Habitat and Microhabitat of Mediterranean Clingfishes (Teleostei: Gobiesociformes: Gobiesocidae)
Abstract
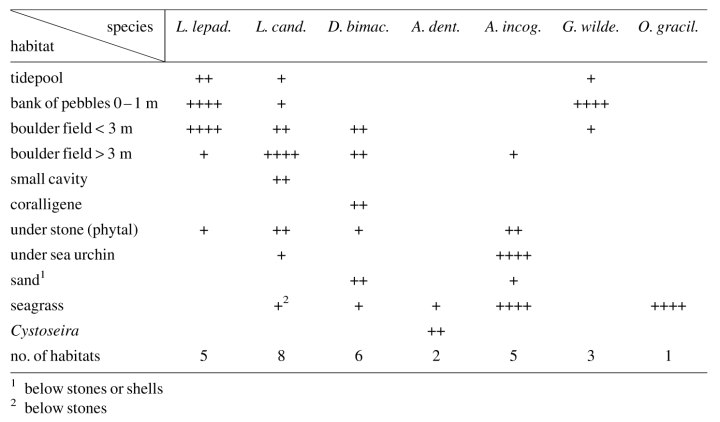
Abstract. This study addresses the habitat and microhabitat of the seven species of gobiesocid fish in the Mediterranean Sea. It is shown that Lepadogaster lepadogaster is closely adapted to large pebbles and boulder fields of rounded stones with a smooth surface. L. candollei is more euryecious and, in addition to inhabiting boulder fields also, occurs close to seagrass meadows, in small cavities and in association with sea urchins. Diplecogaster bimaculata is also euryecious and extends to greater depths. It lives on sand and muddy bottoms as well as on coralline grounds. At some locations this species is found in high abundance during the spawning season under empty bivalve shells or flat stones. Apletodon dentatus is the rarest species of Gobiesocidae in the Mediterranean Sea. It has a close association with seagrass or large brown algae (Cystoseira). Juveniles of A. incognitus are either associated with sea urchins or inhabit Posidonia meadows. Adults prefer the vicinity of seagrass meadows under empty bivalve shells and stones overgrown with red algae. Gouania wildenowi is stenoecious and is restricted to the interstices of roundish coarse gravel near the waterline. Opeatogenys gracilis is also stenoecious and lives only on the leaves of Posidonia and Cymodocea seagrass. The colourations of the different species and their variations are described and discussed.
Problem
Clingfish species of the family Gobiesocidae have a worldwide distribution, occurring in many different habitats in tropical and temperate seas. At present around 150 species in 43 genera are known (Briggs, 1993). In the Mediterranean and the Adriatic Sea, seven species belonging to the subfamily Lepadogastrinae have been recorded: Lepadogaster lepadogaster, L. candollei, Diplecogaster bimaculata, Apletodon dentatus, A. incognitus, Gouania wildenowi and Opeatogenys gracilis (Jordan & Fowler, 1902; Briggs, 1955; Hofrichter & Patzner, 1997). Very little is known about the biology and the habitats of these cryptobenthic fishes (Gibson 1969, 1982; Miller, 1979, 1996; Kovačvič, 1997; Patzner, 1999a). Here we report on the habitat of clingfishes at different sites in the Mediterranean including the Adriatic Sea.
Material and Methods
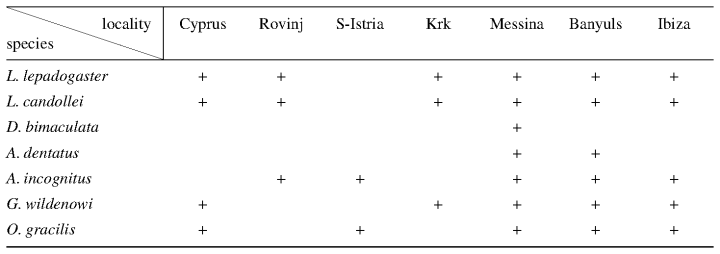
Locations of sites investigated and species collected in the study are given in Fig. 1 and Table 1. Supplementary information on the taxonomy and distribution of the different species was obtained by examining material from different museums and private collections (Hofrichter, 1995).
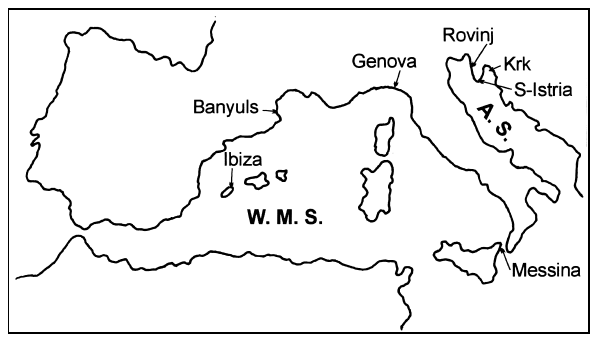
Study sites in the western Mediterranean Sea (W.M.S.) and in the Adriatic Sea (A.S.).
In shallow waters (< 1 m) the field work was undertaken by snorkeling. In deeper regions SCUBA was used. The clingfishes were caught mostly with the aid of Quinaldine (Fluka), diluted to about 1 : 15 with alcohol and squirted under stones and sea urchins as well as into cavities and clefts with a wash-bottle. After a short while the anaesthetized fishes could be captured with a hand net. The relative abundances of the fishes were calculated using transects that ran straight from the waterline towards deeper water. To describe the microhabitat the parameters shown in Fig. 2 were used. Quantitative studies were performed with quadrat frames 25 × 25 cm and 50 × 50 cm. For the quantitative investigation in seagrass meadows a special ‘cube-method’ was developed (Patzner & Hofrichter, unpublished). Thereby a top- and bottomless cube of 50 × 50 × 50 cm was placed on the seagrass and, after squirting Quinaldine, all the floating material was sucked through a net. Non-quantitative samples in the seagrass were taken with a sliding dredge or a hand-net of dimensions 50 × 30 cm.
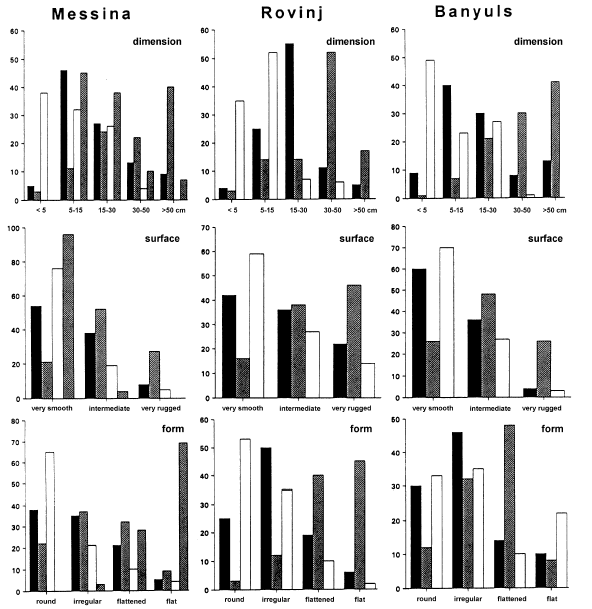
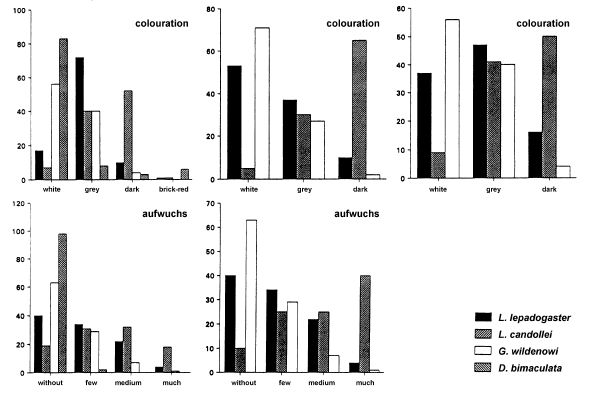
Microhabitat occurrence of gobiesocid species near Messina (Sicily) (n=2000), at Rovinj (Croatia) (n=300) and at Banyuls (France) (n=550). Diplecogaster bimaculata was found only in Messina. Dimension of stones, nature of the surface, form, colouration and aufwuchs of the stones covering the fish are given as percentage of all observations per species.
Results
The greatest number of species and the highest abundances of individuals were found at Messina (Sicily, Italy). This was the only area where all seven Mediterranean clingfish species occurred together. Here, a bank of large pebbles and a boulder field measuring approximately 600 m2 supported nearly 15,000 clingfishes of four species with a total biomass of around 26 kg. Descriptions of the different abiotic and certain biotic factors at Messina are given by Di Geronimo (1987), Di Geronimo & Fredj (1987), Fredj & Giaccone (1987) and Giaccone (1987). Abundances were lower at the other sites. The habitat preferences of the individual species are described below.
1. Lepadogaster lepadogaster (Bonnaterre 1788)
This species occurs on the underside of stones. The cruising radius of L. lepadogaster is small and it has never been observed to swim free between individual stones (Hofrichter & Patzner, unpublished). This species is more closely bound to the substratum than the related L. candollei (see below). The preferences for different microhabitats are given in Fig. 2. In banks of large pebbles and boulder fields in shallow water, L. lepadogaster is more frequent than L. candollei; the highest density is reached at a depth of 0.8 m (Table 2). The maximum abundance was 23 indiv. · m−2 at Messina. No association with seagrass meadows was evident. The body colouration of this species (Fig. 3a) is a green-brown with dark spots and varies only little. There are only some slight morphological differences between individuals or between populations.
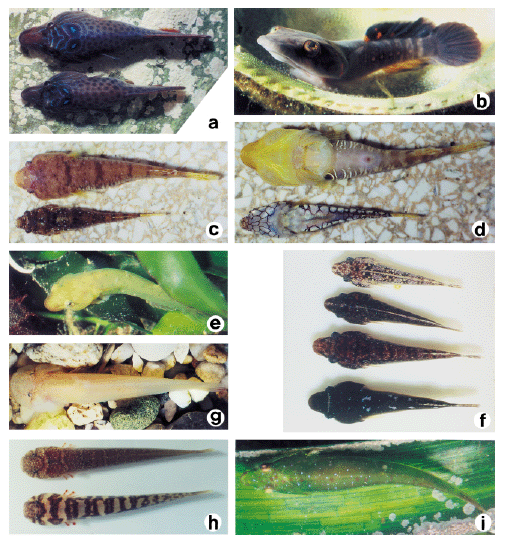
Body colouration and shape of the seven Mediterranean gobiesocid specis. a. Lepadogaster lepadogaster, mature male and female during spawning. b. Lepadogaster candollei, mature male with eggs, in spawning colouration. c. Diplecogaster bimaculata, mature male and female during spawning. d. Diplecogaster bimaculata, mature male and female, ventral aspect. e. Apletodon dentatus, between Caulerpa algae. f. Apletodon incognitus, colour variations. g. Gouania wildenowi, from Krk, Croatia. h. Gouania wildenowi, from Messina. i. Opeatogenys gracilis, on leaves of Posidonia seagrass.
2. Lepadogaster candollei Risso 1810
Lepadogaster candollei is much more mobile than the former species and was often observed swimming freely between stones and clefts in the rocks, covering distances of several meters within a period of one minute. The cruising radius of the males is reduced only during the spawning season. The fish prefer rather flat stones with a diameter larger than 10 cm that are overgrown with algae (Fig. 2). The highest density of L. candollei was counted in depths of around 2 m. In a boulder field at the island of Sv. Katarina (near Rovinj, Croatia) abundances of 10 indiv. · m−2 were found at that depth. They were also often observed beneath flat and overgrown stones in the rhizome zone of Posidonia oceanica but never directly on the leaves. Other microhabitats are given in Fig. 2. On steep slopes of the rocky littoral, especially in overhangs, subadult L. candollei live in small cavities. In this habitat they co-occur with small cryptobenthic gobies down to 20 m. Juvenile L. candollei live under the sea urchins Paracentrotus lividus and Arbacia lixula. The body colouration of this species is a dark reddish brown. During the spawning season males become darker to almost black with 3 to 4 bright red diagonal stripes on each gillcover (Fig. 3b). In some subadults living in or near Posidonia meadows, a whitish line between the eyes is apparent, similar to that in A. incognitus (see below).
3. Diplecogaster bimaculata (Bonaterre 1788)
Within the study site this species was less frequently encountered than L. lepadogaster. In some areas, however, e.g. around Messina or in the Gulf of Genoa (collection of G. Balma), they were caught at all depths down to 200 m depth and more. Preferences for different microhabitats are given in Fig. 2. Diplecogaster bimaculata is occasionally found under the empty bivalve shells lying on sandy bottoms or coralligenous grounds. In Messina this species was very abundant during the spawning season in June. Here the males spawn in a special microhabitat: man-made flat pieces of white marble, between 10 and 50 cm square and 2 to 10 cm thick (Fig. 2). Ninety-eight percent of these were inhabited by D. bimaculata. Thus the abundance of this species appeared to be determined by the number of available white flat stones or similar objects. Only rarely were flattened red stones used as habitat (Fig. 2). The body colouration of the species was variable, with that of each population being adapted to its habitat (Figs. 3c, 3d). Fish living beneath the white marble were pale to whitish while those on coralligenous grounds were reddish to purple red.
4. Apletodon dentatus (Facciola 1887)
This species is regarded as very rare in the Mediterranean Sea. In the present study it was found in a Posidonia oceanica seagrass meadow at Banyuls-sur-Mer (France). Eighty ‘cube’ samples yielded only one specimen of A. dentatus versus nearly 140 O. gracilis and 150 A. incognitus. It could not be determined if the specimen had lived on the leaves or the rhizomes of Posidonia. At Messina A. dentatus also inhabited dense stands of Cystoseira spp. between 0.5 and 2 m. All specimens were olive-green matching the surrounding habitat. The ‘cube-method’ was unsuitable here, so no information on abundance in this habitat can be given. The body colouration of A. dentatus is highly variable and influenced by the substratum (Fig. 3e). Sexual differences in colourations, especially during the spawning season, are reported (see ‘Discussion’).
5. Apletodon incognitus Hofrichter & Patzner 1997
Around 27 % of young and subadult individuals of this species were found on the leaves of Posidonia oceanica and 25 % under different species of sea urchins, reaching an abundance of up to 40 indiv. · m−2. Most remaining habitats were near Posidonia meadows, so that the abundance decreases with increasing distance from the seagrass. Adult animals prefer empty shells of mussels or small flat stones and are found only at relatively low densities. Apletodon incognitus appears to be associated with the sea urchins Paracentrotus lividus, Arbacia lixula and Sphaerechinus granularis in the following habitats: (1) rocky littoral covered with algae at depths of 0.5 to 20 m, maximum abundance in 35 % of the sea urchins, (2) deeper bedrocks with large boulders down to 25 m, maximum abundance in 5 % of the sea urchins, (3) coralligenous bottom with the calcareous red algae Lithothamnion lenormandi and Lithophyllum incrustans, near Posidonia meadows between 0.5 and 8 m, maximum abundance in 15 % of the sea urchins, (4) small sandy areas with empty and broken mussel shells within Posidonia meadows, under large specimens of S. granularis, maximum abundance in 13 % of the sea urchins and (5) Posidonia-meadows between the rhizomes, under small specimens of P. lividus, maximum abundance in 4 % of the sea urchins. The body colouration of A. incognitus is very variable (Figs. 3f) and usually well adapted to the substrate. Some specimens have irregular brown, green and reddish patches,while others have a uniform, rather dark brown colouration with a whitish longitudinal line or a whitish line between the eyes. The latter is not species specific and can also be found in L. candollei.
6. Gouania wildenowi (Risso 1810)
This species lives in the narrow interstices between roundish coarse gravel of diameters between 0.5 and 5 cm, on sheltered as well as exposed shores. It ranges from the waterline down to about 1 m depth, although at extreme low water, individuals even occupy the newly exposed areas of the intertidal zone. Gouania wildenowi reaches the highest abundance in depths to 0.5 m. Up to 24 indiv. · m−2 were counted on the coast of Messina. The deepest records (single specimen) were from 2.0 m, also at Messina. The microhabitats are listed in Fig. 2. No association with seagrass meadows is apparent. The colouration of G. wildenowi can vary between individuals or between populations. All specimens from the northern Adriatic Sea are pale and nearly pigment free (Fig. 3g), whereas those from Messina are strongly pigmented. In the latter region, colouration varied considerably within a single population (Fig. 3h).
7. Opeatogenys gracilis (Canestrini 1864)
This species only lives on the leaves of Posidonia oceanica and Cymodocea nodosa. Its maximum abundance was 8 indiv. · m−2, usually together with A. incognitus. The deepest record of O. gracilis was at 20 m at Banyuls-sur-Mer, although no samples were taken at greater depths. The body colouration is green and, on the dorsal part of the fish, camouflage adaptations to the calcareous red algae growing on the Posidonia leaves is obvious: The irregular whitish spots on the algae are repeated on the skin of O. gracilis (Fig. 3i).
Discussion
All seven Mediterranean clingfish species are small and exhibit a cryptobenthic life style, which affords them with better protection against predators. Small size provides at least two major advantages within the teleosts: better food availability (e.g., small crustaceans) and a great variety of potential microhabitats (Miller, 1979). The sucking disk of the gobiesocids provides extra adaptation to narrow habitats and strong water movements and could also serve during spawning. A comparison with the body shape of Blenniidae and small Gobiidae shows that the trend to the reduction in body height is most advanced in the Gobiesocidae (Abel, 1961). Few data exist on the abundances of cryptic fishes (e.g.,Wells, 1979; Gibson, 1982; Illich & Kotrschal, 1990). Jardas (1985) states that the abundance of individuals is difficult to estimate, especially in cryptic species. In studies without direct underwater observations these clingfishes were often termed ‘rare’ or ‘very rare’ or were missed entirely. For example A. incognitus was long overlooked in regions where marine biological studies were performed over the last century, such as at Rovinj, Messina and Banyuls-sur-Mer. Knowledge of the sea urchin microhabitat is a prerequisite for estimating the true abundance of this species (see below). The highest reported abundances of clingfishes (all sympatric species) are 150 indiv. · m−2 in California (Gibson, 1982) followed by the present study with 45 indiv. · m−2 at Messina (Lepadogaster lepadogaster, L. candollei, Diplecogaster bimaculata and Gouania wildenowi).
The present results may now be conveniently discussed by reference to the habitat types encountered.
1. Banks of large pebbles and boulder fields
These habitats in the upper sublittoral and the mediolittoral are inhabited by most species of clingfishes. The genera Lepadogaster and Gouania are particularly closely associated with this type of habitat. G. wildenowi shows a special adaptation to the narrow interstices between roundish pebbles, used by no other species of fish. Many species of clingfish are adapted to strongly exposed habitats (Gordon et al., 1970; Paine & Palmer, 1978; Paulin & Roberts, 1992); some are regarded as ‘amphibious fish’, including Sicyases sanguineus from the west coast of South America (Ebeling et al., 1970) and Tomicodon abuelorum and T. humeralis from Central America (Szelitowski, 1990; Thomsen et al., 1979; Sayer & Davenport, 1991). Even G. wildenowi has sometimes been found in the littoral zone at low tide, yet it is not ‘amphibious’, because the deeper layers of the pebbles where it lives still contain some water. Lepadogaster zebrina has also been observed under stones exposed at low tide at the Canary Islands (R. Hofrichter, pers. obs.).
2. Empty mussel shells and small flat stones
These microhabitats are typical in habitats as diverse as sand, shell gravel, coralligene, mud and on pebble fields. They are utilized by D. bimaculata and adult A. incognitus and sometimes by L. candollei. At the coast of Messina the abundance of breeding males of D. bimaculata can depend upon the number of available white flat stones or empty mussel shells. Diplecogaster bimaculata is probably the only Mediterranean species which extends beyond coastal regions to waters several hundreds of meters deep. Guitel (1889, 1904) reported it on sandy bottoms with broken mussel shells and coralligene grounds. The discrepancy between the high numbers of juveniles (up to 40 indiv. · m−2) and the low number of adult A. incognitus is not fully understood. One reason might be that the microhabitats of the juveniles are clearly defined whereas those of the adults are more varied.
3. Cavities and caves
The present study shows clearly that, with the exception of L. candollei, no Mediterranean clingfishes regularly inhabit cavities. This is in contrast to Riedl (1966), who counted L. lepadogaster (as L. gouani) and G. wildenowi as cave-dwelling species. In the course of the present work L. lepadogaster was rarely found in rocky clefts or in small cavities, and then only when there was a nearby boulder field. Therefore it seems clear that the main habitat is the underside of stones within a boulder field and caves or clefts may be utilized from there. Lepadogaster candollei inhabits not only small cavities but was also found in caves (Patzner, 1999a).
4. Seagrass meadows
Mediterranean seagrass meadows support three species of gobiesocids: Lepadogaster candollei, Apletodon incognitus and Opeatogenys gracilis. The latter is found only in this habitat. The ventral sucker enables clingfishes to utilize this habitat efficiently (Gibson, 1969; Green & Barber, 1988) by clinging directly to the leaves. The tendency for clingfishes to settle on seagrass has been described worldwide (Jachowski, 1970; Briggs, 1990, 1993; Hutchins, 1991, 1992). In New Zealand, Haplocylix littoreus, Gastrocyathus gracilis and Gastrocyphus hectoris live in seagrass meadows (Paulin & Roberts, 1992). Several publications on Mediterranean fish communities associated with seagrass meadows do not mention clingfishes at all (Khoury, 1984; Harmelin, 1987; Harmelin-Vivien & Francour, 1992), or describe species that were found there by chance and not as typical seagrass inhabitants (Masutti, 1962; Harmelin-Vivien, 1982, 1983; Francour, 1990), or refer only to the family without describing a species (Harmelin-Vivien, 1982, 1983, 1984). The few papers on seagrass meadows that mention O. gracilis in their lists give no further information on their biology (Francour, 1990). Juvenile and subadult A. incognitus are sympatric with O. gracilis, but they are not restricted to that habitat and are also found in other places (see ‘Results’). Apletodon dentatus is also often observed in seagrass meadows, but nothing is known about its mode of life. Lepadogaster candollei (Harmelin-Vivien, 1982) and L. lepadogaster (Masutti, 1962) are sometimes mentioned in lists of seagrass fish species. However, the present study shows that the latter has no direct relationship with seagrass meadows. Individuals from nearby boulder fields were caught probably by chance with a dredge. Lepadogaster candollei is found in Posidonia meadows but is always dependent on the stones under which it lives. Guitel (1904) mentions A. dentatus also in Zostera sp. seagrass meadows. In the Atlantic Ocean at Roscoff this species is quite common, living in hollow bulbs of the brown algae Saccorhiza polyschides (Hofrichter et al., 2000).
5. Association with sea urchins
The association of A. incognitus (as Diplecogaster bimaculata) with different species of sea urchins has been described from the Azores in the Atlantic (Patzner & Santos, 1992;Patzner et al., 1993) and only recently from the Mediterranean Sea (Hofrichter, 1995; Patzner, 1999b). One specimen of Lepadogaster sp. at the Natural History Museum of Stockholm (NRM 26249) was washed out from 127 Arbacia lixula at Brucoli, Sicily (Hofrichter, 1995). Wirtz (1994) shows photographs of L. candollei and A. incognitus (as Diplecogaster bimaculata) associated with sea urchins at one of the Atlantic islands. Some tropical clingfishes have close connections to sea urchins: Diademichthys lineatus is associated with Diadema spp. (Sakashita, 1992), Dellichthys morelandi with Evechinus chloroticus (Dix, 1969), and Acyrtus rubiginosus with Echinometra lucunter (Teytaud, 1971; Schoppe, 1991). The initial reason for using sea urchins as a microhabitat is likely to have been protection from predators. In some clingfish species the association has developed into ectoparasitism (Miller, 1979), as demonstrated by D. lineatus and D. morelandi which feed on the pedicellaria of their hosts (Pfaff, 1942; Sakashita, 1992). In A. incognitus and Acyrtus rubiginosus no such observations have been made (Teytaud, 1971; Patzner & Santos, 1992; Patzner, 1999b).
6. Body colouration
In the course of evolution benthic fish have developed a stronger tendency towards variability of body colouration and changing colouration than pelagic species. Colouration changes are influenced by two factors: substrate colour and fish disposition (Gibson, 1969). The body colouration of most clingfish species is very variable and shows a high tendency for adaptation to habitat. Both Opeatogenys species, O. gracilis and O. cadenati, live exclusively in seagrass and are grass green. Cox & King (1987) report different body colourations of A. dentatus. Dependent upon habitat, some individuals in coralligenous algae are rose-coloured to reddish spotted, while other living in brown algae are olive green. Dieuzeide et al. (1953) found dark green and reddish brown A. dentatus. Aitken (1971) describes one specimen from Scotland as apple green. Holt & Byrne (1898) describe olive green A. dentatus (as Lepadogaster stictopteryx). The D. bimaculata males found in Messina live among white stones and are pale to almost white. On maerl-grounds at Roscoff (Atlantic Ocean) where reddish colours predominate the specimens are intensely red to purple-red (Hofrichter et al., 1999). Ecloniaichthys scylliorhiniceps in South Africa lives mostly on brown algae (Eclonia and Laminaria) and is typically olive green to brown. Where it inhabits the green alga Caulerpa it is light green (Penrith, 1969). In the last twenty years several new species of Australian clingfish have been described in seagrass and algae. Most of these are green, the others brownish (B.J. Hutchins, pers. comm.). Three species living between algae in New Zealand (Haplocylix littoreus, Gastrocyathus gracilis and Gastrocyphus hectoris) have an olive green colouration (Paulin & Roberts, 1992). Acyrtops berillinus from the eastern coast of North and Central America lives in seagrass meadows (Thalassia testudinum) and is also green (Böhlke, 1993). Brownish juvenile A. incognitus caught under sea urchins, but kept for several weeks in aquaria together with the green alga Caulerpa prolifera, acquired a greenish colouration (authors' unpubl. obs.). Several benthic fish species are paler during the night than during the day. Ermin (1948) reported such daily colour change in L. lepadogaster and L. candollei.
Summary
Habitats, microhabitats and colourations of all seven species of Mediterranean gobiesocids are described. Lepadogaster candollei is euryecious and is found in boulder fields, close to seagrass meadows, in small cavities and in association with sea urchins. Diplecogaster bimaculata is also euryecious and extends down to greater depths. It lives on sand, on muddy bottoms, boulder fields and on coralline grounds. Apletodon incognitus is associated with sea urchins or, as juveniles, with Posidonia meadows. Adults are found under empty bivalve shells and under stones, close to seagrass meadows. Apletodon dentatus is closely associated with seagrass or large brown algae (Cystoseira). Lepadogaster lepadogaster is rather stenoecious because it is closely adapted to large pebbles and boulder fields. Gouania wildenowi is stenoecious and is restricted to the interstices between roundish coarse gravel near the waterline. Opeatogenys gracilis is strongly stenoecious and lives solely on seagrass leaves.