Brain potentials associated with the onset and offset of rapid eye movement (REM) during REM sleep
Abstract
Abstract The relationship between dreaming and rapid eye movements (REM) during REM sleep is still controversial. This study records the brain potentials time-locked to the onset and offset of REM in 11 healthy young volunteers. Before the onset of REM, no presaccadic readiness potential was found. Conversely, two positive potentials (P1 and P2) appeared following the offset of REM. The latter potentials were dominant in the parieto-occipital area. These findings suggest that REM is initiated without preparation but elicits some information-processing activities that were speculated to occur in the cortical visual area. The data support the activation–synthesis or association hypothesis of dreaming rather than the scanning hypothesis.
INTRODUCTION
Hypotheses about the relationship between rapid eye movements (REM) during REM sleep and dreaming can be divided roughly into two types. One is the ‘scanning hypothesis’,1 which suggests that REM reflects the dreamer’s voluntary exploration of a visual image in a dream. The other type includes the ‘activation–synthesis hypothesis’2 and the ‘sensory image-free association hypothesis’,3 both of which suggest that REM occurs spontaneously without voluntary control and generate a dream image via the activation of relevant cortical areas.
In the present study, we investigated brain potentials preceding REM in REM sleep. In wakefulness, a negative slow readiness potential occurs before voluntary saccadic eye movements (presaccadic negativity).4 Thus, if REM in sleep occur voluntarily as proposed in the scanning hypothesis, they might be preceded by a similar negative slope. In addition, we recorded brain potentials following REM to confirm whether, as reported previously, REM elicits positive potentials that resemble the λ response in wakefulness, which reflects post-saccadic visual information processing.5,6
SUBJECTS AND METHODS
Eleven healthy university students (age range 20– 22 years) participated in the study. Polysomnogram of nocturnal sleep was recorded following an adaptation night. Electroencephalogram (EEG) was recorded at 25 scalp sites (Fp1, Fp2, F3, F4, FC3, FC4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, FCz, Pz, POz, Oz, A1, and A2) referenced to Cz (τ = 3.2 s; 100 Hz high-cut filter was used). Horizontal and vertical electro-oculograms (EOG) were recorded from the outer canthi of both eyes and from above and below the left eye. The sampling rate was 250 Hz. Only the data during the epoch scored as ‘REM sleep’ according to the standard sleep stage criteria were analysed. The onset and offset of REM were determined by visual inspection. To examine the presaccadic negativity, the epoch between 700 ms before and 300 ms after the onset of REM were averaged. For the λ-like response, the epoch between 500 ms before and 500 ms after the offset of REM were averaged. Epochs containing artifacts were removed. All averaged waveforms were re-referenced to linked earlobes. The baselines were the first 100-ms period for presaccadic negativity and the first 300-ms period for the λ-like response.
RESULTS
Figure 1 shows the grand mean waveforms of the presaccadic negativity and of the λ-like response. Although fluctuation due to background EEG still exists, no reliable presaccadic negativity appears at Cz, which is where this activity should be most dominant. The mean amplitude of the epoch preceding REM was – 0.04 ± 3.29 μV, which did not differ from zero (one-tailed t-test, P > 0.1). Alternatively, two positive potentials (P1 and P2) occurred after the offset of REM with peak latencies of 66.9 ± 22.5 ms and 141.5 ± 19.4 ms. The mean amplitudes of P1 and P2 were 3.7 ± 2.9 μV and 4.2 ± 2.5 μV at POz. Both of these positive potentials were dominant in the parieto-occipital areas.
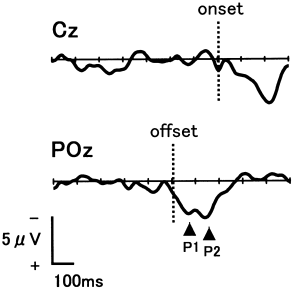
. Grand mean waveforms time-locked to the onset [the average of 290 electroencephalogram (EEG) tracings: Cz] and offset (596 EEG tracings: POz) of rapid eye movements (N = 11).
DISCUSSION
In the present study, no presaccadic negativity was noted before the onset of REM during REM sleep, which indicates that REM in REM sleep occurs spontaneously without voluntary control. Conversely, the λ-like response (P1 and P2) was noted after the offset of REM, which is in agreement with findings of previous studies.5,6 The posterior scalp distribution of these potentials suggests that REM is followed by brain activities similar to those that occur in visual information processing. However, the λ-like response in REM sleep seemed distributed slightly more towards the anterior than the λ response in wakefulness, which should be dominant at occipital sites.
In conclusion, we found new evidence in favour of the activation–synthesis or association hypothesis of dreaming that proposes that dream images do not precede but follow REM in REM sleep.




