Microalgal Communities of the Sea Ice, Ice-Covered and Ice-Free Waters of Wood Bay (Ross Sea, Antarctica) During the Austral Summer 1993 – 94
Abstract
Abstract. During the austral summer 1993 – 94, microalgal density and biomass were investigated in the sea ice, in the underlying water column, in the melt water and during the formation of the sea ice. Of the 96 taxa identified, 59 were diatoms and 32 were dinoflagellates. Among the remaining five species, Pseudopleurochloris antarctica was very abundant both in the sea ice and in the ice-free waters. Cell densities and biomass of microalgae were higher in the bottom of the sea ice and during the formation of the ice than in the seawater, and diatoms were the dominant group at higher microalgal biomass. Among these, Entomoneis kufferathii, Chaetoceros dichaeta and Fragilariopsis species were the most common taxa.
Problem
Sea ice is the major habitat for microorganisms in the Antarctic marine ecosystems and its seasonal development and degradation are a key factor influencing the ecology of microalgal communities in the Southern Ocean. During its development, in fact, most of the microalgae are incorporated into the ice, creating a potential inoculum for the spring algal bloom in the marginal ice-edge zone (Palmisano & Garrison, 1993). However, the concentrations of algae and the microbial assemblages in the sea ice vary according to ice type (Leventer, 1998). For example, diatom assemblages obtained from the fast ice around Prydz Bay, Antarctica, are different from those obtained from the pack ice in the same area (Scott et al., 1994; McMinn, 1996). Moreover, in the pack ice the microbial communities are usually internal or concentrated in a surface or subsurface brine layer. In the land-fast ice, these are often more conspicuous at or near the bottom of the sea ice. These differences are the result of several physical processes (i. e., structural characteristics of the ice, variations in salinity, light levels and nutrient concentrations) which influence near-shore and deepwater areas (Gleitz et al., 1998).
Most investigations of Antarctic sea-ice biota have focused on systematic studies of the ice diatoms, which usually dominate ice-algal assemblages. More recent studies, however, document that sea ice contains a variety of nano- and microflagellates (i. e., Pyramimonas gelidicola), gelatinous colonies of Phaeocystis antarctica, cysts and vegetative cells of chrysophytes and dinoflagellates and cysts of archeomonads (Stoecker et al., 1992; Palmisano & Garrison, 1993). Under the sea ice the presence of microalgae is confined only to the platelet ice. These layers constitute a favourable environment for ice biota development but are confined mainly under the land-fast ice and in regions adjacent to floating ice shelves (Palmisano & Garrison, 1993; Arrigo et al., 1995; Grossmann et al., 1996; Günther & Dieckmann, 1999).
The purpose of this paper is to describe the distribution of microalgal density and biomass in the sea ice, in the water column under the sea ice, in the melt water and during the formation of the sea ice. This study was carried out at the same sampling station to verify the changes in algal assemblages during these phases, which are typical of the Southern Ocean.
Material and Methods
Ice cores were taken from the sea ice of Wood Bay (Ross Sea) (74° 22' 12" S, 165° 24' 06" E) on 29 October 1993 (Fig. 1). Six replicate ice cores were taken 1 m apart using an 11 cm diameter SIPRE corer. After extraction, cores, 3 m in length, were cut into 50 cm sections. Back in the Terra Nova Bay Station, these subsamples were melted in the dark at 4 °C. After melting they were mixed to obtain only one sample for each level. This not gentle melting technique could have caused the loss of naked flagellates. Subsequently, in different periods and in the same sampling site, samples of seawater were collected using Niskin bottles from 6 depths (0, 10, 25, 50, 100 and 250 m). Simultaneously, chlorophyll a was measured by an Idromar Memo 15 multiparametric probe. Three samplings were carried out under the sea ice from the corer hole (7, 19 and 28 November 1993), two in the ice-free water (9 and 30 January 1994) and one during the ice formation (10 February 1994). On these samples and on the melted sea ice ones we identified and counted nano- and microphytoplanktonic species. During the ice core samplings, bad weather and logistical problems did not allow the collection of the seawater samples. Also during the ice formation, the presence of drift ice and bad weather prohibited sampling on the surface.
Sampling station (filled circle) in Wood Bay. The hatched line indicates the edge of the seawater ice.
Melted ice and water samples (500 ml) for the floristic analyses were preserved with glutaraldehyde-Lugol (35 %) solution (final concentration 1 %) and stored in glass bottles in the dark at 4 °C until they were analysed. Quantitative analyses of all microalgae larger than 2 µm were carried out on settled samples using a Zeiss IM35 inverted microscope (Utermöhl, 1958). A distinction between photosynthetic and non-photosynthetic species was made using the information available in the literature (Chrétiennot-Dinet, 1990; Larsen & Sournia, 1991). Cell volumes were calculated by assigning to cells one geometrical body or sometimes a combination of several geometrical bodies, and applying standard formulae. Cell carbon biomass was estimated from cell volumes and cell abundance. The cell volume values were converted to carbon (µg C·l–1) using the equations of Eppley et al. (1970).
We followed Van Landingham (1967 – 1979), Medlin & Priddle (1990), Hasle (1993) and Hasle & Syvertsen (1996) for diatom nomenclature. The books of reference for dinoflagellates were Balech (1976), Dodge (1982) and Steidinger & Tangen (1996), and Throndsen (1993) was used for the other marine flagellates.
Results
Forty samples were examined and 96 taxa were identified. Fifty-nine taxa were diatoms and thirty-two were dinoflagellates. The remaining five species were the xanthophycean Pseudopleurochloris antarctica (Andreoli et al., 1999), the dictyochophycean Dictyocha speculum, the haptophycean Phaeocystis sp., and the prasinophyceans Mantoniella antarctica and Pyramimonas gelidicola (Table 1). Most diatom taxa (44) were in the sea ice and almost all of them were also found in the seawater. Only Actinocyclus cholnokyi, Amphora ovalis, Asteromphalus heptactis, Corethron criophilum, Coscinodiscus bouvet, C. gracilis, C. oculos-iridis, Guinardia cylindrus, Pseudonitzschia turgiduloides, Stauroneis sp., Stellarima microtrias, Synedra tabulata, Thalassiosira australis, T. gravida and T. tumida were found in the water column (Table 1).
| taxa | vol | SI | USI | W | IF | nS | max | min |
|---|---|---|---|---|---|---|---|---|
| Bacillariophyceae | ||||||||
| Achnanthes brevipes Agardh | 12259 | + | – | – | + | 6 | 1600 | 40 |
| Actinocyclus actinochilus (Ehrenberg) Simonsen | 91986 | + | + | + | + | 13 | 200 | 20 |
| Actinocyclus cholnokyi Van Landigham | 74492 | – | + | + | + | 4 | 200 | 15 |
| Amphipleura rutilans (Trentepohl) Cleve | 1967 | + | – | – | – | 4 | 18160 | 40 |
| Amphora ovalis (Kützing) Kützing | 5811 | – | + | – | – | 1 | 10 | |
| Asteromphalus heptactis (Brèbisson) Ralfs | 101108 | – | – | – | + | 2 | 160 | 40 |
| Asteromphalus sp. | 37257 | + | – | – | + | 3 | 600 | 240 |
| Auricola compacta (Hustedt) Medlin | 66801 | + | + | – | – | 3 | 15440 | 40 |
| Chaetoceros atlanticus Cleve | 4568 | + | – | + | + | 11 | 400 | 40 |
| Chaetoceros dichaeta Ehrenberg | 1296 | + | – | – | – | 6 | 345532 | 1240 |
| Chaetoceros sp. | 2329 | + | – | + | + | 8 | 1200 | 40 |
| Cocconeis fasciolata (Ehrenberg) Brown | 20993 | – | – | – | + | 1 | 40 | |
| Corethron criophilum Castracane | 68427 | – | + | – | + | 2 | 40 | 20 |
| Coscinodiscus bouvet Karsten | 358564 | – | – | + | – | 2 | 200 | 120 |
| Coscinodiscus gracilis (Karsten) Hustedt | 6135 | – | + | – | – | 1 | 20 | |
| Coscinodiscus oculos-iridis Ehrenberg | 140603 | – | + | + | – | 7 | 200 | 20 |
| Coscinodiscus tabularis (Grunow) Fryxell & Sims | 14299 | + | + | + | + | 19 | 1440 | 10 |
| Coscinodiscus sp. | 178926 | + | – | + | + | 5 | 160 | 40 |
| Cylindrotheca closterium (Ehrenberg) Reimann & Lewin | 663 | + | – | + | + | 12 | 1520 | 40 |
| Entomoneis kufferathii Mangin | 41306 | + | + | – | – | 5 | 861141 | 680 |
| Eucampia antarctica (Castracane) Mangin | 111182 | + | + | + | + | 12 | 1000 | 10 |
| Fragilariopsis curta (Van Heurck) Hustedt | 848 | + | + | + | + | 40 | 888052 | 60 |
| Fragilariopsis cylindrus (Grunow) Krieger | 658 | + | + | + | + | 40 | 123789 | 40 |
| Fragilariopsis obliquecostata (Van Heurck) Hustedt | 6432 | + | + | + | + | 21 | 2880 | 10 |
| Fragilariopsis rhombica (O’Meara) Hustedt | 2364 | + | + | + | + | 14 | 1600 | 20 |
| Fragilariopsis ritscherii (Hustedt) Hasle | 7215 | + | + | + | + | 27 | 5040 | 10 |
| Fragilariopsis separanda (Hustedt) Hasle | 1942 | + | + | + | – | 11 | 200 | 10 |
| Fragilariopsis sublinearis Hasle | 2440 | + | + | + | + | 20 | 1400 | 10 |
| Fragilariopsis spp. | 900 | + | + | + | + | 35 | 471474 | 20 |
| Guinardia cylindrus (Cleve) Hasle | 1346 | – | – | + | – | 1 | 120 | |
| Navicula cancellata Donkin | 3053 | + | + | – | – | 3 | 2240 | 160 |
| Navicula directa (Wm Smith) Ralfs | 5715 | + | – | – | – | 2 | 80 | 20 |
| Navicula glaciei Van Heurck | 752 | + | – | + | – | 4 | 120 | 40 |
| Navicula spp. | 7967 | + | + | + | + | 9 | 9400 | 40 |
| Nitzschia arctica Cleve | 2925 | + | – | + | – | 2 | 320 | 120 |
| Nitzschia neglecta Hustedt | 2210 | + | – | – | – | 3 | 1840 | 200 |
| Nitzschia stellata Mangin | 2349 | + | + | – | – | 3 | 21040 | 1760 |
| Nitzschia spp. | 8000 | + | + | + | + | 20 | 4320 | 10 |
| Nitzschiella subcurvata Hasle | 129 | + | – | – | + | 3 | 80 | 40 |
| Odontella sp. | 190806 | + | – | – | – | 1 | 20 | |
| Pinnularia quadratarea (A Schmidt) Cleve | 29632 | + | + | – | – | 6 | 11840 | 20 |
| Pleurosigma directum Grunow | 85006 | + | – | – | + | 4 | 35040 | 40 |
| Pleurosigma sp. | 88832 | + | + | – | – | 2 | 40 | 10 |
| Porosira glacialis (Grunow) Jorgensen | 40770 | + | – | – | – | 1 | 40 | |
| Proboscia alata (Brightwell) Sundstrom | 10911 | + | – | – | – | 4 | 320 | 40 |
| Pseudonitzschia seriata (Cleve) Peragallo | 537 | + | – | + | + | 9 | 2600 | 280 |
| Pseudonitzschia turgiduloides Hasle | 132 | – | – | + | + | 12 | 400 | 10 |
| Rhizosolenia styliformis Brightwell | 60043 | + | – | + | – | 5 | 120 | 40 |
| Rhizosolenia truncata (Karsten) Nothig & Ligowski | 15346 | + | – | – | – | 5 | 160 | 20 |
| Rhizosolenia spp. | 58250 | + | – | + | + | 6 | 80 | 40 |
| Stauroneis sp. | 34562 | – | – | + | – | 1 | 160 | |
| Stellarima microtrias (Ehrenberg) Hasle & Sims | 138303 | – | + | + | + | 12 | 520 | 10 |
| Synedra tabulata (Agardh) Kützing | 1275 | – | – | + | + | 2 | 120 | 40 |
| Synedra sp. | 5713 | + | – | + | + | 3 | 1680 | 40 |
| Thalassiosira australis Peragallo | 7256 | – | + | – | – | 2 | 210 | 30 |
| Thalassiosira gravida Cleve | 30293 | – | + | + | – | 2 | 240 | 80 |
| Thalassiosira tumida (Janisch) Hasle | 205487 | – | – | + | + | 12 | 3480 | 40 |
| Thalassiosira sp. | 104827 | + | + | + | + | 8 | 360 | 40 |
| Tropidoneis sp. | 35199 | + | + | – | – | 6 | 10420 | 20 |
| Xanthophyceae | ||||||||
| Pseudopleurochloris antarctica Andreoli et al. | 104 | + | + | + | + | 22 | 236383 | 1614 |
| Dictyochophyceae | ||||||||
| Dictyocha speculum Ehrenberg | 19081 | + | + | + | + | 12 | 32200 | 10 |
| Dinophyceae | ||||||||
| Amphidinium hadai Balech | 1259 | – | + | + | + | 21 | 1520 | 10 |
| Amphidinium sp. | 10870 | – | – | + | + | 15 | 4800 | 10 |
| Dinophysis contracta (Kofoid & Skogs.) Balech | 15904 | – | – | + | – | 2 | 80 | 40 |
| Dinophysis tenuivelata Balech | 24052 | – | – | – | + | 1 | 40 | |
| Gyrodinium glaciale Hada | 20602 | – | + | + | – | 2 | 80 | 60 |
| Gyrodinium lachryma (Meunier) Kofoid & Swezy | 50586 | – | + | + | – | 9 | 1880 | 20 |
| Gyrodinium spp. | 20276 | – | + | + | + | 17 | 13440 | 10 |
| Polarella glacialis Montresor et al. | 1695 | + | + | + | + | 21 | 188912 | 40 |
| Protoperidinium affine (Balech) Balech | 45896 | – | – | + | – | 2 | 240 | 160 |
| Protoperidinium antarcticum (Schimper) Balech | 818507 | – | – | + | – | 1 | 80 | |
| Protoperidinium applanatum (Mangin) Balech | 18691 | – | + | + | + | 23 | 1000 | 10 |
| Protoperidinium archiovatum (Balech) Balech | 15945 | – | – | + | + | 9 | 360 | 20 |
| Protoperidinium areolatum (Peters) Balech | 184419 | – | + | – | – | 1 | 10 | |
| Protoperidinium bellulum (Balech) Balech | 9621 | – | – | + | – | 1 | 40 | |
| Protoperidinium bipatens Balech | 67928 | – | – | + | – | 1 | 80 | |
| Protoperidinium bipes (Paulsen) Balech | 2659 | – | + | + | + | 11 | 19800 | 10 |
| Protoperidinium charcoti (Balech) Balech | 67928 | – | – | – | + | 1 | 40 | |
| Protoperidinium concavum (Mangin) Balech | 106239 | – | – | + | – | 6 | 240 | 40 |
| Protoperidinium curtum (Balech) Balech | 36769 | – | – | + | – | 1 | 40 | |
| Protoperidinium defectum (Balech) Balech | 10602 | – | – | + | – | 1 | 120 | |
| Protoperidinium elegantissimum (Balech) Balech | 11780 | – | – | – | – | 1 | 10 | |
| Protoperidinium incertum (Balech) Balech | 44614 | – | – | + | + | 11 | 1960 | 10 |
| Protoperidinium incognitum (Balech) Balech | 14341 | – | + | + | + | 9 | 600 | 10 |
| Protoperidinium macrapicatum (Balech) Balech | 39082 | – | – | + | – | 2 | 120 | 40 |
| Protoperidinium mediocre (Balech) Balech | 11569 | – | + | + | + | 8 | 200 | 10 |
| Protoperidinium parcum (Balech) Balech | 6273 | – | + | + | – | 2 | 40 | 20 |
| Protoperidinium pseudoantarcticum (Balech) Balech | 566756 | – | – | + | – | 6 | 280 | 40 |
| Protoperidinium raphanum (Balech) Balech | 20200 | – | + | + | – | 4 | 160 | 10 |
| Protoperidinium saltans (Meunier) Balech | 169392 | – | – | + | – | 1 | 160 | |
| Protoperidinium thulesense (Balech) Balech | 26023 | – | – | + | – | 1 | 40 | |
| Protoperidinium spp. | 24818 | – | + | + | + | 28 | 3240 | 20 |
| Zygabikodinium perlata Balech | 17638 | – | – | + | – | 1 | 80 | |
| Haptophyceae | ||||||||
| Phaeocystis sp. | 73 | – | – | + | + | 3 | 15440 | 1320 |
| Prasinophyceae | ||||||||
| Mantoniella antarctica Marchant | 646 | – | – | + | + | 8 | 600 | 40 |
| Pyramimonas gelidicola McFadden | 494 | – | – | + | + | 5 | 11840 | 40 |
In contrast, dinoflagellates were more abundant in the seawater than in the sea ice, where only Polarella glacialis (Montresor et al., 1999) was found both in the vegetative and hypnozygote stages. Most of these taxa, in particular the Protoperidinium species, are heterotrophic (Palmisano & Garrison, 1993). Phaeocystis sp., probably Phaeocystis antarctica (Medlin et al., 1994), was also absent in the sea ice. The low presence of dinoflagellates and the absence of naked flagellates in the sea ice were probably due to the melting technique. However, since the core sampling was carried out in spring, we accepted the loss of these organisms, which make up only about 2 % of total autotrophic biomass in spring sea ice (Garrison & Mathot, 1996; Günther & Dieckmann, 1999).
Other microalgae, not easily identifiable with the light microscope, inhabited both the sea ice and seawater. Pseudopleurochloris antarctica is an example: it was identified only after the analyses of its pigment composition, ultrastructural features, and 18S rDNA gene sequence (Andreoli et al., 1999). Some of these unidentified organisms, like Pseudopleurochloris antarctica, were isolated and taken into culture in order to obtain their exact identification.
The sea ice and the forming ice samples were richer in microalgae than those of both the seawater under sea ice and of the ice-free seawaters. In the sea-ice samples and in the layer near the water, densities reached 4,200 cells·ml–1; in the forming ice samples the density values were 7,900 cells·ml–1 (Fig. 2). In the sea ice, the higher values were produced mainly by Pseudopleurochloris antarctica (2,300 cells·ml–1) and by diatoms (1,800 cells·ml–1) such as Entomoneis kufferathii, Chaetoceros dichaeta, Fragilariopsis curta and F. cylindrus (Fig. 3A). During the ice formation, however, high values were produced only by diatoms (6,800 cells·ml–1), in particular by Fragilariopsis curta (880 cells·ml–1), F. cylindrus (1,200 cells·ml–1) and Fragilariopsis spp. (4,700 cells·ml–1) (Fig. 3B).
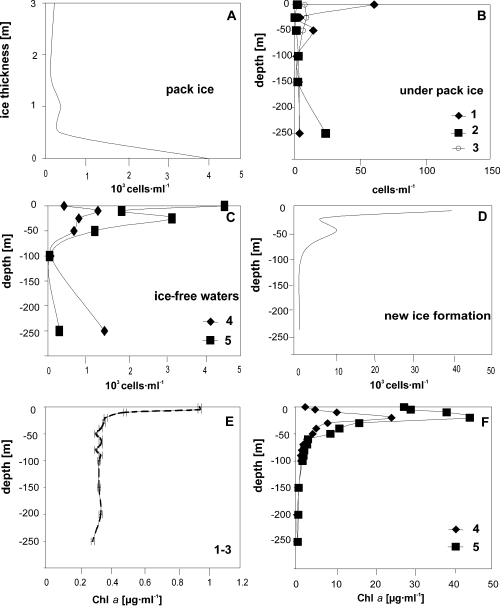
Depth distribution of microalgal densities in the pack ice (A), underlying water column (B), in ice-free seawater column (C) and during the new ice formation (D). In E and F the fluorimetric data of the underlying water column (E) and in ice-free seawaters (F) are reported. Note the lower scales of the density values in the water column under the ice and the higher scale in the sea ice and during the new ice formation (the numbers 1 – 5 refer to the samplings).
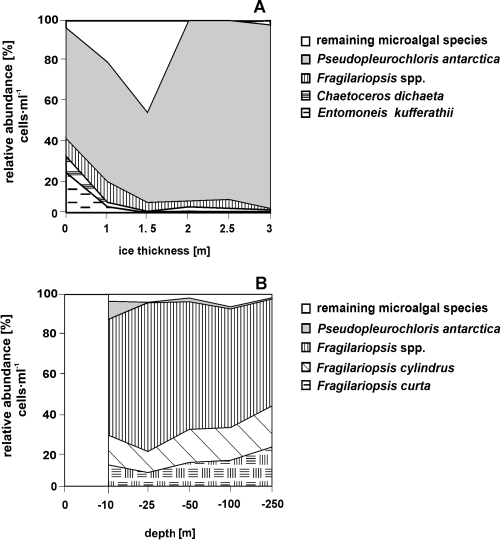
Relative abundance [% cell number] of microalgae in the samples of pack ice (A) and in the seawater column during the new ice formation (B). There is no surface data in this last figure.
In contrast, the phytoplanktonic density, as revealed by the fluorimetric data (Fig. 2E), was very low (5 – 6 cells·ml–1) in the water column underlying the sea ice. The exceptions were 61 cells·ml–1 in the first sampling (at the surface), where diatoms prevailed, and 20 cells·ml–1 (at –250 m) in the second sampling, where Pseudopleurochloris antarctica was the richest species.
In the ice-free waters, also based on the fluorimetric data, high phytoplanktonic densities were registered in the surface layers (1,300 cells·ml–1 at –10 m in the 4th sampling and 4,500 cells·ml–1 on the surface in the 5th sampling) (Fig. 2). The 1,400 cells·ml–1 recorded in the 4th sampling at –250 m, on the other hand, could be the result of sedimentation of phytoplanktonic cells to the bottom. In these samples, Fragilariopsis curta, F. cylindrus and Fragilariopsis spp. were very abundant and, in the 5th sampling in the surface layer, they were also accompanied by a large amount of Pseudopleurochloris antarctica. The dinoflagellate densities, dominated by Protoperidinium spp. and Polarella glacialis (vegetative cells and hypnozygotes), however, were lower and ranged from 0.36 cells·ml–1 (4th sampling at –100 m) to 220 cells·ml–1 (5th sampling in the surface layer). Small flagellate forms were also present with values of about 100 cells·ml–1.
Like the density values, the microalgal biomass confirms that the sea ice, in particular the layer near the sea, is more productive (1.6 µg C·ml–1) than the water under the sea ice (< 0.03 µg C·ml–1) and the ice-free seawaters (< 0.2 µg C·ml–1) (Fig. 4). The biomass values were also high (0.43 µg C·ml–1) during the ice formation and were determined by diatoms (Fig. 5), unlike the density values. In ice-free seawater, however, the biomass values were due to the dinoflagellates (0.02 – 0.12 µg C·ml–1).
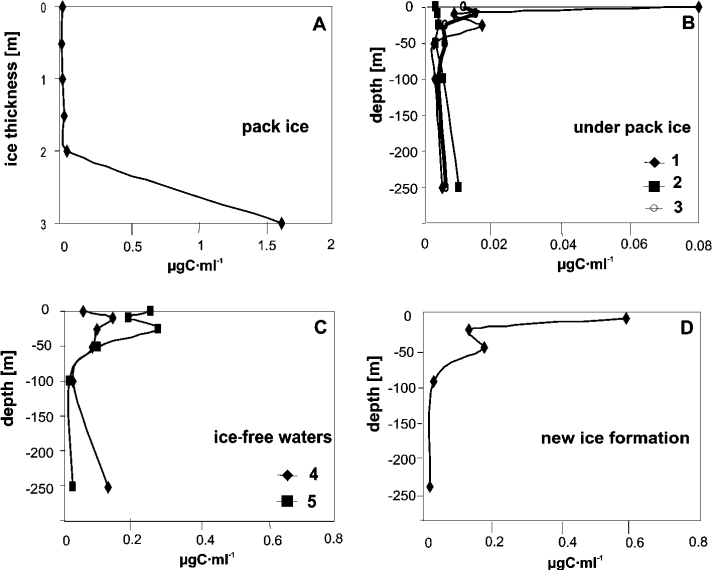
Depth distribution of microalgal biomass in the pack ice (A), underlying water column (B), ice-free seawater column (C) and during the new ice formation (D). Note the lower scales in the water column under the ice and the higher scale of the biomass values in the sea ice and during the new ice formation (the numbers 1 – 5 refer to the samplings).
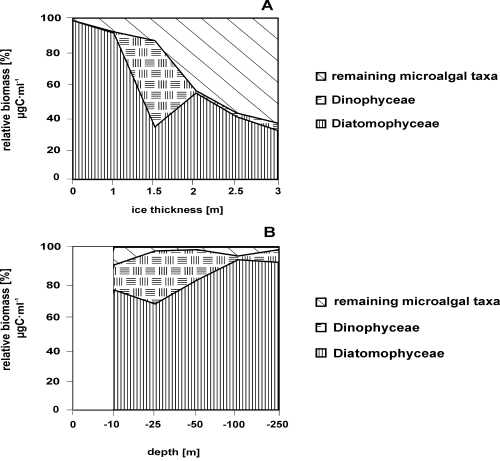
Relative biomass [% µg C·ml–1] of microalgal taxa in the pack ice samples (A) and in the seawater column during the new ice formation (B). There is no surface data in this last figure.
Discussion
Even though many microalgae found in this survey have been reported in the Southern Ocean (El-Sayed & Fryxell, 1993), the community composition of Wood Bay was different from those registered in other coastal areas of Antarctica. The bottom ice, in fact, was dominated, above all in cell densities, by Pseudopleurochloris antarctica, a new Antarctic xanthophycean (Andreoli et al., 1999), rather than by diatoms. In addition to Pseudopleurochloris antarctica, two other microalgae which are unusual for this environment were found both in and under the sea ice. Preliminary data suggested these microalgae were a chlorophycean and another xanthophycean; like Koliella antarctica (Andreoli et al., 1998) and Pseudopleurochloris antarctica, they are apparently derived from terrestrial habitats and adapted to seawaters. Moreover, the Wood Bay samples showed a low presence of various dinoflagellates hypnozygotes and the absence of archeomonads, which are considered resting forms of unknown chrysophytes (Mitchell & Silver, 1982) and are found in the annual land-fast sea ice in McMurdo Sound (Stoecker et al., 1992). Unusual was also he absence of the Parmales, an important component of the nanoplankton in Antarctic seawaters (Booth & Marchant, 1987; Kosman et al., 1993), which was also found at Terra Nova Bay during the austral summer 1995 – 96 (Andreoli, unpublished data).
Most dinoflagellate species occurred in the water, mainly in the surface layer, as was registered in Terra Nova Bay (Andreoli et al., 1995) and in the Weddell Sea (Estrada & Delgado, 1990). This was due both to the presence of a higher surface to colonize than the sea ice and to the grazing activity for heterotrophic species (Garrison & Gowing, 1993).
During our study, most of the microalgae found in the sea ice were also present in the seawater and during new ice formation. The temporal changes concerned mainly concentration and biomass variations of diatoms, in particular Entomoneis kufferathii, Chaetoceros dichaeta and Fragilariopsis species, and Pseudopleurochloris antarctica. This last species was found in all samples, both under the ice and in the ice-free waters. As opposed to sea ice, however, its density and biomass were lower than those of diatoms. Moreover, the microalgal communities in the sea ice of Wood Bay were more characteristic of an annual land-fast-ice than the pack ice. According to Palmisano & Garrison (1993), Scott et al. (1994), Archer et al. (1996) and McMinn (1996), the diatom assemblages were concentrated at the bottom of the sea ice, and the gymnodinioid dinoflagellates (Stoecker et al., 1992), recently assigned to Polarella glacialis (Montresor et al., 1999), were found in the internal layer of the ice. Differently from other land-fast areas, there were no platelet layers in Wood Bay under the sea ice; consequently, the high algal production and biomass accumulation observed both in the Weddell Sea (Smetacek et al., 1992; Grossmann et al., 1996; Günther & Dieckmann, 1999) and in the Ross Sea (Arrigo et al., 1995) were not observed here.
Summary
In this paper we recorded the microalgal density and biomass during the austral summer 1993 – 94 in the sea ice, in the water column under sea ice, in the melting water and during the formation of the sea ice. This research was carried out at the same sampling site in Wood Bay (Ross Sea). The floristic composition showed 96 taxa; 59 of them were diatoms and 32 dinoflagellates. Differently from other coastal areas of the Southern Ocean, a new xanthophycean, Pseudopleurochloris antarctica, was more abundant than diatoms, above all in the sea ice. Cell densities and biomass were higher in the sea ice and during the new formation of the ice than in the seawaters. Among the diatoms, Entomoneis kufferathii, Chaetoceros dichaeta and Fragilariopsis species were the most common taxa. The microalgal communities found in the sea ice of Wood Bay were more characteristic of an annual land-fast-ice than of the pack ice.
Acknowledgement
This research was supported by the Italian National Programme of Antarctic Research (P.N.R.A.).




