Morphology and classification of larval scoli of Saturniinae and Hemileucinae (Lepidoptera: Saturniidae)
Abstract
enThe larval scoli of 15 species of Saturniidae (Saturniinae and Hemileucinae) were investigated by means of scanning electron microscopy. On the basis of morphological findings from surface views and inside views of the scoli, the classification of scoli previously used in the literature has been altered in several points. Two scolus types are united, a new one (blunt-bristly scolus) is proposed, and another one is subdivided. From these morphological data, an improved character set with new state descriptions is derived; it is of potential interest for the systematics and phylogeny of Saturniidae.
Abstract
deMorphologie und Klassifizierung der Scoli von Raupen der Saturniinae und Hemileucinae (Lepidoptera: Saturniidae)
Die Scoli der Raupen von 15 Arten der Saturniidae (Saturniinae und Hemileucinae) wurden mittels Rasterelektronenmikroskopie untersucht. Auf Grundlage der morphologischen Befunde anhand von Außen- und Innenansichten der Scoli wurde die bisher verwendete Scolus-Klassifikation, welche aus der Literatur bekannt ist, in mehrerlei Hinsicht verändert. Zwei Scolustypen wurden zusammengefaßt, ein neuer Typus (Stumpfborstenscolus) aufgestellt und ein weiterer wurde unterteilt. Basierend auf den vorgelegten morphologischen Daten wird ein verbesserter Satz von Merkmalen zusammen mit Beschreibungen deren verschiedener Ableitungsstatus vorgestellt, der von potentiellem Interesse für die Systematik und Phylogenie der Saturniiden ist.
Introduction
In the past decade, considerable efforts have been made to elucidate the defensive habits of caterpillars of the moth family Saturniidae (= Attacidae; emperor moths). These investigations have been stimulated mainly by the economic importance, venomous properties, and the size and beauty of many larvae of this family of Bombycoidea.
Many caterpillars of the Saturniidae bear series of often strikingly coloured, integumental outgrowths on most thoracic and abdominal segments; some of the processes are able to discharge an exocrine secretion on mechanical irritation of the larvae. These so-called `scoli' appear in various phenotypes among the Saturniidae, all of which are phylogenetically derived from dermal outgrowths bearing normal setae. In several species, two sorts of scoli even occur simultaneously on the same larva, or occur successively during larval development.
Nässig (1989 ) was the first who classified seven types of scoli. The following list of these types gives short English terms which we coin herein, along with the original German designations ( Nässig 1989 ) in parentheses, the Latin names ( Deml and Dettner 1997 ) in square brackets, a short description, and typical representatives bearing these scoli (according to Nässig):
1 point-bristly scolus (Stechborstenscolus) [scolus pungentisetosus]: scolus with stinging bristles with or without venom; subfamily Saturniinae, Saturniini: Actias , Loepa , Cricula , Saturnia ( Calosaturnia ); Attacini: some Rothschildia spp.; subfamily Ludiinae;
2 secretory scolus (Sekretstechborstenscolus) [scolus secernentisetosus]: scolus with secretory bristles; subfamily Saturniinae, Saturniini: Caligula , Saturnia ( other subgenera); Attacini: Hyalophora ; Eupackardia ;
3 reduced scolus (sekundär reduzierter Scolus) [scolus reductus]: scolus with secondary reductions, often covered with soft hairs; subfamily Saturniinae, Saturniini: Perisomena , Neoris , some Antheraea spp., some Copaxa spp.; Attacini: some Rothschildia spp.;
4 spraying scolus (Spritzkuppelscolus) [scolus secretospargens]: scolus with spraying cupulae/domes; subfamily Saturniinae, Attacini: some Attacus spp.;
5 horn (Horn) [scolus cornutus]: horn-shaped scolus; two subtypes:
5 (a) derived from star warts (see below) in L1 larvae; subfamily Saturniinae, Saturniini: Rhodinia; Attacini: most species of Attacus, Samia, Epiphora, Coscinocera, Archaeoattacus;
5 (b) not derived from star warts; subfamilies Ceratocampinae, Arsenurinae, Agliinae;
6 thorn scolus (Stechdornscolus) [scolus pungentispinosus]: scolus as spiny thorn; subfamily Saturniinae, most Bunaeini;
7 urticating scolus (Nesselscolus) [scolus urens]: severely irritant, tree-shaped scolus; subfamily Hemileucinae; no scoli (as far as known) in subfamily Salassinae.
In the present study, we investigated the scolus morphology of nine species of last-instar larval Saturniidae by scanning electron microscopy (SEM) for the first time and supplemented it by morphological data given in previous publications on six other saturniid species. In addition, the scoli of younger larvae were inspected in two species. Then we compared our results to the relevant literature data (Nässig 1989) and found it useful to change the previous classification of scoli as well as their stated taxonomic distribution in several points. Furthermore, we combined our observations in order to obtain a character set with appropriate state-descriptions which appears suitable for future phylogenetic analyses.
Materials and methods
Insects
Living specimens of the following species of Saturniidae (origin in brackets) were investigated.
Saturnia (Eriogyna) pinratanai Lampe, 1989 (Doi Inthanon, Chiang Mai, Thailand), S. pyri (Denis and Schiffermüller, 1775) (Castellane, France; Mesopotamia, Iraq), S. ( = Eudia) pavonia (Linnaeus, 1758) (Holzheim and Bad Wurzach, Germany; Basel, Switzerland), Caligula ( =Dictyoploca) simla (Westwood, 1847) (India), Cricula andrei Jordan, 1909 (Bhutan), Antheraea frithi pedunculata Bouvier, 1936 (Surat Thani, Thailand), A. pernyi Guérin-Ménéville, 1855 (China), Antherina suraka (Boisduval, 1833) (Madagascar), Eupackardia calleta Westwood, 1853 (Mexico; Argentina), Hyalophora cecropia (Linnaeus, 1758) (U.S.A.), H. euryalus (Boisduval, 1855) (Escondido/California, U.S.A.), Attacus atlas (Linnaeus, 1758) (Janawa Bangkok, Thailand), Samia cynthia (Drury, 1773) (Oakland/New York, U.S.A.) (possibly the population of this species used for comparison will be given another specific name, based on a revision of the genus Samia which is in preparation [by R. S. Peigler and S. Naumann]; Nässig, pers. comm.), Automeris hamata Schaus, 1906 (Central America), Molippa sabina Walker, 1855 (Maripa, Las Trincheras, Venezuela).
The larvae (ex larva or ex ovo) were each fed with only one species of suitable foodplants (normally Crataegus monogyna, Prunus spinosa, P. laurocerasus, or Ligustrum vulgare) for their whole lives. The animals were reared in aerated plastic boxes at 22°C and 60–70% relative humidity. During the rearings the number of caterpillars per box was reduced constantly due to the increasing body size of the larvae. Last-instar caterpillars of all species as well as L2 larvae of Saturnia pavonia and L1 larvae of Automeris hamata were killed by freezing and stored at −20°C until used.
Scanning electron microscopy
Scoli were excised from the caterpillar integument, macerated in 5% KOH for 24 h, dehydrated with acetone, and critical point dried. Total preparations of the two sorts of younger larvae were not macerated and were dried in an exsiccator for several days above P2O5; after 2 days, a vacuum was applied in addition. All samples were prepared for scanning electron microscopy (SEM) by sputter coating with gold. SEM was carried out with a Stereoscan 90 scanning electron microscope (Cambridge Instruments, Cambridge, UK).
Results
The morphological characteristics of the different scoli in the studied saturniid species (systematics according to Michener 1952) are as follows:
Saturniinae, Saturniini
Saturnia pinratanai has scoli of star-wart-shape bearing short, pointed bristles as well as elongate, soft hairs ( Fig. 1A ). A view into a macerated scolus ( Fig. 1B ) reveals several holes inside the scolus wall, which represent the starting points of channels leading outwards through the hollow hairs and bristles. At higher magnification of such an opening ( Fig. 1C ), one can discern remnants of a cuticular membrane which either only encloses the inner hair base, in vivo , or even extends into a supposed gland cell ( Haffer 1921 ), thereby representing a cuticular lining (intima) of such a cell and a small reservoir for a glandular secretion.
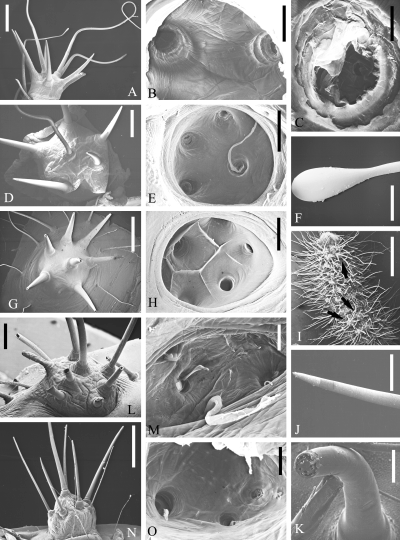
. SEM micrographs of scoli of Saturniinae, Saturniini (part I). Saturnia pinratanai : outside (A), inside (B), inside, single hair base (C); Saturnia pyri : outside (D), inside (E), tip of club hair (F); Saturnia pavonia : outside (G), inside (H); Saturnia pavonia , L 2 larva: dorsal view with star warts (arrows) (I), tips of intact (J) and broken (K) bristles on a star wart; Caligula simla : outside (L), inside (M); Cricula andrei : outside (N), inside (O). Scales: A, D: 1 mm; B, F, H: 200 μ m; C, J, K: 50 μ m; E, G, N: 500 μ m; I: 2 mm; L: 250 μ m; M, O: 100 μ m
Saturnia pyri has scoli that are similar to those of S. pinratanai (see also Deml and Dettner 1993 ). One or several central hairs on the scolus are elongate ( Fig. 1D ), have a particularly long cuticular lining ( Fig. 1E ), and a bulb at their tips ( Fig. 1F ) and are called `club hairs' (Kolbenhaare; Haffer 1921 ).
Saturnia pavonia again has scoli similar to those of the other Saturnia spp. but without elongate hairs ( Fig. 1G ) and with cuticular ridges between the inner bristle openings ( Fig. 1H ) which probably serve for separating and fixing the gland cells. L 2 larvae of S. pavonia are also covered with several scoli of star-wart type ( Fig. 1I, arrows ) but in addition bear many very long setae. The bristles on the scoli of these small larvae ( Fig. 1J ) normally have closed tips (in contrast to those of full-grown caterpillars which are open; Deml and Dettner 1990 , 1993 ). When such bristles are broken and dried prior to SEM, a solid material (containing air bubbles as an artifact) is visible inside the hairs ( Fig. 1K ) and might be the solid substance of the glandular secretion.
Caligula simla bears scoli which resemble those previously described but have bristles that are easily broken, producing splintered edges at the external openings ( Fig. 1L ). In the scolus cavity, the cuticular linings of gland cells are well developed ( Fig. 1M ).
Cricula andrei has scoli which resemble star warts both from the inside ( Fig. 1O ) and from the outside ( Fig. 1N ) but with somewhat finer bristles. Because no delivery of secretion out of the bristles can be observed (in contrast to secretory scoli), Nässig (1989 ) named these scoli point-bristly scoli; however, they do contain a liquid secretion (personal observations).
Antheraea frithi has three differently coloured types of scoli. Bluish-green scoli are very flat and are set with short, reduced, and squat bristles ( Fig. 2A ). Inside the scolus hang clearly discernible intimae ( Figs 2B,C ). Red scoli have a more dome-like shape ( Fig. 2D ) but with an internal anatomy resembling that of the blue ones ( Fig. 2E ). In addition, A. frithi has scoli that are evidently strongly reduced, amounting merely to single, white, very enlarged bristles ( Fig. 2F ); these still have an inner opening ( Fig. 2G, arrow ), but differs from normal hairs due to its size. Only few cuticular remnants of an underlying cell inside such a hole are visible ( Fig. 2H ).
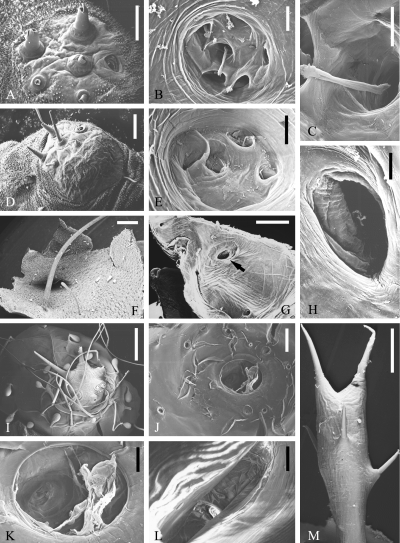
. SEM micrographs of scoli of Saturniinae, Saturniini (part II). Antheraea frithi pedunculata : bluish-green scolus, outside (A), inside (B), inside, single hair base (C); red scolus, outside (D), inside (E); part of integument with long white bristle (probably reduced scolus), outside (F), inside (arrow) (G), inside at larger magnification (H); Antheraea pernyi : outside (I), inside (J), inside at larger magnification (K); Antherina suraka : inside (L), outside (M). Scales: A: 200 μ m; B, E, K, L: 100 μ m; C: 50 μ m; D, F, G, J: 250 μ m; H: 25 μ m; I, M: 500 μ m
Antheraea pernyi again bears scoli which are more similar to star-wart-type scoli of other species ( Fig. 2I ). However, the scoli are encircled by thin, long hairs and by fungiform or drop-like structures with a hitherto unknown function. This concentric arrangement is recognizable also in inside views ( Fig. 2J ). In addition, a large cuticular membrane can be discerned, which is connected to the `ring' at the scolus base (the membrane is no artifact such as a tracheal remnant, which in any case would have a different appearance) and obviously separates the total scolus cavity from the body cavity ( Fig. 2K ).
Antherina suraka has elongated scoli with several short bristles distributed over their length ( Fig. 2M ). Inside the scolus cavity, apart from bristle openings nothing striking can be seen ( Fig. 2L ).
Saturniinae, Attacini
Eupackardia calleta is a representative bearing scoli set with short, easily splintering bristles ( Fig. 3A ) ( Deml and Dettner 1993 ). In interior structure they correspond to secretory scoli ( Fig. 3B ).
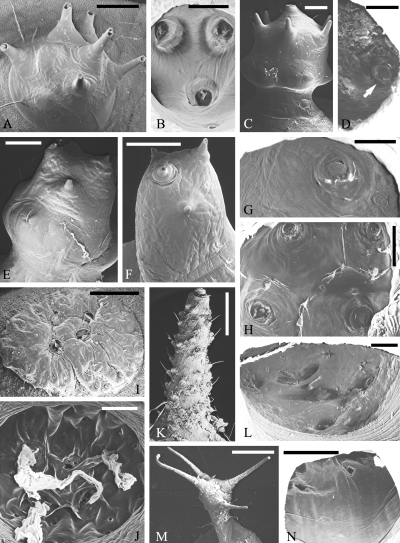
. SEM micrographs of scoli of Saturniinae, Attacini. Eupackardia calleta : outside (A), inside (B; Deml and Dettner 1993 , altered); Hyalophora cecropia : orange scolus, outside (C), inside, half with single hair base (arrow) (D); Hyalophora euryalus : large yellow scolus, outside (E), inside (G); slender yellow scolus, outside (F); white scolus, inside (H); Attacus atlas : spraying scolus, outside (I; Deml and Dettner 1994 , altered), inside (J); horn, outside (K), inside (L); Samia cynthia : outside (M), inside (N) (K-N: altered from Deml 2000 ; by courtesy of Entomologischer Verein Apollo, Frankfurt). Scales: A, C, E, F, I, M: 500 μ m; B, D, J: 250 μ m; G, H: 200 μ m; K: 1 mm; L, N: 100 μ m
Hyalophora cecropia has three distinctly differently coloured groups of scoli (orange, yellow and blue scoli) which are all of the star-wart type but have individual morphological peculiarities (Deml and Dettner, in prep.). For example, the prominent orange scoli are globular structures raised away from the body surface and set with short, thick bristles, the central one of which most easily breaks off ( Fig. 3C ). Externally, the yellow scoli are more slender while the blue scoli are substantially smaller than the others, and both sorts bear fewer bristles than the orange ones. Inside the scoli the bristle openings and, in some cases, cuticular linings as well as membranes ( orange scoli: Fig. 3D ) can regularly be discerned. The scoli of penultimate-instar (L 4 ) larvae also have three colours (red, yellow, blue) but all look much more like star warts than those of last-instars.
Hyalophora euryalus bears scoli which are basically very similar to those of H. cecropia . The colours are yellow (large, swollen ones and slender ones) and white. The bristles on the scoli are even more reduced than in H. cecropia ( large yellow scolus: Fig. 3E; slender yellow scolus: Fig. 3F) but are comparably complex inside the scoli (e.g. large yellow scolus with cuticular membrane across bristle base: Fig. 3G). Inside large yellow and white scoli (Fig. 3H), cuticular ridges between the bristle bases can be seen, similar to those in Saturnia pavonia.
Attacus atlas has two different types of scoli. The spraying scoli are flattened, warty structures ( Fig. 3I ). The bristles are completely reduced to cuticular lids that close the openings and are fixed by cuticular rings ( Deml and Dettner 1994 ). Inside these scoli, distinctly developed ridges and large reservoirs are typical ( Fig. 3J ). When the larvae of A. atlas are irritated they can increase their hemolymph pressure so that the lids are blasted off and the secretion spurts out. The second scolus type of A. atlas , horns, are elongate structures, lengthwise set with many soft hairs ( Fig. 3K ) which have corresponding bases inside the scolus ( Fig. 3L ) and possibly function as sensory hairs ( Deml 2000 ). These horns produce wavy threads of white wax which yield the powder with which the larvae are covered.
Samia cynthia is a second species producing a waxy layer. The scoli responsible for this are elongated and each bears a crown and a belt of fine hairs ( Fig. 3M ); sometimes there are also short and greatly thickened bristles inside the crown ( Deml 2000 ). Inside views show only the hair bases ( Fig. 3N ). Membranes are entirely lacking but this could be caused by the preparation method.
Hemileucinae
Automeris hamata has tree-shaped urticating scoli (see also Deml and Dettner 1997 ). From the trunk, a series of long and thick evaginations with pointed tips (bristles) branches off ( Fig. 4A ), each of which has a circular equivalent inside the scolus ( Fig. 4B ). According to Gilmer's (1925 ) histological investigation of equivalent structures of A. io (Fabricius, 1775) and Hemileuca spp., these evaginations represent spines ending in `points' (i.e. bristles). From our micrographs ( Fig. 4B ) a multitude of bright structures can be discerned inside the trunk and branches, but it is unclear whether they represent residues of cuticular membranes or simply `contaminants' (e.g. residues of tracheae). Therefore it cannot yet be decided whether the gland cells are restricted to the tips of the branches, as was indicated by Gilmer's descriptions and in Nässig's (1989 ) general figure of urticating scoli, or extend farther into the spine cavities. In L 1 larvae of A. hamata , the `trees' are not yet developed. Instead, the long scoli bear short points and fine, pinnate setae ( Fig. 4D ), also detected by Gilmer (1925 ) in A. io and Hemileuca spp.; no clear relationship to star warts is discernible. The tips of the points are closed ( Fig. 4C ). An L 1 larva is protected very well in space by its scoli ( Fig. 4E ).
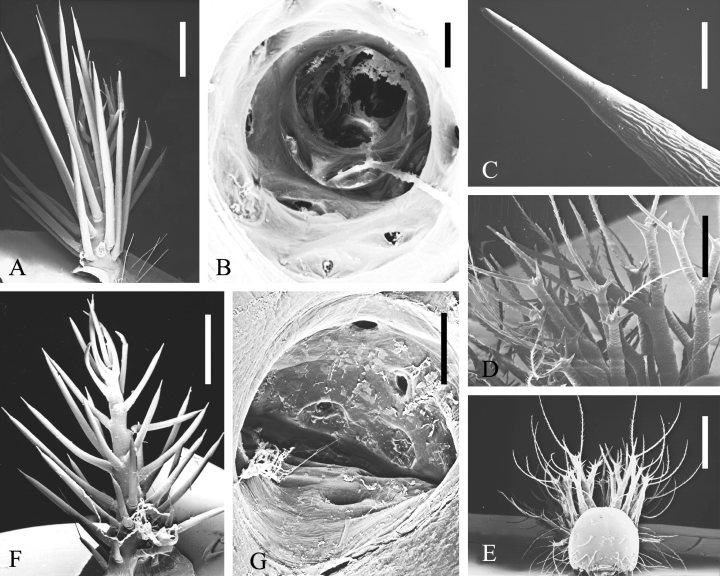
. SEM micrographs of scoli of Hemileucinae. Automeris hamata : outside (A), inside (B); Automeris hamata , L1 larva: tip of point (C), group of scoli (D), front view of larva (E); Molippa sabina : outside (F), inside (G). Scales: A, F: 1 mm; B, C: 100 μ m; D, G: 200 μ m; E: 500 μ m
Molippa sabina bears urticating scoli with spines that stick out further than those of Automeris hamata ( Fig. 4F ). The internal anatomy is similar, but only the base of the `tree' is circular; with increasing height, the sectional area becomes more oval ( Fig. 4G ).
Discussion
General morphology of scoli
Most scoli types of last-instar caterpillars of the Saturniidae are ontogenetically and phylogenetically derived from a basic type, `star wart' [Sternwarze Haffer 1921; (verruca stellata), Deml and Dettner 1997], according to its `star'-like shape. Haffer described this type from Saturnia pyri and other species. Data in the literature (e.g. Nässig 1983; Paukstadt and Paukstadt 1991) and our observations of early instars support this hypothesis. The presence of star warts in first-instar larvae particularly suggests that they are phylogenetically old structures, because in L1 larvae primitive characters may occur which are completely modified after the first moult (Pease 1960).
Haffer (1921 ) identified large gland cells inside the star warts which produce a secretion delivered through the setae. These gland cells are derived from hair-forming cells (trichogen cells). Accumulations and fusions of such single hairs and glands result in the formation of complex glands, star warts. The cuticular intimae ( e.g. in Saturnia pinratanai: Fig. 1C ) at the base of the hairs, normally located inside the gland cells, have already been observed by Haffer, who found them hanging from the old cuticle of moulting larvae. These intimae must be distinguished from membranes like those in Antheraea pernyi ( Fig 2J, 2K) that seemingly delimit the total scolus cavity (not a single hair) and have a hitherto unknown function. The gland cells are type I according to the gland-cell classification of Noirot and Quennedey (1974, 1991), who included glandular hairs, in which the trichogen cell has become a gland cell and pours its secretion into the lumen of the hair, in this category. According to these authors, most type I cells are covered by a cuticle secreted by the gland cells themselves, which may be modified (e.g. by dilated pore canals, inserted slits) in relation to the egress or storage of the secretion (see the intimae). Furthermore, these cells can be distinguished from the partly similar type III cells because they are not connected to the hair via a cuticular canal (tubulus) and are not integrated into a `glandular unit' consisting of several different cells (see Haffer 1921).
We consider the presence of gland cells in scoli, even in scoli without any discharge of secretion and in scoli of species whose L1 larvae do not bear star warts, to be a distinct indication of the common phylogenetic origin of most, if not all, saturniid scoli. This would mean that scolus type 7, which is not ontogenetically derived from a star wart, nevertheless either has phylogenetically originated from a star wart or is a modification of an ancestral, setose scolus common to the other types of saturniid scoli as well; the same is possible for the only remaining type, 5 b (Nässig 1989), which we have not yet morphologically investigated by SEM. Other features that have been described – the presence of gland cells in type 7 scoli (Gilmer 1925; Nässig 1989) and of long, slender setae instead of hard points on scoli of three species of Hemileucinae (Gilmer 1925), in Nässig's (1989) general figure of urticating scoli, and on scoli of L1 larvae of A. hamata (this study) – support this hypothesis regarding type 7.
However, it must be noted in this context that the presence of these scoli types in combination with gland cells has not yet been unequivocally established as an appropriate character for defining the Saturniidae or even a monophyletic group inside this family. On the one hand, it is not known whether all larvae of Salassinae really lack scoli; hitherto only prothoracic and caudal, horn-shaped protuberances (no setose scoli) have been described from Salassa larvae (Nässig 1994). On the other hand, an outgroup comparison of the Saturniidae is difficult because the interrelationships of the families inside the Bombycoidea are still unclear (Minet 1994; Lemaire and Minet 1999). Caterpillars of the Eupterotidae and Bombycidae, which according to Michener (1952) are near relatives of the Saturniidae, have only normal setae but many Bombycidae additionally bear solitary, fleshy horns at their caudal ends. However, these bombycid horns and the saturniid horns (type 5b) could be results of convergent formation, and the same applies, for example, to the spiny scoli found on larvae of Saturniidae, Limacodidae, and certain Nymphalidae (Tuskes et al. 1996). Structures such as elongated processes and, in some cases, scoli have also been observed in the remaining families of the Bombycoidea and may be considered as part of the `groundplan' of bombycoid larvae (Oberprieler and Duke 1994); but it is completely unclear whether some of them contain gland cells and whether they represent a synapomorphy of these species or convergent developments.
Most or all of the widely differing scoli of last-instar larvae of Saturniidae are variations on a single theme. Consequently, a specialization of scoli takes place not only in different species but even in individuals. On the one hand, one larva may bear various scoli of the same type and with relatively similar shape but distinctly different colorations, as is the case in the two Hyalophora species examined. On the other hand, a caterpillar may even have several types of scoli, as can be seen in Antheraea frithi and the extreme case of Attacus atlas, which has two completely different sorts of derived scoli.
The divergent developments of scoli might be attributed to ecological influences. We believe that defence against enemies is the main driving factor for scolus specialization, because a series of bioassays in the laboratory using larval body fluids as well as individual chemical components thereof (summarized in Deml and Dettner 1997) revealed a broad spectrum of potentially repulsed pathogens (bacteria) and predators (ants, birds). For example, the secretory scoli of Saturnia pyri and S. pavonia and the urticating scoli of Hemileucinae are effective injection devices, whereas the spraying scoli of Attacus atlas are excellently adapted for applying the exocrine secretion to the eyes and sensitive mucous membranes of target organisms by spraying. At least in the case of geographically co-occurring species, the presence of different types of scoli in different species, as well as in different larval instars of a given species, could indicate that the animals have different enemies.
Classification of scoli
Having inspected the scoli of these saturniid species, we realized that the scoli types we identified were not always identical with the diagnoses of Nässig (1989). For example, the point-bristly scoli of Cricula andrei are not in principle different from secretory scoli, and Samia cynthia appears to have not true horns but rather a kind of scolus intermediate between Nässig's type 2 and type 5a. The scoli of Antherina suraka also represent a transitional state between type 2 and type 5a (Oberprieler and Nässig 1994; personal observations) but are morphologically unlike those of S. cynthia. Therefore we decided to improve Nässig's classification with regard to a few points (Tables 1 and 2); Roman numerals are used in our system in the tables in order to avoid confusion with Nässig's numbering.
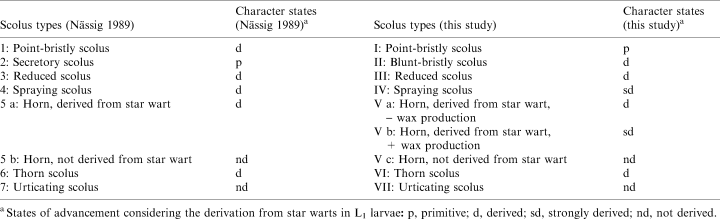
`Point-bristly scoli' (type I) now comprises Nässig's types 2 (secretory scoli, which are identical with star warts) and 1 (point-bristly scoli) because type 1 includes scoli containing a liquid venom in their bristles (e.g. in Cricula andrei). Since the two types are also basically comparable in morphology, we recommend deletion of the term `secretory scolus'.
Instead, we erect a new type II named `blunt-bristly scolus' ([scolus hebetisetosus], scolus with blunt bristles). These scoli make a generally `soft' impression and are normally set with short, blunt bristles which are easily splintered by mechanical stress and thereby might act like `knives' rather than `syringes' (typical of point-bristly scoli), in order to penetrate an enemy's skin. These characteristics indicate that Eupackardia calleta, which hitherto has been classified as having secretory scoli (Deml and Dettner 1993), should be better placed in the new type II because its stinging bristles are substantially shorter (even when undamaged) than those of species with point-bristly scoli (Deml and Dettner 1995). Perhaps the scoli of this species represent a transitional state between type I and the new type II.
The scoli of Antheraea spp., which previously were regarded as members of type 3 (Nässig, pers. comm.), as well as those of Hyalophora spp. (old type 2) can also better be included in the new type II. A separation of Antheraea and Hyalophora from genera such as Saturnia and Caligula is also supported by characters of the respective first-instar larvae (number and position of setae and scoli; Pease 1960). Moreover, Nässig's type 3 (reduced scoli) appears to be too general and extensive, and should be restricted to distinctly reduced scoli (type III) such as those of Antheraea frithi (Figs 2F–H).
Lastly, in a phylogenetic respect, we recommend that `derived' scoli (types II, III, Va, VI) be distinguished from `strongly derived' ones (types IV and Vb). Spraying scoli and horns producing wax are surely the most advanced types, because in order to develop these scoli there have been not only distinct morphological changes but also a complete functional–morphological alteration of their mechanism (spraying by spraying scoli) or even an acquisition of an additional biological function (wax production by horns of type Vb). In order to mark the last-mentioned differentiation of horns, we have also divided Nässig's type 5a into two new types: Va (horns producing no wax) and Vb (horns producing wax).
Implications of scolus classification for the systematics of the Saturniidae
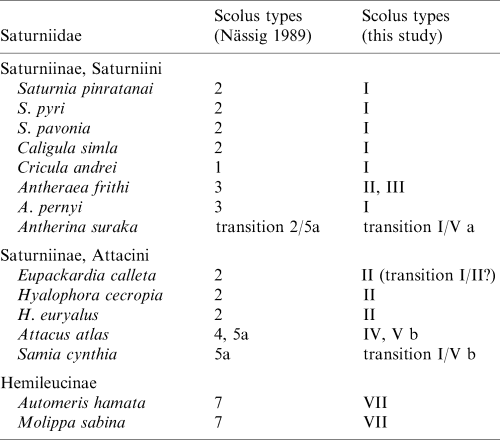
Nässig's (1989 ) scolus classification allows for a good separation of genera and, to some extent, species within the Saturniidae. For example, in the tribe Attacini, Attacus atlas with its highly specialized scoli is clearly different from species which have secretory scoli ( Eupackardia calleta , Hyalophora spp.). On the basis of scolus morphology, Attacus seems to be the most derived and advanced genus and is characterized by the autapomorphy, spraying scoli. Similarly, in the tribe Saturniini, species which retain their secretory scoli (= star warts) until the last larval instar (plesiomorphic state; e.g. Saturnia spp.) appear to be a basal group whereas Cricula andrei , which has point-bristly scoli (derived = apomorphic state), might represent a phylogenetically younger species.
On the other hand, Nässig's (1989) scolus system seems to have limited value for the higher taxonomy of Saturniidae. Type 7 can be explained as an autapomorphy of the subfamily Hemileucinae. Furthermore, the original star wart of L1 larvae seems to be a synapomorphy of Saturniinae and Ludiinae, and Oberprieler and Nässig (1994) consequently placed the Ludiinae as a tribe (Ludiini) within the subfamily Saturniinae. However, in the Saturniinae the tribes Saturniini and Attacini share several scolus types and no scolus autapomorphy of either group can be detected. Therefore, the current taxonomy of these tribes would appear somewhat questionable when based on scolus characters scored according to Nässig's state definitions.
The Saturniini and Attacini are supposedly closely related (Oberprieler and Nässig (1994) even regarded a certain genus (Rhodinia) in Saturniini as the adelphotaxon of Attacini). Although the Attacini are currently looked upon as a monophyletic group (mainly on the basis of imaginal characters), the monophyly of the Saturniini is not at all certain; the relationships within these tribes are also not satisfactorily resolved (Oberprieler and Nässig 1994; Nässig et al. 1996). Our scolus classification (Tables 1 and 2) appears helpful for distinguishing some groups, at least. On the one hand, it seems that the Attacini could be more heterogenous than was hitherto assumed since two groups can be discerned in Table 2. Attacus atlas and Samia cynthia are characterized by strongly derived scoli and the probable synapomorphy horns with wax production. The other Attacini species share the new type, blunt-bristly scoli, but this type was also found in Antheraea frithi pedunculata in the Saturniini. On the other hand, almost all species of Saturniini (apart from A. frithi) share the synapomorphy point-bristly scolus. Antheraea frithi has type-II scoli, which is a synapomorphy with several Attacini; its scoli are also distinctly reduced, which is an autapomorphy as compared with our list of species. Furthermore, A. frithi has scoli that are very different from those of A. pernyi. It seems that A. frithi either is `displaced' in the Saturniini or could actually be the sister-taxon of some Attacini. Because the taxonomy of the genus Antheraea (perhaps only one-quarter of the currently named `Antheraea' species actually belong in this genus) and, even more, of the subgenus Antheraea (Antheraea) (A. frithi as well as A. pernyi are currently placed herein) is still an open field (Pinratana and Lampe 1990; Nässig et al. 1996), the systematic position of A. frithi should be reconsidered, in any case.
The saturniine (and general saturniid) systematics is in urgent need of revision (Oberprieler and Nässig 1994; Tuskes et al. 1996). We expect that the new information presented here regarding the morphology of the larval scoli will contribute to resolving this problem, in particular when potentially equally valuable (but still missing) data on the respective chemical constituents of the secretions and larval hemolymph can be considered as well. Of course, convergent formation of identical scolus types in distantly related species is conceivable but could possibly be detected by chemical comparisons. Furthermore, when additional chemical data are available, genera comprising species that bear different types of scoli (such as Saturnia) or that lack scoli altogether (Antheraea) could be more critically examined with respect to their actual systematic structure.
Footnotes
Acknowledgements
The work of R.D. was supported in part by a grant from the Deutsche Forschungsgemeinschaft, Bonn (De 568/1–1). We thank the following persons who generously donated living specimens of saturniids: Dr A. Attygalle, Universität Erlangen-Nürnberg (Antheraea pernyi); G. Brenner, Eckental/Eschenau (Caligula simla, Cricula andrei); R. Lampe, Nürnberg (Antheraea frithi pedunculata, Hyalophora euryalus, Saturnia pinratanai); H. Lomb, Eltville (Hyalophora cecropia); R. Wanninger, Donaustauf/Sulzbach (Molippa sabina). Furthermore, we are very grateful to Mrs A. Wilcockson, Newbury/Berkshire, England, for performing some of the SEM investigations presented here. Professor Dr K. Fiedler, Universität Bayreuth, kindly gave permission to use his scanner for the SEM negatives, which is also gratefully acknowledged. Finally, we wish to express our thanks to Professor Dr H. Enghoff, Zoologisk Museum København, Denmark, and an anonymous reviewer who gave critical comments and helpful remarks on the manuscript.




