Heavy Metal Contents in Soft-Bottom Marine Macrophytes and Sediments Along the Mediterranean Coast of Spain
Abstract
Abstract. Hg, Cd, Pb and Zn concentrations were determined in sediment and in tissues of five species of soft-bottom marine macrophytes (Posidonia oceanica, Cymodocea nodosa, Zostera noltii, Ruppia cirrhosa and Caulerpa prolifera) along the Spanish Mediterranean coast. Levels of metals were low in most of the sampling stations and similar to those found by other authors in uncontaminated zones. Certain locations, however, showed some degree of contamination (Cambrils, Almassora, Alacant, Mar Menor and El Portús). In Santa Pola we found high contents of metals in one sample of sediment due to the high proportion of the fine fraction (particules < 63µm) and organic matter, but not in the seagrass species. Mercury and zinc concentrations in the sediment are correlated to those in at least some anatomic fractions of Posidonia oceanica, Cymodocea nodosa and Caulerpa prolifera, suggesting that these species reflect the levels of these metals in the environment. Lead contents in the alga Caulerpa prolifera are also correlated to those in the sediment, while no significant correlations were obtained for cadmium. Among the species studied, Posidonia oceanica and Cymodocea nodosa seem to be better biomonitors than Caulerpa prolifera.
Problem
Several organizations have pointed out the need for monitoring heavy metal concentrations in the environment (UNEP/FAO/WHO, 1987, 1996; UNEP/FAO, 1996) because of their toxicity, persistence and accumulation in the biota. In coastal waters, soft-bottom marine macrophytes constitute one of the most extensive, productive and diversified ecosystems, and are the base of a complex food chain through plant detritus (Mann, 1972). Among the plants that are able to colonise marine sediments, seagrasses have attained evolutionary success, forming dense and extensive prairies in warm-temperate seas. Algae usually require hard substrates, but some species are able to grow on soft bottoms forming meadows; examples include those of the genera Caulerpa and Penicillus.
Both seagrasses and algae reflect the concentration of heavy metals present in the environment and are considered as good biomonitors of these contaminants (Augier et al., 1977; Brix et al., 1983; Ward, 1987; Phillips, 1990; Augier et al., 1992; Carter & Eriksen, 1992,; Warnau et al., 1996) as well as of radionuclides (Calmet et al., 1988; Calmet et al., 1991). Soft-bottom macrophytes are able to absorb nutrients and heavy metals both from water and from sediment, and to translocate some of these elements among the different parts of the plant (Williams, 1984; Lyngby & Brix, 1984).
In our area of study, only few studies have been conducted on the levels of heavy metals in seagrasses or algae, all of them are tightly restricted geographically (Nieto & Capdevila, 1986; Sanchiz et al., 1990; Benedito, 1996). Only the latter analyses in more detail the seasonal variation of metal concentrations in Posidonia oceanica.
In the present study we have determined the concentrations of Hg, Cd, Pb and Zn in the sediment, and in five species of soft-bottom marine macrophytes collected in 17 sampling stations along the Mediterranean coast of Spain. The location of stations include open sea coastal areas and estuarine environments. The species collected are three seagrasses (Posidonia oceanica, Cymodocea nodosa and Zostera noltii), a brackish-water phanerogam sensu den Hartog (1970) (Ruppia cirrhosa) and a Chlorophyceae (Caulerpa prolifera).
Some of the species in the present work have been very rarely used in studies dealing with metal concentrations (Caulerpa prolifera and Zostera noltii), and one of them (Ruppia cirrhosa), as far as we are aware, has never before been used to determine heavy metal contamination. These species are potentially useful biomonitors in coastal waters.
Material and Methods
Samples of sediments and macrophytes were collected by SCUBA diving during June and early July 1991 from 17 sampling stations located on the Spanish Mediterranean coast, including the provinces of Tarragona, Castelló, València, Alacant and Murcia (Fig.1). Not all the species were found in all the stations due to their different ecological requirements: Posidonia oceanica, Cymodocea nodosa and Caulerpa prolifera were collected in most of the open sea coastal localities. The two last species were also found in some estuarine environments, while Zostera noltii and Ruppia cirrhosa were only found in brackish-water areas (El Fangar, Alfacs and Mar Menor; in this last station we couldn't find Zostera noltii).
Map of the sampling stations.
In order to determine whether samples within each station varied significantly in their metal concentrations a preliminary study was carried out in Alfacs inlet (Fig.1, station number 4) in January. An abstract of those results can be found in Sanchiz et al. (1991). Six points were randomly selected inside a Cymodocea nodosa patch about 12 metres in diameter. At each point entire plants and surface sediments were collected. The samples from different points were processed and analyzed separately. The results indicate no statistically significant difference in metal concentrations between the samples taken from different points in the meadow. In consequence, samples from one sampling point would be sufficient to assess the metal concentrations of plants and sediments in a seagrass meadow. Nevertheless, in order to obtain more reliable results, in the main sampling campaign 5–6 plant and sediment samples were collected in each station from several points within an area of approximately 15 m diameter. These were then combined following a technique from Phillips & Segar (1986) termed space-bulking.
The thickness of the upper collected layer of sediment was approximately 5 cm. In those stations where different plant species grew on different sites (El Fangar 2, Torre la Sal, Santa Pola and Mar Menor), two sediment samples were collected. At Almassora we collected two sediment samples as well: one of them (Almassora a) was the sediment on which Caulerpa was attached (compacted sand corresponding to old Sabellaria reefs) and the other (Almassora b) was sand surrounding these reefs.
After collection, samples were stored at −20°C. Prior to analysis, leaves of the phanerogams and thalli of the algae were scraped with a glass slide and rinsed with deionized water to remove epibiota. Roots and rhizomes of the phanerogams and rhizoids + stolons of the algae were also rinsed and scraped gently with a glass slide to remove sediment. Plants were divided into the following fractions: roots, rhizomes and leaves for the three seagrasses (Posidonia oceanica, Cymodocea nodosa and Zostera noltii); roots, rhizomes + shoots and leaves for the brackish-water phanerogam Ruppia cirrhosa; and rhizoids + stolons and fronds for the alga Caulerpa prolifera. Each sample was lyophilized prior to digestion. Homogenization of the plant tissues was carried out with an electric mill. Digestion was carried out in triplicate on the sediment fraction < 250 ;µm and on the homogenized plant material in concentrated warm nitric acid. Plant digestion: 0.3 to 0.5 g of ground sample + 5 ml HNO3 in 250 ml erlenmeyer flasks covered with a watch glass; 12 h over heating plate at 75–80°C; then cooled and diluted to 15 ml. Sediment digestion: 1g sediment + 1 ml deionized water + 5 ml conc. HNO3 in Pyrex tubes with teflon closures; 1h in a bath at 100 °C; then cooled and diluted to 25 ml. To prevent contamination, all the glassware in contact with the samples was washed with 10% nitric acid for at least 12 hours. The analyses were performed with a Perkin Elmer model 5000 Atomic Absorption Spectrophotometer, equipped with a deuterium background corrector or Zeeman corrector. Hg was determined by the cold vapour technique, Zn by flame, Cd by graphite furnace (with 0.5% ammonium phosphate as matrix modifier), and Pb by flame in sediments and by graphite furnace in plant tissues (with 1% ammonium phosphate and 0.05% magnesium nitrate as matrix modifiers). A five replicate-recovery assay was run, consisting of the addition of known amounts of each metal to a plant or sediment sample before digestion. The values obtained were: 103 ± 4% for Hg, 100 ± 2% for Cd, 102 ± 6% for Pb (graphite furnace) and 99 ± 5% for Pb (flame), and 105 ± 5% for Zn. The precision of the analyses was determined calculating the coefficient of variation of a five replicate measurement of the metals on Posidonia oceanica leaves (and on a sediment sample for Pb by flame), and was 9.8% for Hg, 2.7% for Cd, 7.0% for Pb by flame, 7.1% for Pb by graphite furnace and 5.6% for Zn. Routine triplicate digestions and analyses of the samples were carried out in order to test analytical reproducibility. Only in the case of Hg, some values were below the detection limit, which was 4 ng · g−1 for sediments and 8 ng · g−1 for plants (dry weight).
The organic matter content of the sediments was determined by the Walkley-Black method (Ministerio de Agricultura, Pesca y Alimentación, 1986), consisting of the oxidation of the organic matter of the sediment with potassium dicromate in acidic medium, and subsequent titration of the excess of dicrotame with a ferrous salt, using diphenilamine as indicator. The fine fraction (grain size < 63 µm) of the sediments was determined by wet sieving. Results for organic matter and fine fraction contents of the sediments are given only in relevant cases.
For data on Posidonia oceanica, Cymodocea nodosa and Caulerpa prolifera, several 2-way related-samples ANOVA were undertaken to compare the concentrations of metals in the different sampling stations. One of the independent variables was the sampling station, and the other the fraction analyzed; the latter was studied in detail in a previous work (Sanchiz et al., 1999) and is not considered here. Samples of the same fraction are considered as related samples. For the statistical analyses, the values below the detection limit have been considered as one half the value of the detection limit (i.e., 4 ng · g−1). A similar data management can be found in Warnau et al. (1995). All the variables were log-transformed in order to fulfil the requirements of the statistical analyses. Data on Zostera noltii and Ruppia cirrhosa are not compared statistically since these plants were found in three sampling stations only. This statistical test cannot be run with the sediment data since there is only one independent variable (the sampling station) with no replicate measurements. Further details on analytical and statistical protocols are given in Sanchiz (1996).
Results
Metal concentrations in the sediment and in plant fractions, at the different sampling locations, are given in 2-5
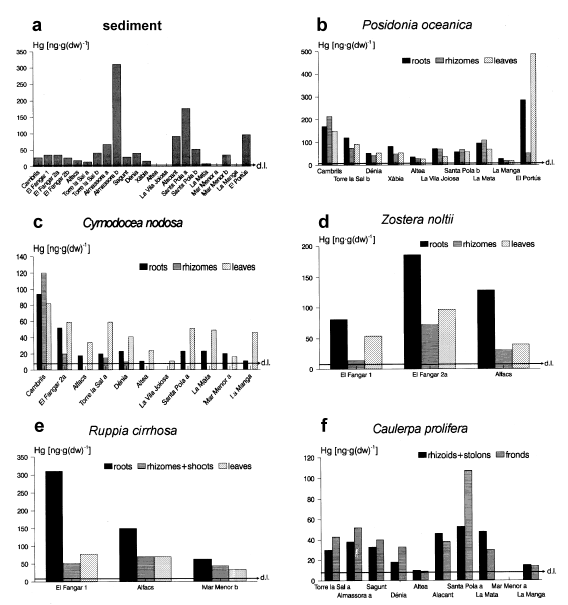
Concentrations of Hg in the sediment and in the marine macrophytes (d.l.: detection limit).
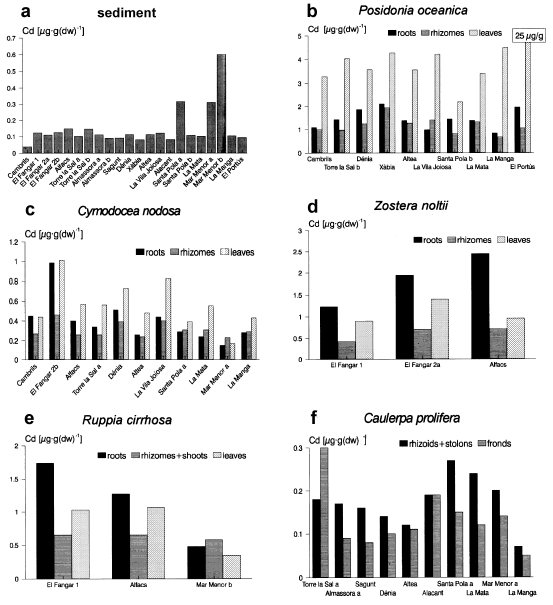
Concentrations of Cd in the sediment and in the marine macrophytes.
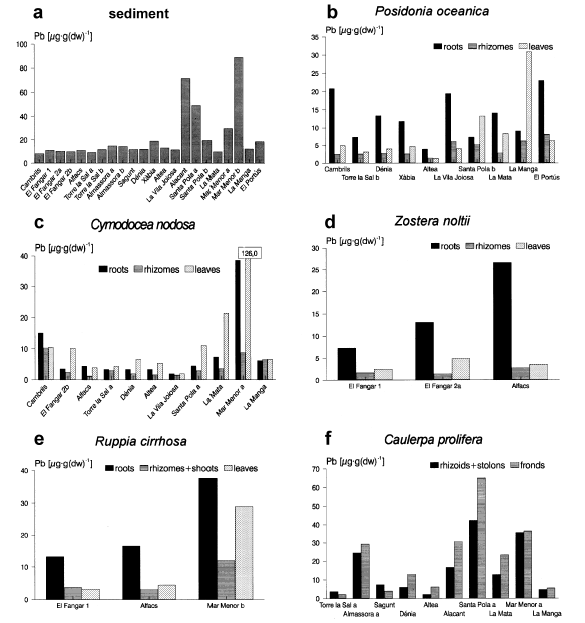
Concentrations of Pb in the sediment and in the marine macrophytes.

Concentrations of Zn in the sediment and in the marine macrophytes.
Table1 gives the results of the ANOVas carried out to determine whether there was a significant difference in metal concentrations at the different stations. These tests were carried out only for the three macrophytes, where more data was available (Posidonia oceanica, Cymodocea nodosa and Caulerpa prolifera).
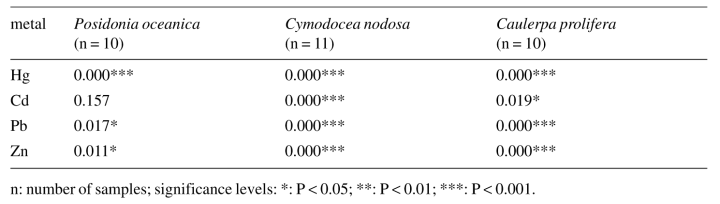
Differences were significant in every case except for Cd concentrations in Posidonia oceanica. 2-5 reveal large differences in the metal concentrations of the three species mentioned above from one station to another. This is especially evident for Hg in all three species (Fig.2) and for Pb and Zn in Cymodocea nodosa and Caulerpa prolifera (Figs 4 and 5).
The exception was Cd concentrations in Posidonia oceanica. Fig.3b shows that in all stations the cadmium concentrations in the three organs of P.oceanica are very similar, except for the leaves from El Portús, with 25 µg·g−1 of Cd. This difference, however, was not statistically significant.
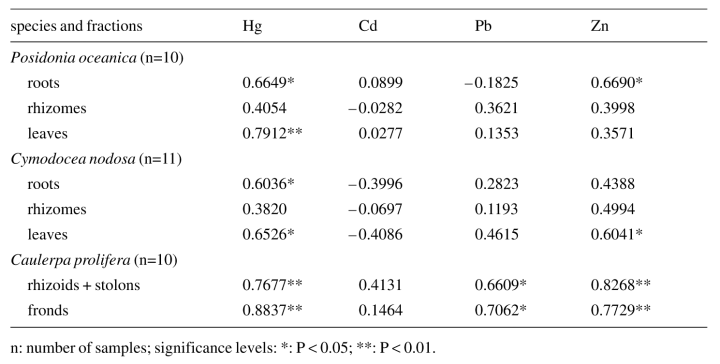
Table 2 shows the Pearson correlation coefficients between heavy metal concentrations in plants and in the sediment, based on the different anatomic fractions of Posidonia oceanica, Cymodocea nodosa and Caulerpa prolifera. Note the significant correlations in the three species (at least in some fractions) for mercury and zinc. Lead contents in Caulerpa prolifera are also correlated with lead in the sediment, whereas for cadmium no significant correlations were found. Figure 6 shows the correlations between Hg concentrations in Posidonia oceanica and in the sediment (Fig.6a) and between Zn contents in Caulerpa prolifera and in the sediment (Fig.6b).
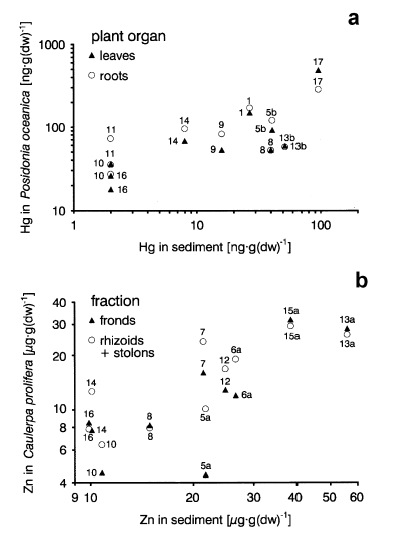
Correlations between metal concentrations in the plants and in the sediment. a. between Hg in Posidonia oceanica and Hg in the sediment; b. between Zn in Caulerpa prolifera and Zn in the sediment.
Discussion
The levels of metals were generally low and similar to those found by other authors in uncontaminated zones (Augier et al., 1980; Castagna et al., 1985; Denton & Burdon-Jones, 1986; UNEP/FAO/WHO, 1987; Sanchiz et al., 1990; Costantini et al., 1991; Giordano et al., 1992; Catsiki & Bei, 1992; Warnau et al., 1995; Gnassia-Barelli et al., 1995; Benedito, 1996; Pergent-Martini, 1998). Nevertheless, the ANOVA tests indicate that, overall, plants from different locations had different metal concentrations (Table 1). Some locations showed higher metal contents, reflecting a contamination in these sites.
In Cambrils, for example, we found relatively high concentrations of Hg in Posidonia oceanica and Cymodocea nodosa (Fig.2) and of Pb and Zn in Cymodocea (Figs4 and 5). The mercury concentrations in Posidonia oceanica at this location are similar to those found by Augier et al. (1977) in the same species in areas of the Mediterranean French coast classified by the authors as “slightly polluted”. The concentration of Hg in Posidonia oceanica leaves (149 ng·g−1) is very similar to that found by Pergent-Martini (1998) at Marseilles-Cortiou, France (176.5 ng·g−1), a location with a high human activity. Other authors have already reported high levels of metals in the sediments of the Cambrils area (Obiols et al., 1984). The slightly higher metal concentrations here are probably due to the influence of the industrial and urban area of Tarragona.
Another station with relatively high concentrations of certain metals in some samples is Almassora. Here we found a high mercury content in one sediment sample (Almassora b, with 311 ng·g−1) but not in the other (Almassora a, with 67 ng·g−1), and in the alga Caulerpa prolifera (38 and 52 ng·g−1 in the two fractions analyzed, see Fig.2). Almassora b corresponds to the loose sand surrounding the Caulerpa meadows, which are attached on sandy sediment (Almassora a) compacted by the polychaete Sabellaria sp. (see Material and Methods). Note that in this station the sediment surrounding the Caulerpa meadow is Hg-contaminated, but not the alga. This can be explained by assuming that the Hg contamination here is associated with sedimentary particules and not in the form of dissolved mercury, and that mercury in the sediment is present in a low leachable form. Augier et al. (1977) proposed a similar model of mercury contamination in low and moderately polluted sites along the Mediterranean French coasts.
As far as the other metals are concerned, only lead shows relatively high concentrations in this station, in C. prolifera, with 24 and 29 µg·g−1 in the two fractions (Fig.4). Almassora station is under the influence of the petrochemical complex of the harbour of Castelló.
In the Alacant station we found relatively high contents of Pb in the sediment (72 µg·g−1) and in C. prolifera (17 and 31 µg·g−1) compared with the remaining stations (Fig.4), indicating an influence of the urban and industrial complex of the city of Alacant.
In Santa Pola, we recorded high contents of the four metals in one sample of sediment: Santa Pola a, which corresponds to a Cymodocea-Caulerpa meadow (176 ng·g−1 Hg, 0.32 µg·g−1 Cd, 49 µg·g−1 Pb and 56 µg·g−1 Zn). These values are above the background levels considered by other authors for the Mediterranean Sea, which are: < 100 ng·g−1 for Hg (UNEP/FAO/WHO, 1987), approximately < 0.20 µg·g−1 for Cd and < 40 µg·g−1 for Pb, as is deduced from Giordano et al. (1992), and < 50 µg·g−1 for Zn (Whitehead et al., 1983).
Relatively high contents of Hg and Pb in Caulerpa prolifera were also detected in this station, but not for the two seagrass species Cymodocea nodosa and Posidonia oceanica (Figs2 and 4). The high levels of metals in the sediment sample ‘Santa Pola a’ are probably due to the high fine fraction (particles < 63µm) and organic matter contents of this sediment (54.5% and 27.3% respectively, while in sandy beach sediments colonized by marine macrophytes the normal values are about < 10% of fine fraction and < 2% of organic matter; own observations). The cause for these unexpected values in ‘Santa Pola a’ is the artificial regeneration of the beach with muddy sediment coming in part from the harbour of Santa Pola. This was carried out several years prior to the sample collection of this study. These two components are known to influence the concentrations of metals in soils and sediments (Added et al., 1982; Adriano, 1986; Persaud & Lomas, 1987). We have already reported a close relationship between some metals and the fine fraction and the organic matter of the sediments in this study area (Sanchiz et al., 1998). Taking into account the metal concentrations in all the samples at this station, no true heavy metal contamination exists here.
In the lagoon of the Mar Menor, high contents of Cd, Pb and Zn are present in the two sediment samples (especially in ‘Mar Menor b’), and of Pb and Zn in Cymodocea nodosa, Caulerpa prolifera and Ruppia cirrhosa (see 3, 4 and 5). These concentrations suggest a moderate contamination at this location. Other authors have already detected this contamination, which is reflected in high metal contents in the sediments and organisms of the lagoon (De León et al., 1982). The metals enter the lagoon via rainwater from the old lead-zinc mines of the Sierra of Cartagena — Portman, situated south of the Mar Menor, and not in use since 1990.
Higher metal contents in the ‘Mar Menor b’ sediment sample (34 ng·g−1 of Hg, 0.60 µg·g−1 of Cd, 89.0 µg·g−1 of Pb and 131.4 µg·g−1 of Zn) versus ‘Mar Menor a’ (<4 ng·g−1 of Hg, 0.31 µg·g−1 of Cd, 30.0 µg·g−1 of Pb and 38.5 µg·g−1 of Zn) are probably due to the higher organic matter content of the first sample (11.17% and 0.37% respectively). In our samples, the plants showed no elevated cadmium content, as was also the case for the sediment (Fig.3); this suggests a low availability of the metal in this sediment. The lead concentrations in the Cymodocea nodosa leaves are particularly high (126 µg·g−1), being similar to those reported by Vicente & Chabert (1981) in whole plants of the same species, in a zone subjected to a strong Pb contamination in Port-Cros (France).
The last station in which a potential metal contamination exists is El Portús. Here, we found high contents of mercury in Posidonia oceanica and, to a lesser extent, in the sediment (Fig.2); elevated cadmium and zinc concentrations were also recorded in the leaves of the phanerogam (Figs3 and 5). The mercury in P. oceanica is of the same order as that reported by Augier et al. (1977) and Catsiki et al. (1987) in the same species in moderately contaminated localities along Mediterranean French and Greek coasts, respectively. The Cd and Zn concentrations in P. oceanica leaves are among the highest concentrations recorded in this species (for Cd, similar to those found by Cristiani (1980); and for Zn — 703.5 µg·g−1— the highest recorded in this species in the Mediterranean). These concentrations are similar to the lowest concentrations found by Olsen (1983) and Ward (1987) in Posidonia australis in some locations of S. Australia subject to a very strong contamination. Compared with literature data there is a moderate contamination of Hg, Cd and Zn in El Portús, probably due to the influence of the urban and industrial complex of Cartagena, situated 8 km east, and possibly to the old Pb-Zn mines of Portman. For Cd and Zn, high contents were found in P. oceanica leaves and not in the sediment, rhizomes or roots. This suggests a contamination of the water, in a dissolved form: these metals are absorbed by the leaves and are not significantly transported to the below-ground parts of the plant.
In Posidonia oceanica there are no studies concerning translocation of metals. In other seagrass species, a translocation has in some cases been observed — for Cd in Zostera marina (Faraday & Churchill, 1979; Brinkhuis et al., 1980), for Cd in Heterozostera tasmanica (Fabris et al., 1982) and for Hg and Zn in Zostera marina (Brix & Lyngby, 1984). The distribution of Cd and Zn reported by Benedito (1996) in Posidonia oceanica in Altea (Spain) suggests a low transport of these elements in this species.
Some authors have determined element losses due to rinsing of Posidonia oceanica intermediate leaves with de-ionized water versus seawater rinsing (Ledent et al., 1995). Thus, results presented here are probably underestimates of total metal concentrations. Many metal concentrations in seagrasses, however, have been determined on distilled water rinsed material (Ward, 1987; Costantini et al., 1991; Catsiki & Panayotidis, 1993; Malea et al., 1994; Malea & Haritonidis, 1995).
The biomonitoring capacity of Posidonia oceanica and Cymodocea nodosa has been reported previously by other authors in field studies (Augier et al., 1977; Vicente & Chabert, 1981; Maserti et al., 1988; Costantini et al., 1991; Catsiki & Bei, 1992; Warnau et al., 1995; Malea & Haritonidis, 1995). Also, Warnau et al. (1996) deduced from uptake and depuration experiments in the laboratory that Posidonia oceanica and the alga Caulerpa taxifolia are good biomonitors of Cd and Zn (and other metals and radionuclides) in the marine environment. It is beyond the scope of this study to examine in depth the validity of these species as biomonitors of heavy metals in coastal waters, but our results indicate that Posidonia oceanica, Cymodocea nodosa and Caulerpa prolifera reflect in their tissues the mercury and zinc concentrations present in the environment, and could be considered as good biomonitors of these elements in coastal waters. This statement could also be applied to C. prolifera for lead.
Several laboratory studies show that the leaves of marine phanerogams reflect the lead concentration in the water (Lyngby & Brix, 1984; Bond et al., 1988). The present study showed no significant correlation between Pb concentrations in the seagrasses and in the sediment: this could indicate a more direct relationship in Posidonia oceanica and in Cymodocea nodosa to lead concentrations in the water than to those in the sediment.
For cadmium, the absence of significant correlations could be explained by the following hypothesis, with two statements: (1) the plants reflect in their organs the concentration of the metal in the water, but not that in the sediment; and (2) there is no correlation between cadmium concentrations in the water and in the sediment. Laboratory experiments demonstrate that cadmium contents in plants reflect concentrations in the water: Morrison & Cohen (1980) and Kabata-Pendias & Pendias (1984) in terrestrial plants, Lyngby & Brix (1984) in the marine phanerogam Zostera marina and Chen & Chen (1993) and Collard & Matagne (1994) in algae. Cadmium in seawater is found mainly as complexes with the chloride ion (CdCl+ and CdCl2), which are poorly adsorbed by the sorbing components of the sediment (McComish & Ong, 1988). These complexes might be easily absorbed by the leaves of the phanerogams and the fronds of the algae.
Figure 3b reveals an especially high cadmium concentration in Posidonia oceanica leaves at El Portús (25 µg·g−1) compared to the remaining stations, whereas the sediment level is similar everywhere (Fig.3a). This is probably due to a high concentration in the water at this station, which is close to several pollution sources. In other sites, a contamination detected in the plants is not observed in the sediment. This is specially true for Posidonia oceanica and Cymodocea nodosa: in Cambrils (Hg in both species and Zn in C. nodosa), and El Portús (Hg, Cd and Zn in P. oceanica).
In Santa Pola, however, we found relatively high metal concentrations in one sediment sample (Santa Pola a) but not in C. nodosa or P. oceanica. This is certainly due to the abnormally high fine fraction and organic matter content, as stated above, which promote a high retention of metals in the sediment. In our opinion, no true contamination of metals exists at this location, based on the concentrations found in the other samples. Accordingly, P. oceanica and C. nodosa would be good biomonitors and would not be significantly influenced by physico-chemical characteristics of the sediment. They reflect the bioavailable metal concentrations in the environment better than total concentrations, and this is one of the requirements of biomonitors (Phillips & Segar, 1986).
Caulerpa prolifera, on the other hand, showed no metal contamination in some stations where sediments presented relatively high concentrations (for instance, mercury in Almassora, and Hg and Pb in Alacant). In other stations, like Mar Menor, metal levels in Caulerpa were higher than at the other stations, but not as elevated as those found in Cymodocea (e.g., for Pb and Zn), which showed a greater biomonitoring capacity. In Santa Pola, levels in Caulerpa seem to be influenced by concentrations in the sediment, probably due to the sediment composition (see above). This is the case for Hg and Pb, and, to a lesser extent, for Cd. The results suggest that Cymodocea nodosa and Posidonia oceanica more exactly reflect the bioavailable metal levels in the water column and in the sediment, in a time-integrated measure, than would Caulerpa prolifera.
For Zostera noltii and Ruppia cirrhosa, the scarcity of the data does not allow us to make an appropriate evaluation of their biomonitoring capacity. Some authors have reported a good biomonitoring value for the congeneric species Zostera marina (Lyngby & Brix, 1983; Carter & Eriksen, 1992). Other authors have utilized these species or congeneric ones as heavy metal biomonitors in coastal waters (Augier et al., 1980; Olsen, 1983; Carruesco et al., 1986). Our results suggest that Zostera noltii and Ruppia cirrhosa could be used as biomonitoring organisms in estuarine environments, but attention must be paid to their tendency to accumulate high concentrations of metals in the roots.
Conclusions
Metal levels in sediments and macrophytes are generally low and similar to those found by other authors in uncontaminated areas. In some locations and some samples, however, metal concentrations are above background levels, indicating a low or moderate contamination at these sites. This is the case for Cambrils (Hg, Pb and Zn), Almassora (Hg and, to a lesser extent, Pb), Alacant (Hg and Pb), Mar Menor (Cd, Pb and Zn) and El Portús (Hg, Cd, Pb and Zn). In one sediment sample from Santa Pola, high concentrations of metals are probably due to the high organic matter and fine fraction content of this sediment, stemming from the artificial regeneration of the beach, and not to a real contamination.
Mercury and zinc levels in at least some fractions of Posidonia oceanica, Cymodocea nodosa and Caulerpa prolifera and lead contents in C. prolifera are correlated to those of the same metals in the sediment, suggesting a good biomonitoring capacity of these species for these metals. For cadmium no significant correlations were observed.
Posidonia oceanica and Cymodocea nodosa seem to be good biomonitors of heavy metal pollution due to their capacity for detecting abnormal metal levels that are not reflected in the sediment. The alga Caulerpa prolifera does not show such a good biomonitoring capacity, because in some cases abnormal metal levels in the sediment are not reflected in the alga.
Acknowledgements
This work has been supported by a grant of the Ministerio de Educación y Ciencia of Spain. We thank Mr. P. Leak for his help in the English revision of the manuscript.




