephala (Latham)), the social organization of relatively few species has been studied in any detail. Furthermore, it has recently become clear that to understand the social organization of any bird one needs to consider both the social mating system and the genetic mating system of the species ( Westneat et al. 1987 ). For example, the Splendid Fairy-wren, Malurus splendens (Quoy & Gaimard), was found to be socially monogamous ( Rowley 1981), but molecular studies revealed a promiscuous mating system with as few as 35% of young raised by a male parent being sired by that individual ( Rowley & Russell 1990). To date, neither the social nor the genetic mating systems has been described for more than a handful of honeyeater species (e.g. Noisy Miner, Dow 1978; New Holland Honeyeater Phylidonyris novaehollandiae (Latham), Paton 1979; Bell Miner Manorina melanophrys (Latham), Clarke (1988); Helmeted Honeyeater Lichenostomus melanops cassidix (Latham), Franklin et al. (1995) ; Regent Honeyeater Xanthomyza phrygia (Shaw), Oliver et al. (1998) ; Geering & French (1998). Molecular analysis of the mating system has been done only for the Noisy Miner ( Poldmaa et al. 1995 ), Bell Miner ( Conrad et al. 1998 ) and Helmeted Honeyeater (N. Murray, unpublished data, 1996). These three species are all sexually monomorphic, predominantly sedentary, largely insectivorous, and have been found to be both socially and genetically monogamous. The New Holland Honeyeater and the Regent Honeyeater are primarily nectarivorous and neither exhibits marked sexual dichromatism or dimorphism. Again, they appear to be socially monogamous but their mating systems have yet to be analysed genetically.
While McFarland & Ford’s (1991) suggestion that Australian honeyeaters are monogamous is consistent with all evidence to hand, the species examined so far are unlikely to represent the total diversity of patterns of social organization honeyeaters exhibit. In particular, very little attention has been given to the sexually dimorphic species of honeyeater, all of which are nectarivorous. Nectarivorous honeyeaters, by the very nature of the seasonal flowering on which they may depend, often display some degree of seasonal mobility ( McFarland & Ford 1991). This mobility makes them much more difficult subjects for intensive study and may, in part, explain why our understanding of their social organization is much poorer than for sedentary, monomorphic, insectivorous species ( Clarke 1997).
In contrast to Australian honeyeaters, most other nectarivorous birds are both sexually dimorphic and dichromatic; the male is usually much larger and more brightly coloured than the female ( Perrins & Middleton 1985). This is particularly true of the hummingbirds, where about 70% of all species exhibit marked sexual dimorphism and dichromatism. Despite obvious differences in morphology and distribution, hummingbirds and the Australian honeyeaters are reliant on both nectar and insects as a food source and also have long bills with tongues adapted for nectar removal ( Pyke 1980). Unlike most nectarivorous honeyeaters, the social behaviour of hummingbirds has been studied intensively (e.g. Pitelka 1942; Wolf 1969; Wolf & Stiles 1970; Wolf & Wolf 1971; Feinsinger 1976). Most hummingbirds that have been studied have been found to be polygynous, with pair-bonds usually restricted to the time of copulation ( Wolf & Stiles 1970). Following copulation, the female is solely responsible for parental care of the young ( Snow & Snow 1973). While males provide no direct care to the young, they defend territories that provide sufficient energy to support themselves and a number of females with which they have mated ( Feinsinger 1976).
The polygynous mating system observed in hummingbirds is consistent with a prediction of sexual selection theory. If a male’s main function in a breeding attempt is to fertilise the female and then defend resources for her during the breeding attempt, conditions exist for intense sexual selection ( Halliday 1983). Evolution of striking, brightly coloured plumages and elaborate displays amongst males may occur if females select mates on the basis of brightness of plumage and display quality. In monogamous mating systems where male–male competition for females is thought to be less, one would predict selective pressures would be less intense, with these species exhibiting sexually monomorphic features and less elaborate displays ( Wickler & Seibit 1977).
The limited analyses of the mating systems of monomorphic honeyeaters are consistent with this pattern. However, if the above hypothesis is correct we might also expect highly sexually dimorphic and dichromatic honeyeaters to exhibit polygynous social and genetic mating systems. The sexually dimorphic and dichromatic nectarivorous Crescent Honeyeater, Phylidonyris pyrrhoptera (Latham) is an ideal candidate for such a study. Despite the species’ wide distribution throughout southeastern Australia, there have been no formal studies of its social organization. The very limited data available are based solely on anecdotal observations of single nesting attempts by unmarked birds ( Campbell 1900; North 1908; Dickison 1926; Cooper 1960).
The broad aim of our study was to examine the social organization of the Crescent Honeyeater to determine whether it exhibited a promiscuous or polygynous system, as has been reported for other sexually dimorphic nectarivores, like the hummingbirds. Specifically our study set out to determine: (i) whether male Crescent Honeyeaters defend discrete breeding territories; (ii) whether they share their territory with one or more breeding females; and (iii) whether they assist in raising the offspring of one or more females simultaneously or sequentially within a breeding season.
METHODS
The social behaviour of Crescent Honeyeaters was intensively studied from 8 August 1996 to 27 February 1997 with additional data being collected infrequently between January 1994 and January 1998. The study site was located on an east-facing slope, 300 m above sea level on Mt Oberon, Wilsons Promontory, Victoria. (39°02′S, 146°20′E). Vegetation characteristics of the site have been described elsewhere (Clarke & Clarke, in press ).
Censuses were conducted to document changes in vocal activity of the Crescent Honeyeater over a 12-month period. Eight fixed census points were selected across the two habitat types within the study area (wet gullies: three sites, dry slopes: five sites). The minimum distance between neighbouring census points was 60 m. All points were censused from August 1996 to February 1997 between the 15th and 20th day of each month and every other month from February 1997 until August 1997. Censuses were conducted between 0700 and 1300 hours Eastern Standard Time (EST). The census technique used was a point count ( Ralph et al. 1993 ). All calls of Crescent Honeyeaters within a 25-m radius of the observer were recorded for a period of 10 min. To control for variation in weather conditions, censuses were not conducted in conditions where winds caused violent movements of branches at canopy height, during moderate to heavy rainfall, and/or when temperatures exceeded 30°C.
A total of 68 Crescent Honeyeaters were colour-banded with unique combinations of three coloured bands and one metal band during the course of this study, with the majority of adults banded in the first 3 weeks of the intensive study period. The identity of colour-banded birds could be determined in the field at distances of up to 70 m with the use of a 25× telescope and 10 × 50 binoculars.
Approximately 3 ha of the site was subdivided into a 20 × 20 m north–south grid. Flagging tape bearing co-ordinates identified each intersection on the grid. Whenever a colour-banded bird was sighted a record was made of: (i) date and time of sighting; (ii) the individual’s identity; (iii) position of the bird relative to the nearest grid point; (iv) height in the forest strata (low, mid, upper); (v) behavioural activity at the time of the sighting; and (vi) vocal activity, including call types, at the time of the sighting.
Sightings of colour-banded birds were recorded opportunistically while conducting other fieldwork. Multiple sightings of the same individual were considered independent of one another provided the bird was out of sight for at least 5 min between observations. Five minutes was considered sufficient to ensure independence of observations, since Crescent Honeyeaters could move from one end of the grid to the other in less than 30 s (RHC, personal observation). Territory maps were constructed by plotting all locations at which each male was observed. The boundary of a male’s territory was determined by recording the locations at which a male sang loudly from an elevated position, the route taken between these singing points and where male–male chases terminated.
At least 4 days during each month of the study were devoted to searching for active nests within the study area. Initially, nests were located by intensive and systematic searches throughout the grid. However, with experience, nests were found more efficiently by carefully monitoring a bird’s behaviour.
Nest watches were carried out to quantify the behaviour of adults at the nest. A nest watch consisted of a 1-h observation period during which the observer remained concealed in a canvas hide, located 4–10 m from the nest, and observed activity at the nest using a 25× telescope. Where possible, nest watches were conducted: (i) during all stages of nest building; (ii) once during early (day 1–5), and once during late (day 8–13) stages of incubation; and (iii) daily during the nestling stage. All nest watches started between 0700 and 1200 hours EST. Nest watches were only conducted when weather conditions were similar to conditions suitable for censusing. An observation period started once a bird had returned to the nest, following the disturbance associated with the observer entering the hide. The identity and time of arrival and departure of all birds approaching to within 2 m of the nest were recorded. Behaviour was assigned to one of nine common categories ( Table 1) or, when it differed greatly from these, was described in detail. All arrivals and departures or changes in behaviour were timed with a stopwatch and recorded to the nearest second on data sheets. In addition to nests at Mt Oberon, three nests located in similar habitat at Squeaky Creek (39°01′S, 146°18′E, 4.25 km west-northwest of the Mt Oberon site) were watched and the data pooled.
| Behavioural state | Definition |
|---|---|
| Arrival (frequency) | approaching to within 0.5 m of the nest |
| Departure (frequency) | leaving from within 0.5 m of the nest |
| Incubating (duration) | sitting on eggs |
| Brooding (duration) | sitting on nestlings |
| Feeding (frequency) | giving food to nestlings |
| Sitting (duration) | sitting on nest rim and not directing gaze towards nest or nestlings |
| Looking (duration) | sitting on nest rim and directing gaze at nest or nestlings |
| Probing nest (duration) | probing nest lining or nest walls with bill |
| Probing nestlings (duration) | probing nestlings with bill |
| Removing faecal sacs (frequency) | consuming faeces or departing with faeces held in bill |
The frequency and duration of behaviours recorded at nests were quantified using The Observer 3.0 ( Noldus Information Technology 1993). Behaviours performed only briefly were quantified as frequencies per 1-h nest-watch; more time-consuming behaviours were quantified as a percentage of the total time per 1-h nest-watch ( Table 1). Data obtained from two or more nests belonging to the same pair were pooled. Hence, sample sizes of nest watch data refer to the total number of different Crescent Honeyeater pairs sampled, rather than to the number of nests observed. Data for the nestling stage were divided into early (days 1–6) or late (days 7–13) stages of nestling development for statistical analysis. Only nest watch data gathered from nests containing three nestlings were included in the analyses presented (n = 16). This was due to the scarcity of broods of other sizes (n = 4) and to control for any effect of brood size upon the behaviours being examined.
Summary statistics are presented as means ± 1 SD throughout.
RESULTS
Space use and social mating system
Male Crescent Honeyeaters occupied and defended discrete territories, rarely straying beyond their territorial boundaries ( Fig. 1). Of 221 sightings of colour-banded males holding territories, only four involved a territory-holding male intruding well into another male’s territory ( Fig. 1a, i, k). Males observed outside their territory boundary behaved inconspicuously, i.e. they did not call and moved through the under-storey rather than through the canopy. Male–male chases were observed on 17 occasions during the breeding season. However, it was usually not possible to identify the males involved in the chases. During the non-breeding season between April and August 1997, all seven males were also observed occupying the same or similar areas to those defended during the breeding season.
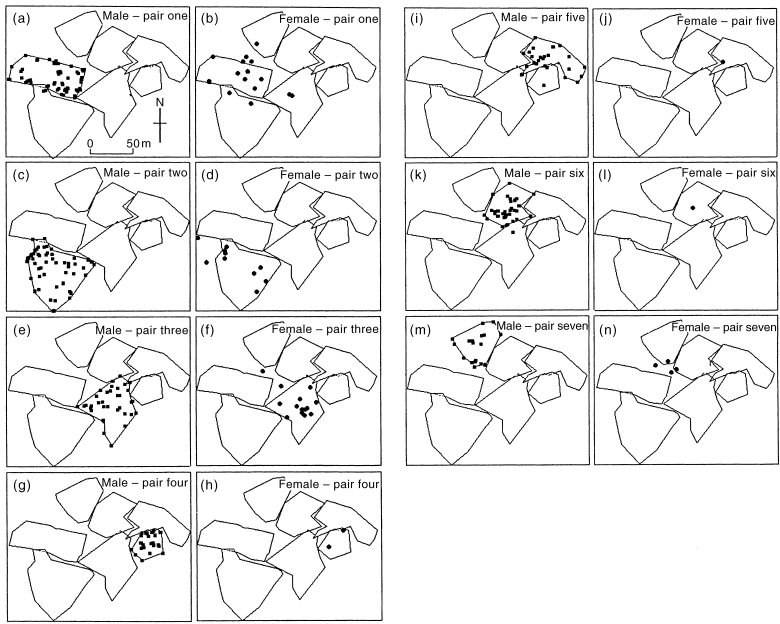
Locations at which colour-banded individuals were observed between August 1996 and February 1997 within the Mt Oberon study area (indicated by dots). Polygons depict the boundaries of male territories.
Male territories typically abutted one another ( Fig. 2). However, certain habitat, such as low, sparse tea tree, Leptospermum sp., was not occupied. Very small areas of most territories overlapped with another (pair one with pair two, pair three with pairs five and six, and pair four with pair five) ( Fig. 2). Only single females were ever observed nesting within a male’s territory at any one time. Paired females mostly confined their activities to within their male’s territory, but because females were less conspicuous or vocal, far fewer observations of females were made. Of 47 sightings of paired females, seven were within the boundaries of a male territory other than their partner’s. Thus females intruded into a non-mate’s territory significantly more often than males intruded into the territories of other males (2 × 2 contingency table χ2 = 16.6; d.f. = 1; P < 0.01). The female of pair one was observed outside the pair one male’s territory during eight out of 15 sightings, five of these being within the boundaries of other males’ territories ( Fig. 1b). Twice, the female of pair one was observed gathering nesting material in the territory of the pair two male. The female of pair two was also sighted out of her male’s territory in an area occupied by an unbanded pair ( Fig. 1d). The remaining two females that occurred outside their corresponding male’s territory did so in areas not known to be occupied by another pair of Crescent Honeyeaters ( Fig. 1f, n). Females of pairs four, five and six were only sighted at active nests ( Fig. 1h, j, l), highlighting the difficulty of locating females away from the nest. Females were never observed chasing other Crescent Honeyeaters.
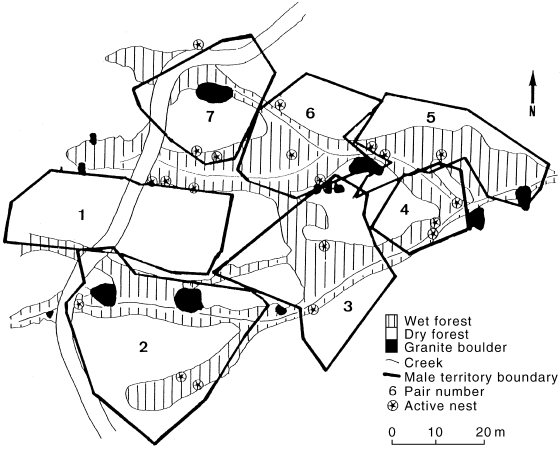
Map of the Mt Oberon study area showing the location of active nests found between August 1996 and February 1997 in relation to the boundaries of male territories and vegetation types.
Among eight breeding pairs, pair-bonds were maintained by all pairs over successive breeding attempts within a season. Among six breeding pairs that were monitored across successive breeding seasons in different years, five were maintained. Just one male (pair 5) was known to have changed mates, and only after the female from the first pairing had disappeared from the study site. Every pair monitored was breeding almost continuously from mid-December 1996 until mid-February 1997. After mid-February, pairs made no apparent attempt to re-nest, such that by late February nesting activity ceased. A single nest was found under construction in June 1997. Pairs were also observed breeding on the same sites during January 1998.
Only the resident pair on each territory contributed to a nesting attempt. No helpers were observed at the nest, although on two occasions unbanded males visited the nests of banded pairs. On the first occasion the intruding male sat passively on the rim of the nest belonging to a marked pair that held a territory off the study site at Mt Oberon and looked at the 5-day-old nestlings for 31 s before departing. On the second occasion an intruding male was observed visiting pair one’s nest. It pecked violently at the 4-day-old nestlings causing them to utter loud calls. The intruder was subsequently chased by the pair-one male. Inspection of the nestlings 40 min later revealed no apparent injuries. However, the three nestlings were taken by an unknown predator in the following 24 h. Unbanded males were not seen again on the pair-one male’s territory during the period of intensive study. In January 1998 pair one was not observed and the territory was occupied by an unbanded pair. At another nest where the parents were unbanded, one of the two nestlings vanished between nest watches on successive days while the other was found dead in the nest with a pin-prick-sized hole in the back of its head.
Roles of the sexes in a nesting attempt
Females alone were responsible for nest construction. When observations started on day 2, visits were made at a rate of 32 per hour (n = 2) and gradually declined to 5.5 visits per hour by day 5 (n = 2), when nests were nearing completion. Males were never observed assisting with nest construction and only on one occasion was a male seen to shadow such a female. The total number of independent observations of females gathering nesting material exceeded 50. Neither parent was observed defending the nest from potential predators in the nest construction, prelaying or laying stages. Although visits by an observer were minimized during the prelaying and laying stages to avoid potential desertion, parents were never seen at the nest during these periods.
Incubation was the sole responsibility of the female. Nest watches in both early and late stages of incubation were made at nests belonging to nine different pairs. In the early stages, females incubated the eggs on average during 79.4 ± 6.1% of an observation period, reducing this to 66.5 ± 3.3% in late stages. Periods of inactivity spent sitting on the nest rim (0.2 ± 0.0% and 0.4 ± 0.2% of the time in early and late stages of incubation) and gentle probing of the nest and eggs (0.4 ± 0.1% and 4.9 ± 0.3% of the time in early and late stages) accounted for the remaining time females spent at the nest during the incubation period. In 65 h of nest observation during the incubation stage at nests belonging to 15 pairs, only twice did the male of a pair visit the nest and then only to look at the contents briefly before departing. On one occasion a male was seen to feed a female approximately 20 m from the nest, when she was in the late stages of incubation.
Both sexes defended nests containing eggs by either flying rapidly at the intruder while uttering harsh scolding notes, or by distracting the intruder with much wing fluttering and scolding while moving erratically through the understorey. However, nest defence was not always employed. Occasionally when the observer was close to an active nest one or both parents would sit passively nearby.
Thirteen pairs attending 16 different nests, each containing three nestlings, were watched for a total of 71 h. Although both sexes attended the nest, females spent more time than males at the nest throughout the nestling period ( Fig. 3). Brooding was solely the role of the female, occupying 50% of a female’s time on day one of the nestling’s development, but steadily declining so that by day eight, brooding had all but ceased ( Fig. 4; Table 2). Females also made more feeding visits to the nest, both in the early and late stages, than did males ( Table 2). Males contributed 30.9% of all feeding visits. Within each sex there was no significant difference in feeding effort between early and late stages of nestling development ( Fig. 5; Table 2). It was not always possible to identify food items brought to nestlings. However, roughly equal numbers of visits were made to bring regurgitated matter, mostly liquid, and invertebrates. Most invertebrates appeared to be flies (Diptera).
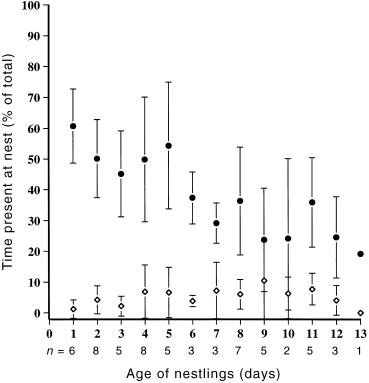
Mean percentage of total time spent at the nest by male (◊) and female (●) Crescent Honeyeaters over the nestling period.
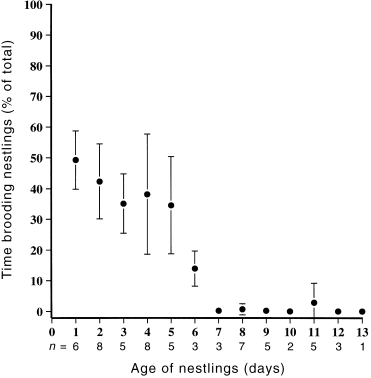
Mean percentage of total time allocated to brooding nestlings by female Crescent Honeyeaters over the nestling period.
| Male | Female | Male versus Female | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early versus Late | Early versus Late | Early (n = 10) | Late (n = 7) | |||||||||||||||||||||||||||||
| Behaviour | Early | Late | Z | P | Early | Late | Z | P | Z | P | Z | P | ||||||||||||||||||||
| Total visits | 2.30 | 2.54 | 0.96 | 0.336 | 5.33 | 6.24 | 2.56 | 0.010 | 2.80 | 0.005 | 2.37 | 0.018 | ||||||||||||||||||||
| (frequency) | (1.23) | (0.87) | (1.08) | (2.29) | ||||||||||||||||||||||||||||
| Brooding bouts | – | – | – | – | 36.36 | 0.59 | 2.91 | 0.004 | – | – | – | – | ||||||||||||||||||||
| (duration) | (11.17) | (1.33) | ||||||||||||||||||||||||||||||
| Feeding visits | 2.23 | 2.54 | 1.12 | 0.262 | 4.41 | 5.79 | 1.21 | 0.228 | 2.29 | 0.022 | 1.99 | 0.046 | ||||||||||||||||||||
| (frequency) | (1.27) | (0.86) | (1.26) | (2.10) | ||||||||||||||||||||||||||||
| Removal of faecal sacs | 0.46 | 0.69 | 0.81 | 0.421 | 1.01 | 2.07 | 0.97 | 0.334 | 2.55 | 0.011 | 2.21 | 0.027 | ||||||||||||||||||||
| (frequency) | (0.48) | (0.88) | (0.93) | (1.06) | ||||||||||||||||||||||||||||
| Nest probing bouts | – | – | – | – | 1.95 | 2.35 | 0.80 | 0.423 | – | – | – | – | ||||||||||||||||||||
| (duration) | (2.27) | (1.87) | ||||||||||||||||||||||||||||||
| Nestling probing | 0.11 | 0.53 | 1.54 | 0.124 | 7.68 | 16.27 | 2.40 | 0.016 | 2.80 | 0.005 | 2.37 | 0.018 | ||||||||||||||||||||
| (duration) | (0.22) | (0.89) | (6.20) | (12.43) | ||||||||||||||||||||||||||||
| Looking bouts | 2.71 | 3.16 | 0.80 | 0.423 | 2.32 | 5.51 | 1.60 | 0.109 | 0.15 | 0.879 | 0.85 | 0.398 | ||||||||||||||||||||
| (duration) | (4.61) | (2.88) | (2.63) | (3.66) | ||||||||||||||||||||||||||||
| Sitting bouts | 0.45 | 1.28 | 0.57 | 0.568 | 0.25 | 1.49 | 1.37 | 0.172 | 0.93 | 0.352 | 0.17 | 0.866 | ||||||||||||||||||||
| (duration) | (0.93) | (2.15) | (0.48) | (1.98) | ||||||||||||||||||||||||||||
- Numbers in parentheses are standard deviations. Comparisons of the behaviours performed during early (n = 6) and late
- (n = 6) stages of nestling development were made using Mann–Whitney U-tests. To achieve independence of samples, no individual was included both in the early and in the late data sets. Z-values were corrected for ties where necessary. Comparisons of the behaviours of male and female parents at the same stages in the nestling period were made using Wilcoxon Matched Pairs Signed Rank tests. Dashes indicate where a behaviour was not observed.
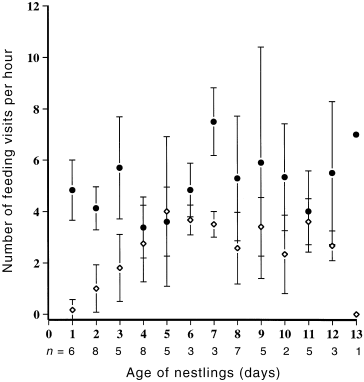
Mean frequency of feeding visits to nestlings by male (◊) and female (●) Crescent Honeyeaters over the nestling period.
The removal of faecal sacs was performed by both sexes, but more often by the female ( Table 2). Only females probed the nest walls and floor. While both sexes probed nestlings, females did so more often and spent more time probing in the later stage of nestling development than in the early stage ( Table 2; Fig. 6). Probing of nestlings was seen to remove remains of faecal sacs, spilt liquid food and feather scales. Bouts of probing were usually preceded by bouts of looking. Periods of inactivity, when little appeared to be done to benefit the nestlings, were spent sitting at the nest. Whilst sitting at the nest birds often closed their eyes and rarely appeared alert. This was the only behaviour at the nest where male effort was approximately equal to that of the female ( Table 2). Both sexes defended nestlings, by using the same methods they used during the incubation stage.
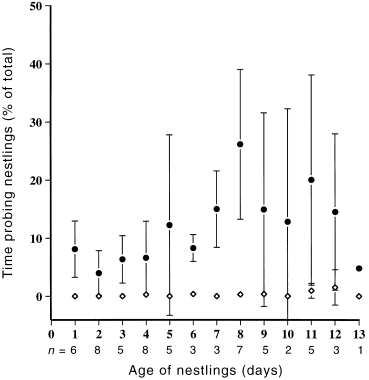
Mean percentage of total time allocated to probing nestlings by the male (◊) and female (●) Crescent Honeyeaters over the nestling period.
Both males and females were observed attending fledglings but parental roles could not be determined, as fledglings were difficult to observe for long. If the female re-nested before fledglings were feeding for themselves (n = 4), the male appeared to be largely responsible for their feeding in the final days of dependence. Although some male fledglings (n = 5) and a female fledgling (n = 1) were known to be alive at the time of their parents’ next breeding attempt, they were not seen to help with these subsequent breeding attempts.
Although males were not as heavily involved in parental care as females, they devoted considerably more time to vocalising than did females. Observations of colour-banded birds revealed that only males gave loud single-, double- and multisyllable calls. Of the 251 observations of colour-banded males, 166 (66%) were of males uttering loud calls and a further 20 (7.9%) were of males uttering soft chattering notes. In contrast, of the 64 sightings of females, five (7.8%) were heard uttering soft chattering notes, the remainder (92.2%) were not calling at all. Males sang from fixed points that remained constant over months. Calling males were observed significantly more often in the upper layer than in mid or low vegetation strata. Males were observed on two occasions performing song flights, uttering a continuous piping call while making a quivering wing flight between singing points.
The calling behaviour of males changed considerably during the breeding season. To examine the nature of changes in calling activity and in what habitats they were occurring, calls were classified as a single syllable, calls with two syllables and calls with three or more syllables (‘multisyllable’). The most significant change in call rates occurred in the number of multisyllable calls given in wet gullies ( Table 3, Fig. 7). Multisyllable calls were most commonly given in January, the height of the breeding season, but were absent in February, when no new nesting attempts were started. Double-syllable call rates also peaked in wet gullies over the spring–summer period ( Table 3, Fig. 7). The majority of double-syllable calling in wet gullies occurred between November and January, with a smaller peak in June. By contrast, there were no significant changes between months in the frequency of single-syllable calls in both habitats ( Table 3, Fig. 7).
| Call type | Habitat | Differences between | ||
|---|---|---|---|---|
| months | ||||
| d.f. | F | P-value | ||
| Single-syllable calls | Wet gullies | 9 | 1.782 | 0.142 |
| Dry slopes | 9 | 2.049 | 0.062 | |
| Double-syllable calls | Wet gullies | 9 | 2.928 | 0.025 |
| Dry slopes | 9 | 1.476 | 0.164 | |
| Multisyllable calls | Wet gullies | 9 | 5.115 | 0.002 |
| Dry slopes | 9 | 0.601 | 0.787 | |
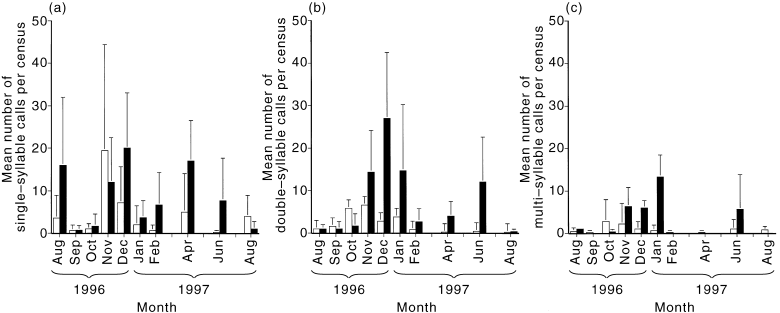
Mean number of (a) single-syllable, (b) double-syllable and (c) multisyllable Crescent Honeyeater calls per 10-min census in wet gullies (n = 3) and dry slopes (n = 5) at Mt Oberon between August 1996 and August 1997.
DISCUSSION
Social mating system
Male Crescent Honeyeaters occupied discrete but abutting territories throughout the year on Mt Oberon. Unlike the socially promiscuous or polygynous sexually dimorphic hummingbirds (e.g. Wolf & Wolf 1971; Snow 1974), male Crescent Honeyeaters were only ever observed to have a single female nesting within their territory at any time. The male’s defence both of the nest and of the feeding resources within a single contiguous boundary was similar to that reported for congeneric New Holland Honeyeaters occupying similar forested habitats ( McFarland 1986). However, Crescent Honeyeater territories were more clumped, forming a loose colony. Unfortunately, we do not have sufficient data to determine whether territorial boundaries were as intensely defended and defined in the non-breeding season as in the breeding season. Nevertheless, males clearly remained in the same locations within the site throughout the year.
While only a single female was ever observed nesting within each male’s territory, females were observed intruding into the territories of neighbouring males much more frequently than males. Intrusions included two cases where the female was conspicuously gathering nesting material – a stage in the reproductive cycle when fertilization typically occurs in passerines ( Murton & Westwood 1977). Females were never observed being chased from the territories of neighbouring males. This could be because females were not being detected by their neighbours. However, while only four males were identified intruding on the territories of others, 17 male–male chases were observed, suggesting that territory-holding males are quite capable of detecting intruders long before the observer can. Of the four males observed intruding none was observed to call whilst intruding. It is therefore more likely that males permit non-mate females to enter and remain in their territories. Such behaviour may increase the male’s chances of mating with multiple females. Studies of the Red-winged Blackbird, Agelaius phoeniceus, have shown that polygynous males enjoy increased reproductive success compared with monogamous males ( Weatherhead & Boag 1997).
Not only do males appear to tolerate neighbouring females in their territories, it appears that some males may attempt to kill their neighbour’s offspring. Although not confirmed, the attack on a nestling by an intruding male we observed closely resembled an incidence of infanticide reported in the Noisy Miner by Whitmore (1986). The single dead nestling found in another nest had sustained injuries consistent with those reported for infanticide in North American passerines ( Chek & Robertson 1991).
Pairs remained together across several successive breeding attempts (often unsuccessful) within a breeding season and across at least three breeding seasons. No cases of ‘divorce’ were observed within or across breeding seasons (Clarke & Clarke, in press). Such long-term persistence of social pair-bonds has also been observed in monomorphic nectarivorous (e.g. New Holland Honeyeaters, Paton (1979)) and insectivorous honeyeaters (e.g. Bell Miner- Clarke 1988)).
Parental care
Male Crescent Honeyeaters provided parental care only to the young of the female which nested in his territory. Females performed the majority of parental activities more often and for longer than males. The contribution by the male Crescent Honeyeater, relative to females (30.9 cf., 69.1%), was substantially smaller than has been described for monomorphic honeyeaters. The rate of feeding visits to nestlings by male and female parents was the same in the sexually monomorphic Brown-backed Honeyeater ( Maher 1996), but male Bell Miners made 38.6% of visits ( Clarke 1988), while in the New Holland Honeyeater, males made 39.3% of visits ( Paton 1979). We found no evidence of helpers at the nest, in contrast to the common occurrence of this behaviour among sedentary monomorphic honeyeaters ( Clarke 1995).
Our estimate of the male’s contribution to parental care may be biased by observations restricted to the nest area. We once saw a male feeding his mate 20 m from the nest in the late stage of incubation, which suggests one possible route by which males may have contributed to the pair’s reproductive effort. However, feeding of mates away from the nest has not been previously reported in other intensive studies of honeyeaters, except in courtship feeding in the prenesting stages ( Dow 1978), and is unlikely to be a common occurrence.
Male Crescent Honeyeaters did not contribute as much to raising young as the females but they devoted considerably more time to territorial defence and singing than did females. The male’s repertoire is diverse and very loud in comparison to the female’s. We assigned all calls to three categories because investigating their functions in detail was beyond the scope of this study (at least 18 different calls have been recorded from two males, M. Clarke, unpublished data, 1996). We found that increased rates of double- and multisyllable calling by males coincided with the timing of breeding and defence of territories, suggesting they may have both a territorial advertisement and a courtship function. This strong association provides a previously unreported but useful cue when trying to determine whether Crescent Honeyeaters are breeding in an area. Single-syllable calls given by males throughout the year may be to maintain contact with their mate.
Song flights have not previously been reported in the Crescent Honeyeater and appear to be rare. However, song flights have been observed being performed by the congeneric New Holland Honeyeater in heathland and open woodland ( Recher 1977; Rooke 1979). Jurisevic and Sanderson (1994) argued that song flights were most often performed by species that had reduced access to elevated singing posts (e.g. Noisy Miner and Yellow-plumed Honeyeater (Lichenostomus ornatus)). In well-wooded areas such as those inhabited by the Crescent Honeyeaters in this study, singing from elevated posts may replace song flights as a more energy-efficient method of advertising territories.
Male Crescent Honeyeaters only occasionally chased other males, which may reflect the infrequency with which neighbouring males enter one-another’s territories. As all neighbouring male territories remained stable during the period of intensive study, there was no opportunity to investigate behaviour associated with the establishment of territory boundaries. If all males know their neighbours’ boundaries and regularly reinforce their own through singing, it would be energetically inefficient for a male to intrude and subsequently be involved in a chase unless the benefit of doing so was high. As unbanded males were also rarely observed within banded males’ territories, the frequency with which apparent floaters enter territories appears low. Studies of the North American Hooded Warbler, Wilsonia citrina, have shown disputes between males of adjoining territories occur less frequently between those that have been long-term neighbours, compared with those that have only recently become established, and that recognition is at least in part based on the neighbour’s song ( Godard 1991).
In conclusion, the pattern of social organization we observed, where pairs occupied discrete territories and exhibited bi-parental care, is typical of a socially monogamous species, and unlike that of other sexually dimorphic nectarivores such as the hummingbirds. However, male Crescent Honeyeaters contributed far less towards parental care than females, possibly engaged in infanticide and allowed neighbouring females onto their territories. It is therefore quite likely that the genetic mating system in this population may not be monogamy. Genetic analysis of the parentage of the young raised by these social pairs will enable the testing of this hypothesis. It would appear that the Crescent Honeyeaters we studied were part of a sedentary population. This species is clearly not a year-round resident throughout all of its range ( Keast 1968; Loyn 1985) and it remains to be seen whether non-sedentary populations of this species exhibit a similar form of social organization and whether either is typical of other sexually dimorphic honeyeaters.
Acknowledgements
We are grateful to Jim Whelan of the Department of Natural Resources and Environment (DNRE) Wilsons Promontory, for his enthusiastic support of the project from its inception and Craig McKenzie, DNRE Wilsons Promontory, for arranging access to a number of field sites. Peter Attiwill from the University of Melbourne arranged accommodation at Wilsons Promontory throughout the intensive study period. We are grateful to Keith Clarke and George Appleby for assisting with nest watches, and to Floyd Connor for recording and Susan Maxwell for tabulating the range of calls of the Crescent Honeyeater on the site. Peter Griffioen assisted greatly in the mapping of the survey sites. We are grateful to Merilyn Grey for helpful comments on early drafts of this paper and to Ian Rowley and Hugh Ford who reviewed the manuscript.
This study was carried out in accordance with the conditions of permits from the La Trobe University Animal Experimentation and Ethics Committee, the Australian Bird and Bat Banding Scheme and the Victorian Department of Natural Resources and Environment.




