Dynamics Behind Standoff Interactions in Three Reef Sponge Species and the Coral Montastraea cavernosa
Abstract
Abstract. Benthic organisms compete for space, and standoff interactions, i. e. interactions with no clearly observable outcome such as win or loss, are very common in marine hard substratum communities. Standoffs were more common than overgrowths among all sponge-coral interactions observed in a coral reef community off Santa Marta, NE Colombia. The dynamics of these standoffs and the role played by each of the interacting organisms were examined in a series of standard observations of sponge-coral interactions involving the sponge species Niphates erecta, Rhaphidophlus venosus, Scopalina ruetzleri and the coral Montastraea cavernosa. Most sponge-coral standoffs in this study displayed rapid changes in number of polyps along the coral border, sponge area and sponge-coral distance. The outcome of sponge-coral standoffs depended on the ecological stategy (e.g., growth) of the sponge species. Scopalina ruetzleri and N. erecta did not show any signs of direct competition with Montastraea cavernosa. R. venosus, however, was often observed to take over vacant space formed by dead coral polyps (54 % of interactions). The impact of coral damage (lesions) on the interaction process between R. venosus and M. cavernosa was shown by a significant increase in coral polyp death in damaged compared with undamaged M. cavernosa colonies (22.7 % versus 6.9 %). This study demonstrates that (1) sponge-coral standoffs are actually quite dynamic because of the continually changing distance between the organisms, (2) the dynamic nature of sponge-coral standoffs depends on growth-related strategies (growth form, growth rate) of the competing sponge species, (3) the actual frequency of coral overgrowth by (thinly encrusting) sponge species can only be determined by means of intermittent observations; space gained at the cost of coral tissue cannot be seen by single observations, (4) coral damage (lesions) on reefs may enhance deterioration of corals by increasing their susceptibility to sponge overgrowth.
Problem
A very visible form of spatial competition is direct overgrowth of organisms already occupying space. However, competition for space is not always visible, especially when chemical aggression is involved. Sponges are known to possess a wide variety of chemical substances (Faulkner, 1984) which, among other functions, can be used in competition for space (Jackson & Buss, 1975; Aerts & van Soest, unpublished). Studies on sponge interactions with other reef organisms mainly focused on the competitive mechanism employed by sponges (Sullivan et al., 1983; Porter & Targett, 1988) or on the occurrence of overgrowth (Suchanek et al., 1983; Vicente, 1990; Rützler & Muzik, 1993; Aerts & van Soest, 1997). However, standoffs (defined as interactions without a clearly observable outcome such as win or loss) also occur frequently. The observed frequency of sponges overgrowing corals has never been correlated to the number of standoff interactions between the two interacting species. Although the frequency of overgrowth is important, the most frequent interactions are standoffs such as peripheral contact, tissue contact and non-contact interactions (Aerts & van Soest, 1997). It is unknown if any exchange occurs in these standoffs. There may be cessation of growth at the border of contact between the two species (Karlson, 1980; Schmidt & Warner, 1986) or these interactions may involve repeated reversals, by which the coral and sponge advance and retreat alternately (Chornesky, 1989). Establishment of a competitive hierarchy between species may be perfectly transitive when standoffs are ignored (Rubin, 1982; Schmidt & Warner, 1986). In species assemblages where standoffs are frequently encountered, however, they cannot be ignored and should obtain a status equal to that of wins and losses when determining competitive hierarchies (Schmidt & Warner, 1986; Tanaka & Nandakumar, 1994).
In this study I examined the dynamics behind standoff interactions between three sponge species (Niphates erecta, Rhaphidophlus venosus, Scopalina ruetzleri) and one coral species (Montastraea cavernosa) on a Caribbean reef through intermittent observations. Standoffs are defined as contact or non-contact interactions, with a sponge-coral distance of < 5 cm, without a clearly observable outcome such as overgrowth. Selected sponge-coral standoffs were observed at three reef localities, each characterised by a different sediment load. Furthermore, I investigated if other factors such as coral damage (in the form of lesions) increase the susceptibility of coral colonies to sponge competition. Since the recovery from damage reduces the biological functions of corals and because energy needed to recover from lesions is derived from the surrounding polyps (Meesters et al., 1994), the competitive ability of the coral may decrease locally.
Questions I address in this study are: (1) are sponge-coral standoffs dynamic; i. e. is there a cessation of growth along the border of both organisms or are repeated reversals occurring; (2) do environmental conditions influence the dynamic nature of the standoffs; (3) do conditional factors such as coral damage increase susceptibility to sponge competition.
Material and Methods
1. Study area
Standoff interactions between individuals of three sponge species and one coral species were repeatedly observed along the coast of the Santa Marta area, Caribbean Colombia (11°15’ N, 74°13’ W; Fig. 1). Because sedimentation is an important environmental factor causing coral stress on reefs (Rogers, 1990), sponge-coral standoffs were followed at three localities, each characterised by a different sedimentation load. Sedimentation, sampled in a former study (see Aerts & van Soest, 1997), was measured using PVC sediment traps with a length/diameter ratio of 5:1 (length: 30 cm, diameter: 6 cm; after Larsson et al., 1986). The traps (one per station) were left in situ for two months over a one-year period, yielding 6 samples per station. Sedimentation was expressed in g·m–2·d–1, dry weight. Sedimentation rates are presented in Fig. 1.
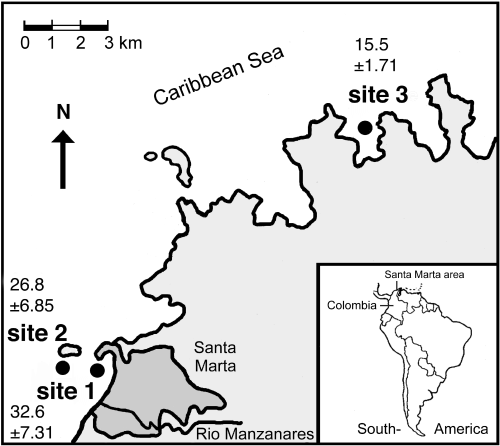
Map of the study area with the three localities in Colombia. Site 1 = Punta de Betin, site 2 = El Morro and site 3 = Chengue. Sedimentation data in g·m–2·d–1± SD for each locality (plain figures) were derived from Aerts & van Soest (1997).
2. Observations of interactions
At 20 m depth (where most interactions were encountered) a total of 141 sponge-coral standoff situations were marked, each with two steel nails, and photographed using a Nikonos V with a 1:3 extension tube. The size of the photo frame was 9 by 13 cm. The sponge species Niphates erecta Duchassaing & Michelotti 1864, Scopalina ruetzleri Wiedenmayer 1977 and Rhaphidophlus venosus Alcolado 1984 and the coral species Montastraea cavernosa Linnaeus were chosen because they were the most common in the study area. Stand-off interactions were selected randomly. Sample sizes were not equal because the occurrence on the reef differed between the three sponge species. A total of 55 interactions between S. ruetzleri and M. cavernosa (n = 16, site 1; n = 22, site 2; n = 17, site 3), 53 interactions between R. venosus and M. cavernosa (n = 16, site 1; n = 19, site 2; n = 18 site 3) and 33 interactions between N. erecta and M. cavernosa (n = 12, site 1; n = 11, site 2; n = 10 site 3) were photographed in February 1994 (one-month interval), August 1994 (six-months interval), October 1994, December 1994, February 1995 and April 1995.
In a series of parallel observations, the effect of coral damage on the outcome of sponge-coral standoffs was studied. A series of additionally selected interactions of Montastraea cavernosa colonies and specimens of the sponge Rhaphidophlus venosus was monitored from February to April 1995. Using a pneumatic drill, lesions of about 10 mm diameter and an average depth of 2.5 mm, were made into the tissue of selected corals. These lesions were located approximately one polyp (= 5 – 10 mm) away from the outer coral tissue boundary, leaving a space of living coral tissue between the damaged part (totally devoid of live tissue) and the coral boundary. This experiment was performed only with R. venosus. The reaction of both sponge and coral species was followed by photographic sampling of each interaction after 7, 28, 56 and 77 days. To make sure that the same area was photographed each time, a photo-frame was used and the interacting area was marked. Two localities with different sedimentation load (site 1, n = 10 and site 3, n = 34) were compared to investigate the possible role of sedimentation stress on the outcome of the experimental interactions. At the same localities and depth, undamaged M. cavernosa colonies interacting with R. venosus specimens served as controls (site 1, n = 16 and site 3, n = 18).
3. Interpretation of the photographs
Because all photo-frame positions were fixed, photo prints of individual interactions taken at different times were similar and could be compared easily by image analysing. The photographic frame did not cover the whole coral and sponge colony. Observed changes in sponge specimens and coral colonies refer only to the interaction area and the specimens’ borders within the image. Changes outside the frame were not considered. From each photograph I recorded the following features:
1. Number of polyps along the coral border within the photo-frame; polyps were chosen as the unit of measure because they are the basic module of a coral and because retreat or growth occurred per polyp. Length of the coral border was the same for each site and sponge species (10.5 ± 1.3 cm).
2. Changes in sponge area [cm2] within the photo-frame.
3. Minimum distance [mm] between sponge and live coral tissue. If the sponge was growing parallel to the coral border, the length [mm] of the contact area between sponge and coral border was measured.
4. Details of sponge-coral interactions (e.g., tissue damage, overgrowth, necrosis) which occurred during the 15-month period within the photo-frame.
5. Lesion condition (e.g., regenerated, overgrown, damaged) for interactions with experimental lesions.
4. Statistics
Data on the three variables (coral border changes, sponge area and sponge-coral distance) were analysed in two different ways, relative and absolute. Relative data refer to the number of interactions displaying changes and are expressed in percentage of the total number of interactions (Table 1A). Absolute data refer to actual changes expressed as number of border polyps, sponge area [cm2·a–1] or sponge-coral distance [mm·a–1] (Table 1B). To test whether the occurrence of changes of the three variables differed significantly among localities and sponge species, R × C tests of independence using the G-test were performed (Sokal & Rohlf, 1981). Absolute values of both transformed and untransformed data were not normally distributed (normality plot and Kolmogorov-Smirnov test; Sokal & Rohlf, 1981) and the variances were all heteroscedastic (Barlett’s test; Sokal & Rohlf, 1981). As a consequence medians and ranges of the changes in sponges and corals were used (Table 1B, Fig. 2) and tested among localities, species and periods with the non-parametric Kruskal-Wallis one-way analysis of variance (Sokal & Rohlf, 1981). Correlations between changes of polyp number and sponge area were tested with Pearson’s correlation coefficient (Sokal & Rohlf, 1981). Sedimentation data of the three localities (derived from Aerts & van Soest, 1997) were analysed with the Wilcoxon signed ranks test (Sokal & Rohlf, 1981) taking dates as blocks.
| A | S1 | S2 | S2 | Sr | Ne | Rv | B | S1 | S2 | S3 | Sr | Ne | Rv |
| coral polyps | coral polyps | ||||||||||||
| decrease | 38.6 | 59.6 | 64.4 | 56.4 | 48.5 | 56.6 | decrease | 1.7 | 3.0 | 5.6 | 2.4 | 1.8 | 4.8 |
| increase | 47.7 | 32.7 | 26.7 | 34.5 | 36.4 | 35.8 | increase | 1.6 | 1.6 | 1.9 | 1.2 | 1.6 | 1.6 |
| no change | 13.6 | 7.7 | 8.9 | 9.1 | 15.2 | 7.5 | average | 0.0 | –1.0 | –1.3 | –0.5 | 0.0 | –1.3 |
| range | 15.1 | 20.3 | 34.1 | 31.7 | 26.3 | 36.0 | |||||||
| sponge area | sponge area | ||||||||||||
| decrease | 45.5 | 50.0 | 41.5 | 58.2 | 48.5 | 30.6 | decrease | 6.4 | 5.0 | 8.9 | 8.2 | 1.7 | 4.6 |
| increase | 50.0 | 40.4 | 53.7 | 41.8 | 45.5 | 55.1 | increase | 4.5 | 6.0 | 6.8 | 4.2 | 5.2 | 8.3 |
| no change | 4.5 | 9.6 | 4.9 | 0.0 | 6.1 | 14.3 | average | 0.7 | –0.1 | 0.8 | –0.8 | 0.0 | 0.7 |
| range | 54.8 | 129.6 | 89.1 | 88.3 | 33.7 | 129.6 | |||||||
| sponge-coral distance | sponge-coral distance | ||||||||||||
| decrease | 32.6 | 26.9 | 24.4 | 20.0 | 33.3 | 32.7 | decrease | 3.6 | 3.0 | 3.6 | 3.6 | 2.4 | 3.6 |
| increase | 27.9 | 48.1 | 37.8 | 52.7 | 42.4 | 21.2 | increase | 6.0 | 6.0 | 8.4 | 8.4 | 6.0 | 3.6 |
| no change | 39.5 | 25.0 | 37.8 | 27.3 | 24.2 | 46.2 | average | 0.0 | 0.0 | 0.0 | 1.2 | 0.0 | 0.0 |
| range | 36.0 | 44.4 | 96.0 | 102.0 | 33.6 | 57.6 | |||||||
| total n | 44 | 52 | 45 | 55 | 33 | 53 | |||||||
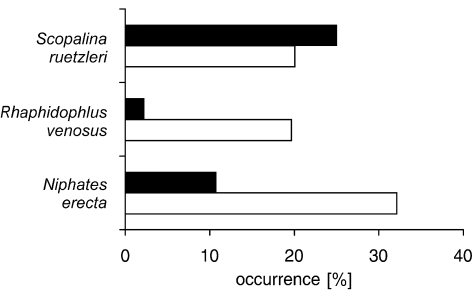
Occurrence [%] of the change of non-contact interactions between M. cavernosa and three sponges into contact interactions (white bars) and vice versa (black bars) over a 15-month period for each sponge species. Total number of interactions involved is 28, 46 and 40, respectively.
Results
1. Dynamics of sponge-coral standoffs
The relative occurrence of decrease and increase of coral border polyps was similar for each locality (R × C independence test, P > 0.05; Table 1A). Absolute changes in number of border polyps differed among localities; the highest decrease in number of border polyps was found at sites 2 and 3 (Kruskal-Wallis, P < 0.01; Table 1B). Both the occurrence and magnitude of changes in number of coral border polyps demonstrated no significant difference among sponge species. Apparently, the three sponge species had a similar effect on the coral border polyps.
Among localities, no differences were apparent between the occurrence of decrease and increase of sponge area and sponge-coral distance (R × C independence test, P > 0.05; Table 1A). It apparently did depend on the competing sponge species. Sponge area [cm2·a–1] decreased most frequently in Scopalina ruetzleri (R × C independence test, P < 0.001; Table 1A).
This decrease was significantly greater in S. ruetzleri than in R. venosus (Kruskal-Wallis, P = 0.011; Table 1B). As a consequence, an increase of sponge-coral distance between Montastraea cavernosa and S. ruetzleri occurred more frequently than between M. cavernosa and R. venosus (R × C independence test, P < 0.03; Table 1A). Also the extent to which the sponge-coral distance (in mm·a–1) increased was significantly higher for S. ruetzleri (Kruskal-Wallis, P = 0.015; Table 1B). The low ranges in area decrease and increase and the average area changes of 0 cm2·a–1 in N. erecta reflect its relative stability in terms of space occupancy compared with the other two sponges (Table 1B).
Because a decrease of sponge area and thus increase in sponge-coral distance was not uncommon for S. ruetzleri, 25 % of all contact interactions ended after 15 months as non-contact interactions. This was not the case for R. venosus and N. erecta; growth towards the coral colony occurred more often than the reverse (Fig. 2).
The dynamics of the sponge-coral standoffs mentioned above were calculated over a 15-month period. Relative and absolute changes of the three variables were also calculated at 2-month intervals to determine the impact of seasonal influences. No differences in the occurrence of changes existed for each variable between time periods (R × C independence test, P > 0.05). Most selected interactions displayed no change in sponge-coral distance, although both sponge area and coral border were subject to changes. Apparently, decreases and increases of sponge area and coral border did not affect the distance between the two organisms (Table 2). The absolute values of sponge area and sponge-coral distance were similar for each period (Kruskal-Wallis, P > 0.05; Fig. 3B, 3C). The number of coral border polyps, however, decreased significantly more during the period February-August 1994 compared to other time periods (Kruskal-Wallis, P = 0.003; Fig. 3A). Although the absolute values of each variable showed wide ranges, the medians of the changes were all around zero (Fig. 3).
| Jan–Feb’94 | Feb–Aug’94 | Aug–Oct’94 | Oct–Dec’94 | Dec’94–Feb’95 | Feb–Apr’95 | |
| coral border | ||||||
| decrease | 23.0 | 57.6 | 39.0 | 39.7 | 32.8 | 35.6 |
| increase | 16.4 | 19.6 | 16.9 | 27.3 | 28.6 | 23.7 |
| no change | 60.7 | 22.8 | 44.1 | 33.1 | 38.7 | 40.7 |
| n | 61 | 92 | 118 | 121 | 119 | 118 |
| sponge area | ||||||
| decrease | 46.9 | 38.3 | 33.3 | 39.3 | 46.8 | 49.5 |
| increase | 43.8 | 61.7 | 61.3 | 54.7 | 41.3 | 41.4 |
| no change | 9.4 | 0.0 | 5.4 | 6.0 | 11.9 | 9.0 |
| n | 31 | 47 | 111 | 117 | 109 | 111 |
| sponge-coral distance | ||||||
| decrease | 16.4 | 25.3 | 21.1 | 22.4 | 8.9 | 23.0 |
| increase | 18.0 | 27.3 | 25.4 | 26.7 | 29.5 | 21.2 |
| no change | 65.6 | 47.5 | 53.5 | 50.9 | 61.6 | 55.8 |
| n | 61 | 99 | 114 | 116 | 112 | 113 |
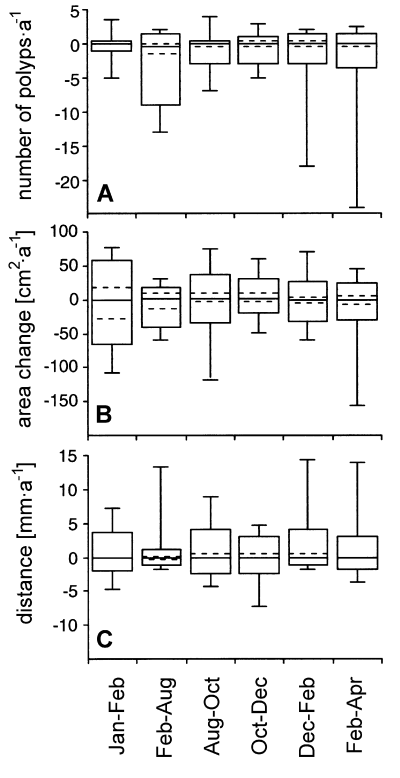
Percentile plots of changes in A. number of coral border polyps, B. sponge area and C. sponge-coral distance for each time period. The boxes comprise 90 % of all data. The vertical lines show the ranges of the data. The median of the data is displayed by the horizontal solid line.
The reaction of sponges to changes in the number of coral border polyps appeared to be species-specific:
(1) Area changes of both N. erecta and S. ruetzleri were not correlated to the number of coral border polyps (Pearson’s correlation coefficient, P = 0.699 and P = 0.546, respectively). Niphates erecta displayed 3 cases of overtopping (9 %). This means that the sponge was growing over the coral but was not attached to the coral tissue. In one case (3 %) the border of the coral showed some signs of bleaching. Neither overgrowth nor other signs of damage to the coral occurred in any of the interactions between S. ruetzleri and M. cavernosa.
(2) Area changes of R. venosus were negatively correlated with changes in the number of border polyps (Pearson’s correlation coefficient, P < 0.001; Fig. 4). In contrast with the other two sponge species, R. venosus often took over vacant space caused by polyp death (54 % of the selected interactions).
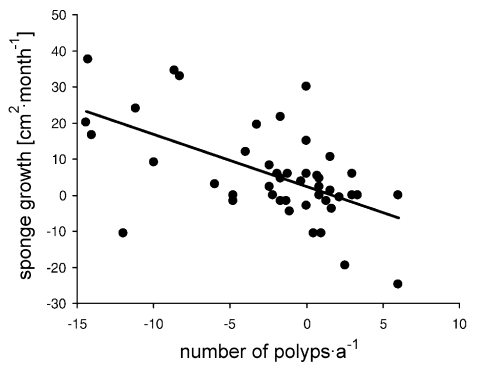
Correlation between changes in number of coral border polyps (per year) and sponge growth [cm2·month] for R. venosus. r = Pearson’s correlation coefficient and P = probability that rejection of H0 (there is no correlation) is incorrect.
2. Coral damage experiment
Of all interactions involving coral colonies with experimental lesions, 22.7 % showed overgrowth of living coral polyps by the sponge R. venosus. The overall occurrence of sponge overgrowth of living polyps of the control colonies was 6.9 % (Fig. 5). This difference was also observed for each locality separately. At site 1, overgrowth of living polyps of damaged coral colonies by R. venosus was higher than overgrowth of control colonies (20 % versus 0 %). At site 3 the corresponding values were 23.5 % for damaged corals and 13.3 % for control colonies. To determine if infliction of experimental lesions led to a greater decrease of border polyps, colonies with experimental lesions and the controls were compared. No significant differences in polyp changes were found (Fig. 6).

Occurrence of overgrowth (in % of total number of interactions) of the coral M. cavernosa by the sponge R. venosus for each locality. Black bars show the results of coral colonies with lesion infliction and white bars the control colonies. S1 = site 1, S3 = site 3 and S1 + S3 = average of data from sites 1 and 3.
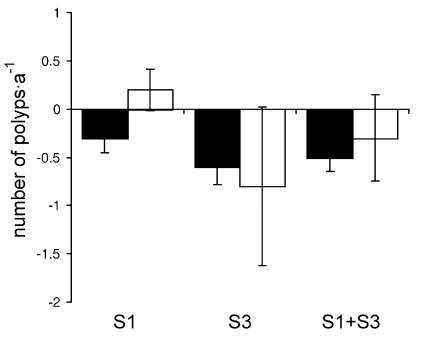
Changes in number of coral border polyps (per year) ± SE for M. cavernosa colonies with and without lesion infliction at each locality. Explanations and abbreviation of localities as in Fig. 5.
Discussion
1. Dynamics of sponge-coral standoffs
Most studies on competition for space used data collected at a particular point in time (e.g.,Jackson, 1979; Rubin, 1982; Lopez Gappa, 1989; Steneck et al., 1991). If the purpose of the study is to establish competitive dominance, such single observations can easily lead to misinterpretations, especially when standoff interactions are involved (Tanaka & Nandakumar, 1994). In a former study (Aerts & van Soest, 1997) most sponge-coral interactions on the reef were considered as standoffs. The present study demonstrates that such standoffs were actually quite dynamic. There was no evidence of cessation of growth along adjacent edges between interacting species (as observed by Karlson, 1980). In most cases, both competitors alternately lost and gained tissue and space during the 15-month interval, which conformed with the repeated reversals pattern of coral-coral interactions described by Chornesky (1989). Coral colonies showed an overall decline in number of border polyps, which was related more to locality than to sponge species. Apparently, local environmental conditions influenced border polyp loss. High sedimentation load appeared to play no role since most polyp loss occurred at localities with less sedimentation. In some cases, especially at the low sedimentation site, macroalgae of the genus Dictyota colonised vacant space caused by recession of the sponge or coral. Partial mortality of coral colonies could be due to smothering by macroalgae (Hughes, 1996), but from our photographs the potential role of algae in the loss of border polyps was not clearly observable. Variation in coral size and morphology among localities may also contribute to the observed differences in polyp loss, whereas the susceptibility of corals to competition depends on their surface-perimeter ratio (Hughes, 1996; Meesters et al., 1997). The decrease in number of border polyps was certainly not caused by competitive activity of neighbouring sponges because such polyp loss was similar for each sponge species.
2. Competition strategies of sponges
The necessity for sponges to compete with corals in order to gain space probably depends on their growth form in combination with other growth characteristics, such as growth rate (Sebens, 1986; Becerro et al., 1994). The three sponge species clearly reacted differently towards changes in the number of coral border polyps. Rhaphidophlus venosus, with its thinly encrusting growth form and variable, fast area changes, was often observed to take over space vacated by polyp death. Because the extent of polyp decrease along the coral border was similar in interactions with all three sponges, R. venosus was the only species benefiting from this decline yet did not cause this retreat. In general, the advance and retreat movements of R. venosus colonies resembled those described for encrusting colonial ascidians (Birkeland et al., 1981). Scopalina ruetzleri, although also encrusting, forms thick cushions on the substratum and can therefore easily expand into height. Despite its fast and variable area changes, S. ruetzleri was never observed to occupy space vacated by dead coral polyps. In most cases, either S. ruetzleri or the coral border receded, apparently without any utilization of the available space, which explains the very low frequency of overgrowth displayed by S. ruetzleri (Aerts & van Soest, 1997). The same appears to apply to N. erecta. During this study N. erecta escaped direct competition with the coral by growing taller (thereby overshadowing the coral colony in most cases). Probably due to its minor and relatively slow area changes, N. erecta never took over available space caused by retreated coral tissue. Direct competition in terms of coral overgrowth was not observed, probably due to the elevated growth form of N. erecta. Whether the sponge and coral specimens studied here redirect their growth away from the zone of interaction (Romano, 1990) is difficult to determine because observations were focused on the interacting area and did not include the whole sponge and coral colony.
In summary, the standoff interactions between sponges and corals were dynamic. The outcome of sponge-coral standoffs depends mainly on the growth strategy displayed by sponge species. The necessity and/or ability of sponges to overgrow corals or take over vacant space caused by the death of coral tissue appears to depend on their growth rate and form.
3. Coral damage and competitive outcome
Several field studies have revealed that regeneration of coral damage is influenced by environmental conditions (e.g.,Meesters et al., 1992; Meesters & Bak, 1993; van Veghel & Bak, 1994; Ward, 1995). Available energy is often limited (e.g.,Bak, 1983) and must be divided between several biological functions. Competition with other organisms can severely reduce the physiological functioning of corals (Tanner, 1995). The repair of damage may locally reduce the competitive ability of the coral. Standoff interactions between R. venosus and M. cavernosa show an apparent equilibrium at the border of contact (Karlson, 1980) when observed at one point in time. When observed over a period of time, however, R. venosus often overgrows dead coral polyps at such borders and sporadically also living coral polyps. In damaged coral colonies, living coral polyps were more frequently overgrown by R. venosus. Interestingly, overgrowth of living coral tissue of control colonies occurred in corals which appeared to be in a very bad condition (mean polyp decrease of 22 and 12 polyps·a–1). Due to the encrusting growth form of R. venosus, only observations over time can distinguish overgrowth activity. Very likely, a certain proportion of the peripheral growth interactions observed in a former study (Aerts & van Soest, 1997) were actually overgrowth interactions.
Overgrowth of living border polyps by R. venosus was an active process because lesion infliction did not influence the decrease of the border polyps. The active role of the sponge is also demonstrated in Fig. 7 where sponge growth is directed towards the damaged area. Because bio-assays of R. venosus extracts demonstrated biological activity (J. C. Braekman, pers. comm.), this sponge may use its chemicals to compete with corals. Possession and use of chemicals in competition for space has been observed for several other reef organisms (Jackson & Buss, 1975; Coll et al., 1982; Porter & Targett, 1988).
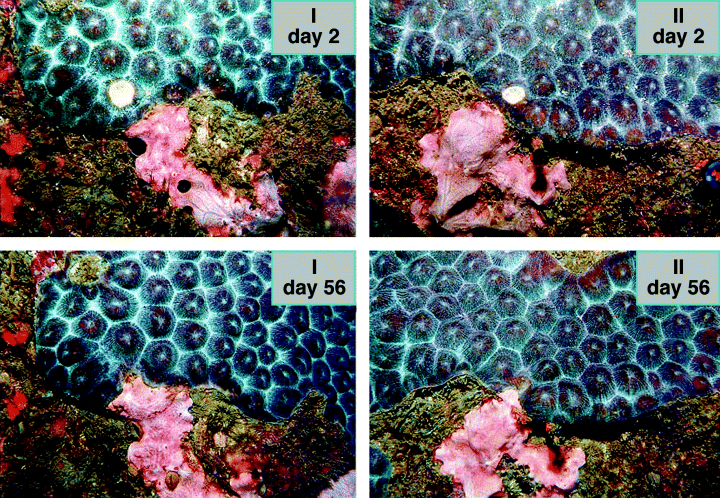
Photographs of 2 interactions (I, II) showing the sponge R. venosus and the coral M. cavernosa at days 2 and 56. The corals have an experimental lesion (white spot at day 2). Growth of R. venosus is directed towards the experimentally damaged area of the coral.
From the lesion experiment we can conclude that damage to M. cavernosa alters the interaction process with R. venosus. Apparently, besides reducing coral growth and reproduction (Tanner, 1995), damage reduces the competitive ability of the coral in such a way that sponges can benefit from it by actively overgrowing live coral tissue. This means that coral damage on reefs could enhance deterioration of corals by increasing their susceptibility to competing organisms.
Conclusion
Standoffs are characterised by the fact that no visible interaction is evident between the two organisms. Despite the apparent stable situation, however, they are actually very dynamic. The dynamic nature can only be determined by repeated observations. It depends mainly on the growth strategy of the sponge species involved, such as growth form and growth rate. Damage to coral colonies in the form of lesions influences the dynamics of standoff interactions because damaged colonies are more susceptible to sponge overgrowth than unharmed colonies.
Acknowledgements
I would like to express my gratitude to Dr. R. W. M. van Soest and Prof. R. P. M. Bak for their support and for critically reading the manuscript. Furthermore I thank Dr. S. Zea for his practical advice, Dr. L. Botero (director of the INVEMAR) for logistic support, P. A. J. van der Wolf for material aid and suggestions and boatsman J. Gonzalez for his help during the field trips. This research was made possible by a grant from the Netherlands Organisation for Scientific Research in the tropics (WOTRO, project number W84 – 342).




