Phylogenetic analysis of the Malacostraca (Crustacea)
Abstract
enThe Malacostraca comprises about 28 000 species with a broad disparity in morphology, anatomy, embryology, behaviour and ecology. The phylogenetic relationships of the major taxa are still under debate. Is the Leptostraca the sister group of the remaining Malacostraca, or is this taxon more closely related to other Crustacea? Does the Stomatopoda or the Bathynellacea represent the most basal taxon within the remaining taxa? Is the Peracarida monophyletic or are some peracarid taxa more closely related to other ‘caridoid’ taxa? Is the Thermosbaenacea part of the Peracarida or its sister group, and how much support is there for a taxon Amphipoda + Isopoda? To answer these questions a phylogenetic analysis of the Malacostraca combining different phylogenetic approaches was undertaken. In a first step, the monophyly of the Malacostraca including the Leptostraca is shown using the ‘Hennigian approach’. A computer cladistic analysis of the Malacostraca was carried out with NONA and PEE-WEE, based on 93 characters from morphology, anatomy and embryology. Nineteen higher malacostracan taxa are included in our analysis. Taxa whose representatives are exclusively fossils were not included. The Leptostraca was used as an operational out-group. The present analysis supports the basal position of the Stomatopoda. Syncarida and Peracarida (including Thermosbaenacea) are supported as monophyletic, the Eucarida is not. Instead a sister-group relationship is suggested between Euphausiacea and Peracarida (including Thermosbaenacea), with the Syncarida as the sister group to both taxa. Certain embryonic characters are interpreted as support for the monophyly of the Peracarida (without Thermosbaenacea) because convergences or reversals of these characters seem implausible. Within the Peracarida, the Mysidacea (Lophogastrida + Mysida) represents the sister group to the remaining taxa. A sister-group relationship between Amphipoda and Isopoda is not supported.
Abstract
deEine phylogenetische Analyse der Malacostraca (Crustacea)
Das Taxon Malacostraca umfaßt ungefähr 28000 Arten, die sich vielfältigst in ihrer Morphologie, Anatomie, Entwicklung, Ökologie und ihrem Verhalten unterscheiden. Die phylogenetischen Verwandtschaftsbeziehungen der einzelnen Taxa zueinander werden weiterhin kontrovers diskutiert. Wichtige Fragen betreffen dabei die Leptostraca und ihre Zugehörigkeit zu den Malacostraca, die Mono-bzw. Polyphylie der Peracarida und die möglichen engeren Verwandtschaftsbeziehungen der beiden artenreichsten Taxa innerhalb der Peracarida, Amphipoda und Isopoda. Unsere phylogenetische Analyse verbindet den klassischen ‘Hennig’schen Ansatz’ mit einer computergestützten cladistischen Analyse. In der computergestützten Analyse unter Verwendung der Programme NONA und PEE-WEE fanden 93 Merkmale aus Morphologie, Anatomie und Entwicklung ihren Einsatz. Wir berücksichtigten 19 ‘höhere’ Taxa der Malacostraca, dabei ausschließlich Taxa mit noch rezenten Vertretern. Unsere Analyse unterstützt die Monophylie der Malacostraca. Die Leptostraca sind die Schwestergruppe aller übrigen Taxa, die als Eumalacostraca zusammengefaßt werden können. Innerhalb der Eumalacostraca sind die Stomatopoda das Schwestertaxon zu den übrigen Gruppen. Ein Monophylum Eucarida findet keine Unterstützung. Stattdessen favorisiert unsere Analyse eine Schwestergruppenbeziehung zwischen Euphausiacea und Peracarida (inklusive Thermosbaenacea). Bezüglich der Position der Thermosbaenacea ergibt sich aus der cladistischen Analyse keine eindeutige Hypothese. Wir favorisieren eine Schwestergruppenbeziehung zwischen Thermosbaenacea und Peracarida s. str., Insbesondere aufgrund der Verteilung einiger embryologischer Merkmale, die im Falle einer Position der Thermosbaenacea innerhalb der Peracarida als Konvergenzen zwischen Mysidacea und den übrigen Peracarida oder als Reversionen der Thermosbaenacea zu interpretieren wären. Innerhalb der Peracarida s. str. Werden die Mysidacea (Mysida + Lophogastrida) als monophyletisch unterstützt. Sie sind die Schwestergruppe der übrigen Peracarida s. str. Innerhalb dieses Taxons wird eine Schwestergruppenbeziehung zwischen Amphipoda und Isopoda nicht favorisiert.
Introduction
The Malacostraca is a very speciose and morphologically and ecologically highly diverse taxon. The traditional view places the Malacostraca in the Crustacea. However, the relationships of the Malacostraca to other crustacean taxa are highly controversial (e.g. Lauterbach 1975; Schram 1986; Walossek 1993; Moura and Christoffersen 1996; Schram and Hof 1998; Ax 1999). A sister-group relationship between Malacostraca and Insecta has even been proposed (Hanström 1926; Nilsson and Osorio 1998; Wilson et al. 2000). Here, we focus on the monophyly of the Malacostraca and the phylogenetic relationship of the higher taxa.
The Malacostraca was introduced in 1802 by Latreille, and initially this did not include the Leptostraca. After the studies of Claus (1888), the Leptostraca was for a long time accepted as part of the Malacostraca. Within the Malacostraca, the Schizopoda Latreille 1817 – comprising Mysidacea and Euphausiacea – occupied a central position. This taxon was strongly supported by Claus (1885). Thomson (1892, 1894) described the first recent representative of the Syncarida –Anaspides tasmaniae– and put this species in the Schizopoda. However, 10 years earlier Boas (1883) favoured a separate position of the Mysidacea and Euphausiacea in addition to the Cumacea, Amphipoda, Isopoda, Decapoda and Squillacea (= Stomatopoda). Hansen (1893) created the basis for the most accepted classification of the Malacostraca until today. He suggested the Leptostraca as being opposed to the remaining taxa, which were at this time united under the name Eumalacostraca, introduced by Grobben (1892). Hansen (1893) distinguished three major taxa within the Eumalacostraca: the first comprised Mysidacea, Cumacea, Isopoda and Amphipoda, the second Euphausiacea and Decapoda, and the third the Stomatopoda. Calman (1904, 1909) finally established the most common classification (e.g. Zimmer 1927; Kaestner 1959; Brusca and Brusca 1990; Gruner 1993). He created three new divisions, Peracarida (Mysidacea, Cumacea, Tanaidacea, Isopoda and Amphipoda), Eucarida (Euphausiacea and Decapoda) and Hoplocarida (Stomatopoda). Together with the fourth division, the Syncarida (Anaspidacea), the three new divisions constituted the Eumalacostraca, as opposed to the Leptostraca. Calman (1909) also recognized Bathynella natans Vejdovsky, 1882 as a member of the Syncarida (now Syncarida – Bathynellacea). Thermosbaenacea, Mictacea, and Spelaeogriphacea were discovered more recently.
Thus, at the beginning of the twentieth century, the classification of the Malacostraca could be summarized as follows (slightly modified after Calman 1909):
Malacostraca Latreille 1802
Leptostraca CLAUS 1880
Eumalacostraca GROBBEN 1892
Syncarida PACKARD 1885
Peracarida CALMAN 1904
Eucarida CALMAN 1904
Hoplocarida CALMAN 1904
Former approaches
It is beyond the scope of this work to give an overview of all phylogenetic hypotheses concerning the relationships of the Malacostraca. Too many suggestions – often based on the study of single character complexes – have been made. Here, we refer only to comprehensive approaches dealing with the major relationships of the Malacostraca.
In the middle of the twentieth century Siewing contributed in several publications (e.g. Siewing 1951, 1952, 1953, 1955, 1956, 1958, 1959, 1960, 1963) to our knowledge of malacostracan phylogeny. Unfortunately, Siewing ignored Hennig’s methods, but his character analyses and homology decisions were carefully done and his suggestions on the phylogenetic relationships of the Malacostraca can easily be translated into a cladogram (Fig. 1). According to Siewing, the Peracarida is monophyletic and the Thermosbaenacea (Siewing’s Pancarida) is the sister group to the Peracarida. Both taxa together constitute the sister group to the Eucarida, and the Syncarida is the sister group to the Eucarida, Pancarida and Peracarida, and all these taxa together constitute a monophylum. The sister group to this taxon is the Stomatopoda. The Leptostraca represents the most basal taxon within the Malacostraca. Within the Peracarida, Siewing (1951; 1953) argued for a Cumacea-Tanaidacea-Isopoda clade and against a sister-group relationship between Amphipoda and Isopoda.
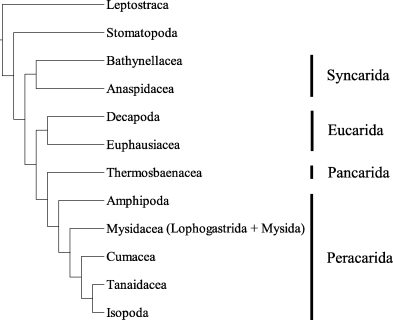
Phylogenetic relationships of the Malacostraca modified from Siewing (1 956), characters omitted
Hessler (1982a, 1983) established the taxon Caridoida for the Eumalacostraca with the exception of the Stomatopoda and gave some support for this taxon (his characters are included in our data matrix). His main interest at that time was the defence of the ‘caridoid facies’. Schram (1982) proposed a scheme essentially similar to that of Hessler except that he used the term Eumalacostraca sensu stricto to apply to all non-hoplocarid/phyllocarid malacostracans. Thus, he recognized three malacostracan subclasses: Phyllocarida, Hoplocarida, and Eumalacostraca.
Some decades after Siewing, Schram presented several computer cladistic analyses of the Malacostraca (Schram 1981, 1984) and of the entire Crustacea (Schram 1986), a subject which was revisited by him recently (Schram and Hof 1998; see also Emerson and Schram 1998). Here, we only refer to the recent analysis by Schram and Hof (1998). According to this analysis, the monophyly of the Malacostraca depends on the inclusion or exclusion of extinct taxa. When all groups that are exclusively fossil are excluded, the Leptostraca is more closely related to the Branchiopoda (Fig. 2a). In their 50% majority rule consensus of the entire data set, the Leptostraca (together with some extinct taxa) is the sister group to the remaining Malacostraca (Fig. 2b). One important result of the analysis by Schram and Hof (1998) is that the Bathynellacea, and not the Stomatopoda, is the sister group of the remaining Eumalacostraca. This is true for both analyses, independent of the inclusion or exclusion of extinct taxa.
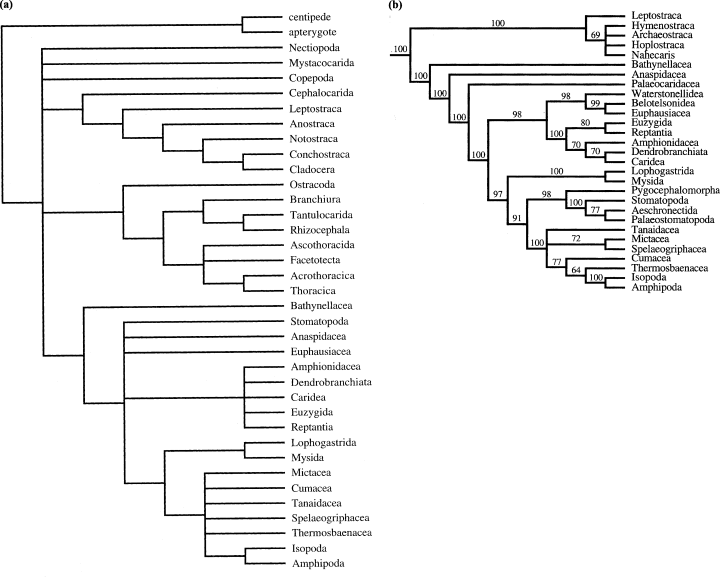
(a) Modified strict consensus tree by Schram and Hof (1998) resulting from their analysis of 90 characters using only the 35 taxa that are not exclusively fossil groups. The Leptostraca is the sister group to the Branchiopoda and not to the Eumalacostraca. (b) Phylogenetic relationships of the Malacostraca based on Schram and Hof (1998). This cladogram represents the malacostracan part of their 50% majority rule consensus of the entire data set (their Fig. 6.8). Here, the Leptostraca (together with some extinct taxa) is the sister group to the remaining malacostracan taxa. The numbers on the branches refer to the percentage of most parsimonious cladograms which support a particular clade
Wills (1998) presented an analysis of the entire Crustacea. He included 135 characters and 62 taxa. According to Wills, the Malacostraca is not monophyletic. The Leptostraca is the sister group to the Cephalocarida. Within the Eumalacostraca, Wills (1998) proposed a sister-group relationship between Stomatopoda and Eucarida. The Bathynellacea represents the most basal clade within the Eumalacostraca. The Peracarida is monophyletic but comprises the Thermosbaenacea, and as in Schram & Hof’s analysis, Amphipoda and Isopoda are sister groups (Fig. 3).
Phylogenetic relationships of the Malacostraca according to Wills (1998) from his preferred ordering and weighting of the data
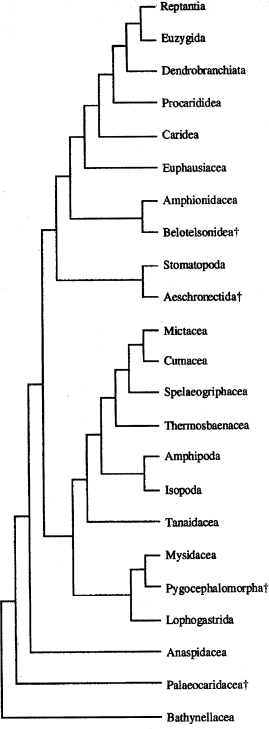
A different, more traditional approach was used by Watling (1981, 1983, 1999). The main result of his studies is that the Peracarida is not monophyletic. Lophogastrida and Mysida are more closely related to the Eucarida, and the remaining Peracarida (inclusive the Thermosbaenacea) constitute a separate clade (Fig. 4). Watling et al. (2000) included the Stomatopoda in the proposed malacostracan relationships. Richter (1994b, 1999) proposed phylogenetic relationships of the major malacostracan taxa based on the compound eye ultrastructure and various other characters (see also Ax 1999; based partly on Richter 1994b). Kobusch (1999) presented a cladogram of the Peracarida mainly based on foregut characters. He included also other malacostracan taxa as out-groups. Recently, Hessler and Watling (1999) gave a detailed overview about the different approaches dealing with the peracarid phylogeny.
Phylogenetic relationships of the Malacostraca according to Watling (1999). Characters omitted
Until today, there are only two published molecular analyses, using 18S rDNA data, dealing with the relationships of the major malacostracan taxa (Spears and Abele 1998, 1999). According to Spears and Abele (1998, 1999) the Malacostraca is monophyletic, and the Leptostraca and Eumalacostraca are sister groups. Within the Eumalacostraca the Stomatopoda is the sister group to Eucarida plus Syncarida although this result seems not to be entirely clear. Representatives of the Peracarida were not considered in these analyses. Concerning the Malacostraca, the ‘total evidence’ analysis by Wheeler (1998) is based on morphological data only (data matrix by Schram and Hof 1998).
In this publication, we focus on the relationships of the Eumalacostraca (used in the original sense as introduced by Grobben 1892). In particular, the following questions are crucial. Is the Stomatopoda or the Bathynellacea the sister group to the remaining Eumalacostraca? Is the Peracarida monophyletic and is the Thermosbaenacea a subordinate part of it or does it represent its sister group? Are the Syncarida and the Eucarida, respectively, monophyletic? Do Amphipoda and Isopoda constitute a clade?
Materials and methods
Theoretical aspects
A phylogenetic analysis of a taxon such as the Malacostraca with about 27 700 species (Gruner 1993) implies some severe problems. The two main problems concern the choice of the terminal taxa and the coding of characters for these taxa, which are rarely single species (see also Yeates 1995; for a discussion of this problem but with a different solution).
The selection of the terminal taxa is more or less a matter of practical considerations when using morphological characters. Including all species of the Malacostraca or only Calman’s five ‘divisions’ would be the two extremes: the first is impractical, the second would make too many a priori assumptions about monophyly. Therefore, the number of terminal taxa will be between these extremes. One important point is the monophyly of the terminal taxa. Of course, one could argue that it is reasonable to accept a priori the monophyly of well-established taxa. However, we consider it better to support the monophyly of each terminal taxon with unique apomorphic characters prior to cladistic analysis because the para- or polyphyly of a terminal taxon can never be a result of such analysis (although a cladistic analysis can give additional support for the monophyly of the terminal taxa). For example, if we use the Mysidacea as a terminal taxon, the cladistic analysis could not prove whether the sister-group relationship between its constituent taxa, Lophogastrida and Mysida, is supported or not. If there is serious doubt about the monophyly of one of the terminal taxa, this taxon should be split into monophyletic subunits. If the monophyly of terminal taxa is supported by unique apomorphic characters, this step is completely independent from the following cladistic analysis, because the inclusion of these characters would have no effect concerning the relationships between the taxa.
The other severe problem concerns the character coding of ‘higher’ terminal taxa. When higher terminal taxa are used, we are interested in the character pattern of the stem species of these taxa. The use of the stem species of the terminal taxa – in the hypothetical case where we could go back in time and could collect these animals – would solve the problems of using higher taxa. However, although these stem species really existed in former times, the actual character scores are hypothetical constructs and the methods for inferring hypothetical ground patterns are under dispute (see e.g. Wägele 1994; Wilson 1996). This is one of the most serious problems of any cladistic analysis focused at a higher level.
The simplest case is if all species within the terminal taxon possess the same character state. Then this character state can be also assumed for the stem species. A particular problem concerns anatomical or embryonic characters. Many features of these character complexes are only known from one or a few species. If one wants to use these characters in a phylogenetic analysis, one has to accept these characters as part of the ground pattern. Of course, the confidence in these characters increases with the number of species studied and depends also on the phylogenetic position of the species studied.
The difficulties increase if characters are ‘polymorphic’ in the terminal taxon. Wilson (1996) suggested four ways to handle polymorphic characters. Two of them are relevant for our analysis, the ‘multistate’ option and the ‘plesiomorphic character state’ option. In our opinion, it should be carefully considered when the usage of the ‘multistate option’ can be supported. For example, within many malacostracan taxa there are blind representatives, such as cave-dwelling species. We think that it would be unwise to score all these taxa as polymorphic with respect to the absence or presence of compound eyes. The result would simply be a loss of information. The multistate option should be only used if there is major doubt which character state belongs to the ground pattern. We generally prefer the ‘plesiomorphic character state’ option. The problem is how to determine this state:
If there are congruent hypotheses about the phylogenetic relationships of the terminal taxa, the basal node character states (ground pattern) can be used for the actual phylogenetic analysis. This approach was criticized by Wilson (1996) who argued that the result might not be ‘globally parsimonious’. This can sometimes be the case but should not lead to ignoring existing phylogenetic hypotheses. If no phylogenetic hypothesis indicates which of the character states is plesiomorphic, we suggest out-group comparison (Watrous and Wheeler 1981; Maddison et al. 1984). If only one character state of the in-group is known in the out-group (one or more of the other terminal taxa), this character state should be used in the ground pattern of the terminal taxon. Incorrect homology decisions lead to incorrect character scores in the data matrix. It should be emphasized that this is the ‘traditional’a priori out-group comparison with fixed in-and out-groups prior to the compilation of the data matrix (we are convinced that the criticism of this approach by Nixon and Carpenter (1993) is not justified in this case). Nevertheless, in our opinion, the terminal taxa should be scored as polymorphic only in problematical cases. We will discuss this in the character section.
We decided to exclude all taxa with exclusively fossil representatives. This decision does not imply that we generally believe that extinct taxa should be excluded. The reason is that we are introducing new anatomical and embryological characters which cannot be scored for extinct taxa and the number of unknown character states is already high enough if one uses only extant taxa. We also wanted to avoid repeating the analyses of Schram and Hof (1998) and Wills (1998).
In order to maximize the number of applicable character scores, only malacostracan taxa are included in our analysis. The monophyly of at least the Eumalacostraca (including the Stomatopoda) has never really been questioned. Only the sister-group relationship between the Leptostraca and the Eumalacostraca is under dispute, although in our opinion there is convincing character support for the monophyly of the Malacostraca (Leptostraca + Eumalacostraca). Our cladistic analysis mainly concerns the Eumalacostraca (with the Leptostraca as a single out-group). The proposed sister-group relationship between Leptostraca and Eumalacostraca is the result of a traditional Hennigian approach. Our analysis is thus a synthesis between a computer cladistic and a manual, Hennigian approach. We are aware of the problem that the use of only one out-group implies that the in-group cannot be supported by unambiguous (in a cladistic sense) characters. We will deal with this point separately. Our cladistic analysis is an unrooted analysis excluding a hypothetical ancestor of the Malacostraca and the root was chosen after the analysis between the operational out-group (Leptostraca) and the operational in-group (Eumalacostraca). The inclusion of more and more distantly related out-groups was abandoned because it would result in more debatable homology decisions. Furthermore, the choice of additional out-groups would be subjective if one would not include more or less all crustacean taxa as well as insects which have also been suggested as being the sister group of the Malacostraca (e.g. Wilson et al. 2000).
In our analysis we included 19 malacostracan taxa: Leptostraca, Stomatopoda, Bathynellacea, Anaspidacea, Euphausiacea, Amphionidacea, Dendrobranchiata, Caridea, Stenopodidea, Reptantia, Thermosbaenacea, Lophogastrida, Mysida, Amphipoda, Mictacea, Spelaeogriphacea, Cumacea, Tanaidacea, and Isopoda. These are the same taxa used by Schram and Hof (1998) and by Wills (1998). Wills (1998) additionally included the Procaridea, which is in our opinion part of the Caridea (see Holthuis 1993).
In our analysis, there is a total of 93 characters, 87 characters are parsimony informative.
Methods
In the first and second part of our analysis, we deal with the monophyly of the 19 terminal taxa and the Malacostraca. Here, we used the ‘Hennigian approach’ (Hennig 1950, 1966) to establish monophyla. The third part of our study – a numerical cladistic analysis of the Eumalacostraca – was performed with the programs NONA (Goloboff 1993a) and PEE-WEE (Goloboff 1993–1997) using WINCLADA (Nixon 1999–2000) as the shell program. Almost all characters were treated as unordered (with the exception of character 14 where character state 2 is nested in state 1) and equally weighted. The analysis using NONA was carried out with ‘hold 1000’, ‘hold/1000’, ‘mult* 100’, using tbr-branch swapping. Trees with clades supported by unambiguous changes (‘amb-’) were considered as well as trees with all clades supported under at least one optimization (‘amb=’; and checking of all trees using WINCLADA). A second set of analyses was carried out with PEE-WEE (Goloboff 1993–1997) a program which implements a non-iterative method for weighting characters according to their homoplasy. It is based on searching for trees with maximum total fit, with character fits defined as a concave function of homoplasy. Then, when comparing trees, differences in steps occurring in characters which show more homoplasy on the trees are less influential in tree choice as they contribute less to the overall fit of the tree. In PEE-WEE a modification of the consistency index is used as measure of fit. The function is also modified to be less steep by a constant of concavity, k. For higher values of k the function weights less drastically against characters with homoplasy (Goloboff 1993b). We ran six analyses using different values of k (conc 1, 2, 3, 4, 5, 6, each analysis with ‘mult* 50’).
Results
Monophyly of the terminal taxa
For all of the 19 terminal taxa at least some support for their monophyly can be given. We preferred characters which are unique for the particular terminal taxon. Therefore, we here present only a selection of apomorphies of the terminal taxa. Further apomorphies will appear as results of the cladistic analysis.
Leptostraca
Martin et al. (1996) presented a key to the two families of the Leptostraca (Nebaliopsidae, Nebaliidae) and all known extant genera. Olesen (1999) proposed phylogenetic relationships of the leptostracan genera including apomorphies of the entire taxon. The monophyly of the Leptostraca is supported by the vestigial and uniramous pleopods of the fifth and sixth pleomeres (see Siewing 1956; Hessler 1982a) and the elongated palp of the first maxilla which functions as a cleaning appendage (Olesen 1999). A further potential apomorphy, the uniramous second antenna is included in our data matrix and will be discussed below.
Stomatopoda
Ahyong (1997) and Hof (1998) presented cladistic analyses of the Stomatopoda (Bathysquilloidea, Erythrosquilloidea, Gonodactyloidea, Lysiosquilloidea, Squilloidea), also including extinct taxa, with quite different results. Here, only some unique synapomorphies will be mentioned which support at least the extant taxa: bilobed eyes, with a central band of ommatidia, and the differentiation of the thoracopods into five ‘maxillipeds’ (see also characters 21 and 22) and three walking legs. The subspherical or faintly bilobed eyes as well as the absence of the central band in some taxa were interpreted as derived within the Stomatopoda (Ahyong 1997). Ahyong (1997) emphasized that in comparison to archaeostomatopods and palaeostomatopods, the second maxilliped of crown group stomatopods is specialized as the main raptorial claw and ‘maxillipeds’ 3–5 have become reduced in size.
Anaspidacea
The Anaspidacea comprises the Anaspididae, Koonungidae, Psammaspididae and Stygocarididae (Gruner 1993; Lopretto and Morrone 1998). Schminke (1975, 1978) suggested several apomorphies of the Anaspidacea. However, petasma, statocyst and transformation of the first thoracopod into a maxilliped are also known from other malacostracans, although Schminke (1978) mentioned specific details of these structure which are unique to the Anaspidacea. These characters are included in our data matrix. An autapomorphy of the Anaspidacea is the position of the eighth thoracopod, which is opposite to the other thoracopods: the claw of the dactylus is oriented towards the anterior, in the other thoracopods it points posteriorly. In addition, there are no exopods and epipodites on the eighth thoracopod. Many characters, in particular anatomical characters, are known only from one species, Anaspides tasmaniae (Thomson, 1892).
Bathynellacea
The Bathynellacea comprises the Bathynellidae and the Parabathynellidae (Gruner 1993). Schminke (1975) suggested that the Bathynellacea is monophyletic, based on the transformation of the eighth thoracopod into a copulation organ in males, and the fusion of the mandibular molar process with the accessory incisor process to a tooth row.
Lophogastrida
The Lophogastrida consists traditionally of two families, the Lophogastridae and the Eucopidae (Gruner 1993; Nouvel et al. 1999). Richter (1994a) mentioned some autapomorphies which support the monophyly of the Lophogastrida: e.g. exopod of the maxilliped lancet-shaped and unsegmented, incisor process of the left mandible of semicircular form, with a few relatively large teeth, and left and right paragnath of different shape. Casanova et al. (1998) and Taylor et al. (1998) discussed relationships within the Lophogastrida.
Mysida
The Mysida comprises the Petalophthalmidae, Lepidomysidae, Stygiomysidae and Mysidae (Nouvel et al. 1999). Only the Mysidae possess statocysts in their uropods, therefore this character does not support the monophyly of the Mysida. Richter (1994a) suggested the vestigial pleopods of the females as an autapomorphy of the Mysida. As a second potential autapomorphy, the absence of epipodites on the thoracopods 2–8, was mentioned (Richter 1994a), but the interpretation of this character depends on the monophyly of the Mysidacea. It is included in our data matrix. Kobusch (1998)– based on a study of the mysid foregut – found additional autapomorphies of the Mysida: the bulbous cardia with its dorsal fold, the armature of the lateralia, and the construction of the funnel region.
Amphipoda
The Amphipoda consists of four major taxa: Gammaridea, Ingolfiellidea, Caprellidea, and Hyperiidea (Gruner 1993). Coleman (1994) identified some foregut characters which support the monophyly of the otherwise not well-justified Hyperiidea. The Gammaridea are probably a paraphyletic taxon (see Kim and Kim 1993). The fusion of the coxae of the first thoracopods (maxillipeds) and the differentiation of thoracopods and of the pleopods are suggested as autapomorphies of the Amphipoda. The pleon is differentiated into a pleosome with pleopods and a urosome with three pairs of uropods.
Mictacea
Only four species are known is this taxon: Mictocaris halope, Bowman and Iliffe 1985; Hirsutia bathyalis, Sanders, Hessler and Garner 1985; Hirsutia sandersetalia, Just and Poore 1988 and Thetispelecaris remexGutu and Iliffe 1998. Bowman et al. (1985), based on the description of the first two discovered species, gave a diagnosis of the new order Mictacea. Most of the characters given in the diagnosis are plesiomorphic, some potential apomorphies (maxilliped without epipodite, see character 24) are not unique for the Mictacea and are included in our analysis. We suggest as an autapomorphy the loss of the lacinia mobilis of the right mandible, although this character state is also known for example from the Phreatoicidae (Isopoda), but which is proposed as an apomorphy of this taxon within isopods (Wägele 1989; Brusca and Wilson 1991).
Spelaeogriphacea
Three spelaeogriphacean species in one single extant family (see Boxshall 1999) are described: Spelaeogriphus lepidops, Gordon 1957, Potiicoara brasiliensis, Pires 1987, and Mangkurtu mityula, Poore and Humphreys 1998. Pires (1987) presented a phylogenetic analysis of the Peracarida mentioning the following as a synapomorphy of P. brasiliensis and S. lepidops: pereiopods IV–VI with exopod reduced, fleshy, respiratory. Such respiratory exopods also seem to occur on the pereiopods (at least IV–V) of the recently discovered third species, M. mityula (see Poore and Humphreys 1998). Yan-bin et al. (1998) have questioned the monophyly of the Spelaeogriphacea.
Gutu and Iliffe (1998) and Gutu (1998) suggested a reorganization of spelaeogriphacean and mictacean taxa. However, the suggestions presented in these papers seem to be quite problematic. Thus, we do not follow them for now.
Cumacea
The Cumacea consists of nine families (Lampropidae, Bodotriidae, Leuconidae, Nannastacidae, Pseudocumatidae, Ceratocumatidae, Gynodistylidae, Diastylidae, Archaeocumatidae) with as yet unclear phylogenetic relationships (Gruner 1993; Bacescu and Petrescu 1999). It seems that Nannastacus shows a plesiomorphic character state compared with all other cumaceans: the separated eyes which are – when present – fused in all other taxa (Schram 1986; Gruner 1993). As potential autapomorphies of the Cumacea we suggest the paired pseudorostrum and the very specialized shape of the epipodite of the first maxilliped (its function as a respiratory organ is included in our data matrix; character 24).
Tanaidacea
The Tanaidacea consists of three major extant taxa: Apseudomorpha, Neotanaidomorpha, and Tanaidomorpha (Gruner 1993; Gutu and Sieg 1999). Sieg (1984) suggested as unique apomorphies of the Tanaidacea the existence of a small distal palp on the hypopharynx, a short ring shaped ischium of the pereiopods, and the second thoracopod forming a large claw. A further character, the fusion of two thoracomeres with the head, is included in our data matrix.
Isopoda
The most recent and detailed analyses on the Isopoda are by Wägele (1989) and Brusca and Wilson (1991) with partly contradictory results. Brusca and Wilson (1991) mentioned 13 apomorphies of the Isopoda, eight of them are unique (in comparison to other Peracarida, but not necessarily to other Malacostraca) for the Isopoda. Here, we refer only to the characters which are not included in our analysis: biphasic moulting, striated muscles with unique myofribril ultrastructure, uropodal rami always uniarticulate.
Thermosbaenacea
In his recent revision and cladistic analysis of the Thermosbaenacea, Wagner (1994) recognized 34 species in four families (Thermosbaenidae, Monodellidae, Halosbaenidae, Tulumellidae; see also Monod and Cals 1999). Unfortunately, Wagner did not present a list of the autapomorphies of the Thermosbaenacea. An obvious autapomorphy is the dorsal brood pouch (e.g. Pires 1987).
Euphausiacea
The Euphausiacea comprises traditionally two families: the monotypic Bentheuphausiidae with Bentheuphausia amblyops, G.O. Sars 1885 and the Euphausiidae with about 85 species (Baker et al. 1990). A ‘typical’ euphausiacean character, the luminescent organs, is absent in Bentheuphausia amblyops, and the presence of such organs is possibly an apomorphy of the Euphausiidae (other characters see below). Christoffersen (1988) suggested the reduced eighth thoracopod, with an endopod containing only four segments in adults, as the only apomorphy of the entire Euphausiacea. However, this character state is only true for Bentheuphausia. In the other species the eighth thoracopod is even more reduced. We suggest as a unique autapomorphy the special (snail-like) shape of the lateral branch of the gills.
Amphionidacea
The Amphionidacea comprises probably only one species, Amphionides reynaudii, H. Milne Edwards 1833. The modified first pleopod together with the carapace forming a brood pouch in females is an autapomorphy of this species.
Dendrobranchiata
The most recent revision and classification of the Dendrobranchiata has been undertaken by Pérez Farfante and Kensley (1997). They proposed seven families within the Dendrobranchiata: Aristeidae, Benthesicyminidae, Penaeidae, Sicyoniidae, Solenoceridae, Luciferidae and Sergestidae. Unfortunately, they did not provide any phylogenetic hypothesis. Christoffersen (1988) suggested four characters as support for a monophyletic Dendrobranchiata: dendrobranchiate gills, endopod of the first pleopod transformed to a petasma attached to the proximal portion of the protopodite, appendices internae absent from the pleopods, postlarval mandibular palp expanded and lamellar. The characters ‘petasma’ and ‘missing appendices internae’ are included in our data matrix because they are not unique to the Dendrobranchiata. Felgenhauer and Abele (1983) suggested that the trichobranchiate gills are derived from dendrobranchiae, however, this hypothesis is not supported by any observation. The expanded mandibular palp is possibly not a valid character, since different parts of the palps are expanded in the different taxa (see Christoffersen 1988).
Caridea
A list and a key of the recent genera in 28 families of caridean shrimps are given by Holthuis (1993). Gruner (1993) suggested the Procaridoidea as sister group to the remaining Caridea. Christoffersen (1990) suggested a cladogram of eight recognized superfamilies (without Procaridoidea) with the Atyoidea as sister group to the remaining seven taxa. As an autapomorphy of the entire Caridea, we propose the phyllobranchiate gills with two efferent blood vessels (Taylor and Taylor 1992). In Procaris ascensionis Chace and Manning 1972, the gills are also phyllobranchiate and they have the same shape as the gills of other Caridea (Abele and Felgenhauer 1985) but nothing is known about the blood circulation in the gills. Phyllobranchiate gills are also known from some taxa within the Reptantia. This has to be interpreted as convergent. In these taxa, only one efferent blood vessel is present, and the shape is different (Taylor and Taylor 1992).
Stenopodidea
The Stenopodidea comprises the Stenopodidae with four genera and the Spongicolidae with five genera (Schram 1986; Holthuis 1993). Christoffersen (1988) suggested as an autapomorphy that the sixth thoracopods (third pereiopods) are much more strongly developed than the seventh and eighth.
Reptantia
The most recent phylogenetic analysis of the Decapoda Reptantia is that of Scholtz and Richter (1995). They suggested nine apomorphies which support the monophyly of the Reptantia: e.g. depressed body, in particular the pleon, fifth pereiopods as specialized chelate or subchelate grooming appendages, brain wider than long, due to the lateral orientation of the neuropil of the second antenna, and head-to-head orientation, with their ventral surfaces opposed, during copulation. Within the Reptantia a sister-group relationship between the Polychelida and the remaining taxa, the Eureptantia is proposed. Within the Eureptantia the Achelata (Palinuridae, Scyllaridae) is the sister group to the other taxa, the Macrochelata.
Characters
The characters used in this analysis come from different sources including our own, in some cases unpublished, studies. We include a comparatively high number of anatomical and embryological characters. One could argue that some character complexes are overweighted. This might be the case but it should be considered that this is also true for earlier analyses which were mainly based on external characters. One more important point has to be stressed. We often use a nested (encaptic) system of characters which implies the a priori exclusion that different characters from the same character complex support the same node in the cladogram. This can be seen in the resulting cladograms. In general, we suggest all characters used and numbered here are independent from each other. We are aware that it might be difficult to prove this in every case.
[1] Number of free pleon segments: (0) seven; (1) six; (2) five (6th pleomere fused with telson to form a pleotelson). In malacostracans the number of thorax segments is always eight, the number of pleomeres differs to some extent. The Leptostraca possess seven free pleon segments. Most other Malacostraca show, at least in their ground pattern, six free pleon segments. Brusca and Wilson (1991) discussed the occurrence of a pleotelson (a fusion of pleon segments with the telson) within the Peracarida. According to these authors a pleotelson is part of the ground pattern only in the Isopoda; the pleotelson in Tanaidacea (Schram 1986), some Cumacea and some Thermosbaenacea is thought to have evolved within these taxa. Following Brusca and Wilson (1991) we scored only the Isopoda (2) within the Peracarida. There is also a pleotelson in the Bathynellacea. The fusion of other pleomeres is not considered (see Brusca and Wilson 1991).
[2] Pleomere size: (0) first pleomere fully developed, of similar size and appearence as the more posterior pleomeres; (1) first pleomere reduced, smaller than the second pleomere; (2) more pleomeres reduced. In the Stenopodidea and the Reptantia the first pleomere is reduced in size (1). In the Caridea and Dendrobranchiata it is fully developed (0). Within the Peracarida several pleomeres are reduced in Tanaidacea and Isopoda resulting in a relatively short pleon (2).
[3] Pleura of the second pleon segment: (0) pleura not overlapping other pleura, or pleura overlapping only those of the third pleomere, or pleura absent; (1) pleura of the 2nd pleomere overlapping those of the first and third pleomere. This character refers to the Decapoda and was discussed by Scholtz and Richter (1995). In the Caridea (including the Procaridoida) and in several Reptantia such as in the Polychelida, Homarida, Astacida, and the thalassinid genus Naushonia, the second pleura overlap those of the first and third pleomere. Based on the distribution within the Reptantia this taxon is also scored (1). The second pleura overlap only those of the third pleomere of Dendrobranchiata and Stenopodidea. Non-decapod taxa were also scored (0).
[4] Number of pleon appendages: (0) six; (1) three (third to fifth pleopods missing). In the Malacostraca, the number of thoracopods is constant (with a few exceptions within the terminal taxa such as the reduced eighth thoracopods in euphausiids), and only the number of the pleopods (all appendages of the pleon, including the uropods) differs. In the Bathynellacea and the Thermosbaenacea, the third to fifth pleopods are missing. Also in other taxa different pleopods can be reduced (e.g. within the Reptantia, Brachyura), but in these cases this seems not to be part of the ground pattern of the taxa of our analysis.
[5] Carapace: (0) present; (1) absent. Wills (1998) and Schram and Hof (1998) suggested different characters concerning the carapace. However, the main and general character is the presence or absence of a carapace. The ‘carapace’ is a quite theory-laden character. Different opinions about the origin and development of the carapace exist. However, there is no reason to exclude a homology of this structure in the Malacostraca a priori independent of whether one favours the origin of a carapace fold from the head segments (Calman 1909; Lauterbach 1974; Newman and Knight 1984; Casanova 1991, 1993; Scholtz 1998) or whether one thinks that the carapace is not much more than the ‘dorsal/branchiostegal folds’ fused with the thoracomeres (Dahl 1983, 1991; but see Newman and Knight 1984). We disagree with Watling (1999) who emphasized the differences in the development of the carapace as arguments for convergent evolution. Different modes in development do not a priori exclude the homology of characters. Also, Dahl (1991:18) suggested a ‘basic malacostracan pattern’.
[6] Carapace: (0) bivalved, enveloping most of the body; (1) well-developed, covering the thorax; (2) short, covering only the anteriormost thoracic segments. This character follows the suggestions of Wills (1998) and Schram and Hof (1998). The taxa without a carapace were scored as inapplicable.
[7] Carapace: (0) nonrespiratory; (1) with respiratory function. A respiratory carapace is known to occur in Cumacea (Siewing 1952), Tanaidacea, Spelaeogriphacea, Mysida, and Thermosbaenacea (Grindley and Hessler 1971). The carapace in Lophogastrida seems to be non-respiratory (Dahl 1991). Wägele (1994) suggested this character as a synapomorphy of the Mysida and the peracarid taxa with a manca stage. However, a respiratory function of the carapace occurs also outside the Peracarida at least in Leptostraca and Dendrobranchiata (Vannier et al. 1997; Taylor and Taylor 1992) and within the Reptantia in the terrestrial hermit crab Birgus latro (Linnaeus 1767) and some terrestrial brachyurans. The condition in the Amphionidacea is unknown (Gruner 1993). This character is not applicable for taxa without a carapace.
[8] Carapace adductor muscles: (0) present; (1) absent. The character is uninformative (in a cladistic sense) in our data matrix. Carapace adductor muscles are only present in the Leptostraca. However, their absence is a potential apomorphy of the Eumalacostraca. Therefore, we decided to include this character in our matrix.
[9] Number of thoracomeres involved in forming the cephalothorax: (0) No thoracomere fused with the head; (1) one thoracomere fused with head and carapace (if present); (2) two thoracomeres fused with head and carapace; (3) three thoracomeres fused with head and carapace; (4) eight thoracomeres fused with head and carapace. A different number of thoracomeres is fused with the head to form a cephalothorax. In the taxa possessing a carapace, the number of fused thoracomeres corresponds to the number of segments which are fused with the carapace (Casanova 1991, 1993), or the number of segments giving rise to dorsal or branchiostegal folds (Dahl 1991). Therefore, we decided to introduce only one character here. The advantage is that this character is applicable for taxa both with and without a carapace. According to Claus (1888) and Newman and Knight (1984), the carapace is not fused to a thoracomere in Leptostraca. Dahl (1991) suggested that a ‘cephalothoracic shield’ covers the cephalon and the three anterior thoracic segments. Our own observations show a connection between the carapace and the dorso-median parts of at least two thoracomeres but this is not a true fusion. Therefore, we score the Leptostraca (0). The conditions in the Stomatopoda are different. According to Balss (1927) the thoracomeres 1–4 are fused, but there is no fusion between the anterior part of the thorax and the head (although there is a close connection) (see also Lauterbach 1972). However, Casanova (1993) suggested that the carapace is connected to the first thoracomere like in other Malacostraca. We decided to score this character (1) in the Stomatopoda. Within Lophogastrida, Cumacea, Tanaidacea, and Isopoda a different number of segments is involved in forming the cephalothorax. We decided to score the lowest number of segments involved in the formation of the cephalothorax in every taxon (see for example Brusca and Wilson 1991 for the Isopoda).
[10] Rostrum: (0) absent; (1) present and fused to head; (2) present and hinged to head. Leptostraca and Stomatopoda possess a movable rostrum (Siewing 1956). A rostrum seems to be absent in Bathynellacea and Thermosbaenacea. In the Cumacea, there is a pair of anterior processes the so-called ‘pseudorostrum’ which is not homologous to the true rostrum. Therefore, we scored the Cumacea (0). Most Isopoda and Amphipoda do not possess a rostrum, but some do. We scored both taxa polymorphic (0/1).
[11] First antenna: (0) biramous, both rami as flagella; (1) biramous, outer ramus a scale; (2) biramous, inner ramus a scale; (3) triramous; (4) uniramous. Schram and Hof (1998) used the character state ‘uniramous’ for scoring the Isopoda and the Amphipoda (the latter scored polymorphic, also ‘biramous’). Indeed, many amphipods possess clearly biramous first antennae, and this state can be assumed for the ground pattern of the Amphipoda (following our guidelines in the section ‘Theoretical aspects’). Brusca and Wilson (1991) discussed the ‘minute, uniarticulate structures’ in the isopod taxa Epicaridea and Limnoriidae as possible homologues of a second flagellum and decided to score these taxa as ‘biramous’. However, one of their results is that this ‘scale’ evolved within the Isopoda. We decided to score the Isopoda as ‘uniramous’. In the Leptostraca, the outer ramus forms a scale (scored 1). An uniarticulate second flagellum is described for the Bathynellacea by Siewing (1959). However, in contrast to the Leptostraca, this knob-like structure is the inner ramus (scored 2). The taxon Stomatopoda is characterized by a triramous first antenna, a character which it shares with representatives of its fossil relatives (see Schram and Hof 1998). This character is uninformative in our analysis.
[12] Second antenna: (0) biramous, outer ramous represented by a scale (scaphocerite); (1) uniramous. The Leptostraca possess a uniramous second antenna. In many other taxa, the second ramus forms a scale, the scaphocerite. Schram and Hof (1998) used two separate character states for ‘scale with a separate basal segment’ in stomatopods and ‘simple scale’ in other Eumalacostraca. However, except for this difference, both scales are similar enough to suggest homology. Within the Isopoda only in the Asellota (Brusca and Wilson 1991) and within the Thermosbaenacea only in Tulumella (Wagner 1994) do we find a scale-like exopod. Homology of these structures with a scaphocerite can be questioned (see Brusca and Wilson 1991 for the Isopoda). In all other representatives of these two taxa the second antenna is uniramous (we scored both taxa 0/1). The second antenna is uniramous in Cumacea and Amphipoda (scored 1).
[13] Mandibular palp: (0) present (1) absent. A reduction of the originally three-articulated mandibular palp (to one or two articles) and its complete loss has occurred in many groups. We decided to score all taxa (0) where at least some representatives possess a mandibular palp. Only in the Cumacea the palp is absent in all species, and in Amphionidacea a mandibular palp is missing too (both scored 1).
[14] Base of adult mandible: (0) only with incisor and molar process; (1) a spine row or a field of setae between the two processes; (2) in addition to the spine row, a lacinia mobilis on at least one mandible [additive]. In many of our terminal taxa reductions and transformations of different parts of the mandible are known, for example as an adaptation to parasitic life. These derivations are not considered here. It is suggested that in the ground pattern of every taxon in this analysis, at least the incisor and the molar process were present. In addition, it is important to distinguish between the adult and larval mandible. A lacinia mobilis in the adults is found only in the peracaridan taxa and the Thermosbaenacea, although a larval lacinia mobilis is also known from other taxa (see character 15). However, according to Hennig’s (1950) semaphoront concept, larval and adult characters must be treated independently. Within the Anaspidacea, the Stygocarididae is not considered, since the mandibles of its representatives probably show many larval characters (Schminke 1978). According to Schminke (1981), the bathynellacean mandible shows larval characters. We scored the Bathynellacea (1), because a homology of one of the setae between incisor and molar process with a lacinia mobilis is doubtful (see Schminke 1972 for a detailed discussion). In the Mictacea, a lacinia mobilis occurs only on the left mandible (Bowman et al. 1985; Hessler 1999), which is also true for some isopod taxa (e.g. Wägele 1989). A lacinia mobilis homologue occurs on both mandibles of the Lophogastrida (Richter 1994a). We are aware that a lacinia mobilis also occurs in some insect taxa and in the Remipedia (e.g. Schram et al. 1986; Moura and Christoffersen 1996).
[15] Lacinia mobilis on the larval (embryonic) mandible: (0) absent; (1) present. Dahl and Hessler (1982) drew our attention to the fact that the lacinia mobilis in adult and larval (embryonic) malacostracans shows a different distribution. Little seems to be known about the lacinia mobilis in marsupial juveniles of the peracarid taxa. However, Dahl and Hessler (1982) show a drawing of a marsupial young of Gammarus pulex (Linnaeus 1758) with laciniae on both mandibles, which in general do not differ from those of adults. Thus, we scored (1) for all taxa which possess a lacinia mobilis as adults. Weigmann-Haas (1977) found a spine row and a lacinia mobilis in Euphausia hanseni Zimmer 1915 calyptopis and furcilia larvae. Dahl and Hessler (1982) confirmed this observation for Meganyctiphanes norvegica (M. Sars 1857). In a few decapod larvae, a lacinia mobilis is present on the left mandible between incisor and molar process; however, it seems that all species with a larval lacinia belong to the Caridea (Balss 1940–44; Dahl and Hessler 1982), therefore only this taxon is scored (1), and Dendrobranchiata, Stenopodidea, and Reptantia are scored (0). Our knowledge about the mandibles of the embryos or newly hatched anaspidaceans is limited. Hickman (1937) stated that the mandibles of newly hatched Anaspides tasmaniae do not differ from those of the adults. Unfortunately, his drawing (Fig. 39) is not sufficient. We scored the Anaspidacea as (?). Nothing seems to be known about the mandibles of the bathynellacean larvae (also scored?).
[16] Labrum: (0) without posterior tooth; (1) with posterior tooth. Character state (1) is found in some Lophogastrida (for example in Gnathophausia sp.) and Mysida (for example Boreomysis sp.). We suggest that this posterior tooth belongs to the ground pattern of both taxa (see also Richter 1994a). To the best of our knowledge this kind of labrum tooth is not known from any other taxon.
[17] First maxilla: (0) palp present; (1) palp absent. In the Leptostraca, except Nebaliopsis, the palp of the first maxilla is flagellate. It functions as a cleaning appendage reaching into the carapace chamber (Gruner 1993). In the other malacostracan taxa the palp is small or absent (see for example Brusca and Wilson 1991 for the Isopoda). In Amphionidacea, in the Spelaeogriphacea, and in the Mictacea (Gruner 1993; Boxshall 1999; Hessler 1999) the palp is absent (1). Two setae were discussed as the vestigial palps in the Bathynellacea by Siewing (1959). We scored the Bathynellacea (0).
[18] Second maxilla exopod: (0) present, forming a broad plate; (1) modified to scaphognathite; (2) absent. The outer part of the second maxilla is transformed into a scaphognathite as part of the respiratory system in Dendrobranchiata, Caridea, Stenopodidea, Reptantia and Amphionidacea. Balss (1940–44) discussed the homology of the scaphognathite to the exopod versus to a fusion of exopod and epipodite.
[19] Second maxilla palp: (0) present; (1) vestigial or absent. A palp of the second maxilla is missing in the peracaridan taxa with the exception of Lophogastrida and Mysida (Brusca and Wilson 1991). The palp is present in the Thermosbaenacea (Fryer 1965). The homology of the ‘palp’ in the Bathynellacea is under discussion (Siewing 1959).
[20] Thoracopods: (0) phyllopodous (1) stenopodous. Character state (0) characterizes only the Leptostraca within the Malacostraca. This character is uninformative in our analysis.
[21] First thoracopod: (0) normal; (1) transformed into a maxilliped. Schram and Hof (1998) suggested the ‘number of maxillipeds’ as a character. However, this scoring seems to be problematic. We decided to use two characters: the first distinguishes between taxa with at least one (first) maxilliped, and taxa without any maxillipeds. With this kind of scoring we propose a general homology between all first maxillipeds in Malacostraca although detailed differences between the taxa are obvious. Only the Leptostraca, Bathynellacea, and Euphausiacea are scored (0).
[22] Additional thoracopods transformed to maxillipeds: (0) no; (1) 2nd (and 3rd) thoracopod are maxillipeds; (2) five ‘maxillipeds’. A putative homology between the additional maxillipeds seems more questionable. In the Stomatopoda, the second ‘maxilliped’ is the main raptorial leg, and the third to fifth thoracopods are also quite different from maxillipeds in other taxa. We scored the Stomatopoda with a separate character state (2). Often three maxillipeds are mentioned as generally occurring in decapod taxa (e.g. Holthuis 1993; Pérez Farfante and Kensley 1997). However, in the Dendrobranchiata the third thoracopod, which seems to function as a maxilliped, shows no important morphological transformations to a maxilliped and differs little from the more posterior thoracopods. Within the Caridea, the Procaridoidea, the suggested sister group to the other Caridea (Gruner 1993), also possesses a leglike third maxilliped. This is also true for the Stenopodidea. Because of the different interpretation we used only one character state (1) for Dendrobranchiata, Caridoidea, Stenopodidea, and Reptantia. Within the peracarid taxa, the Cumacea possesses three and the Mysida possesses two maxillipeds (both taxa scored 1).
[23] First thoracopod: exopod: (0) present; (1) absent. Most taxa possess a more or less flagelliform exopod, but in the Leptostraca the exopod is a broad unarticulated paddle (Cannon 1960). The exopod in the Lophogastrida is lancet-shaped (Richter 1994a). It is absent in Tanaidacea, Mictacea, Spelaeogriphacea, Isopoda, and Amphipoda. In the Cumacea the siphon is discussed as exopodal (Schram 1986), but Gruner (1993) interpreted this structure as an epipodite. We followed Gruner (1993) and scored the Cumacea (1).
[24] First thoracopod: (0) epipodite(s) respiratory and differ(s) in general not from the epipodites of more posterior thoracopods; (1) epipodite produces a respiratory current; it is respiratory itself or nonrespiratory; (2) epipodite present, but not producing a respiratory current and nonrespiratory; (3) epipodite absent. The epipodite(s) of most taxa play a role in the respiratory system (Grindley and Hessler 1971). However, their function differs. In the Leptostraca, Stomatopoda, Anaspidacea, Bathynellacea, Amphionidacea, and Euphausiacea the epipodites of the first thoracopod differ little from the epipodites of the posterior thoracopods; they all are respiratory. Within the Decapoda, and here in particular the Reptantia, the structure and function of the epipodite may change (for example as a gill cleaning flabellum within the Brachyura), but for the ground pattern of the Dendrobanchiata, Caridea, Stenopodidea, and Reptantia an epipodite which functions as a gill seems to be most probable. The epipodite is carried under the carapace and produces a respiratory current in the Lophogastrida, Mysida, Spelaeogriphacea, Cumacea, Tanaidacea, and Thermosbaenacea (scored 1). The epipodite itself functions additionally as a gill in the Cumacea, Tanaidacea, and Spelaeogriphacea (scored 1). The epipodite of the Isopoda never functions as a gill (Brusca and Wilson 1991; scored 2). The epipodite is absent in Mictacea and Amphipoda (3).
Thoracopods 2–8:
Schram and Hof (1998) scored characters concerning the thoracopods in two parts, the ‘anterior trunk limbs’, which comprise the thoracopods 1–5 and the ‘posterior trunk limbs’. On the other hand, Wills (1998) used different characters for the first to fourth thoracopods. Both approaches may be valuable in dealing with all crustaceans. However, in our case a further differentiation of the thoracopods does not seem justified, although in the Stomatopoda in particular, a clear separation of the thorax into two parts exists. We agree with Schram and Hof (1998) that a separate set of characters for all thoracopods would put too much weight on this region. Therefore, we decided to score only the ‘general characters’ and not whether these general characters occur on every thoracopod. An exception is the oöstegites (see characters 27–29).
[25] Thoracopods 2–8, number of epipodites (without oöstegites) on at least some thoracopods (0) one; (1) two (often two branches, developing from one anlage) present. (2) more than two epipodites, as arthrobranchiae and pleurobranchiae, or in another way; (3) none. A single epipodite is present on all thoracopods of the Leptostraca; this is true for the thoracopods 1–5 in the Stomatopoda. The Anaspidacea and Bathynellacea (at least in Bathynella) possess two epipodites on the thoracopods 2–7, in some Euphausiacea (Bentheuphausia, Thysanopoda) the epipodites are two-branched, a lateral part (‘Schnecke’), and a second part which is carried under the thorax (Zimmer and Gruner 1956). In representatives of the Benthesicyminidae within the Dendrobranchiata, the podobranchia and the unfeathered epipodite develop from the same anlage (Gurney 1942). We suggest that these two structures are homologous to the two epipodites of the other taxa. In addition to these two epipodites, two arthrobranchiae and one pleurobranchia can occur. There are no epipodites on the thoracopods 2–8 in the Thermosbaenacea and most peracarid taxa (except the Lophogastrida and Amphipoda). One epipodial gill is present in Amphipoda (0). More than two epipodites occur within the Lophogastrida (for example in Gnathophausia sp.) and in the Dendrobranchiata, Caridea, Stenopodidea, and Reptantia.
[26] Thoracopods 2–8: position of epipodites: (0) lateral; (1) at least one branch carried under the thorax. Although the epipodites are quite different in their shape, in some Euphausiacea and in Lophogastrida one epipodial branch is carried under the thorax. Also, the epipodial gills in Amphipoda are carried under the thorax. In all other taxa, the epipodites are lateral and never reach under the thorax. This character is inapplicable for taxa without epipodites (apart from the oöstegites; see below).
[27] Oöstegites: (0) absent; (1) present. Oöstegites are coxal structures which form the marsupium for the development of the embryos. Although the oöstegites are probably homologous to epipodites (Claus 1885; Siewing 1956; Dahl 1983), we deal with both structures separately. The different development of the oöstegites in the peracarid taxa (see Watling 1999) is not important enough to exclude a priori their homology. The structures are very similar in shape and position in the different taxa and also the function is identical.
[28] Oöstegites: (0) on thoracopods 2–8; (1) on thoracopods 2–7; (2) on thoracopods 2–6; (3) on thoracopods 3–6. This character is only applicable for taxa with oöstegites, all other taxa are scored as inapplicable. The taxa are scored with the maximum number of oöstegites appearing within each taxon. For example, within the Mysida only the Petalophthalmidae, Lepidomysidae, Stygiomysidae and the genus Boreomysis within the Mysidae possess seven pairs of oöstegites (e.g. Gruner 1993). The oöstegites of the second thoracopod are vestigial in Cumacea and Tanaidacea, but for a comparison between the taxa it is necessary to score both (2). In some groups within the Isopoda oöstegites may form on the thoracopods 2–8 (Brusca and Wilson 1991). An additional oöstegite is present on the maxilliped of Mictocaris and some Isopoda (Brusca and Wilson 1991); at least in the Isopoda, this is probably not part of the ground pattern.
[29] Reduction of the oöstegites after every brood: (0) no reduction; (1) yes. Siewing (1953) introduced this character, which only refers to the taxa with oöstegites. All other taxa are scored as inapplicable. According to Siewing (1953), in the Cumacea, Tanaidacea, and Isopoda the oöstegites are reduced after every brood with a moult (Zwischenhäutung). They develop again in an additional (marsupial) moult before the next brood starts. The conditions in Spelaeogriphacea and Mictacea are unknown.
[30] Thoracopods 2–8: exopod: (0) present, at least on some thoracopods, flagellate or vestigial; (1) completely absent from all thoracopods. Only in the Amphipoda and Isopoda are the exopods completely absent in all thoracopods. The Reptantia is scored (0) because the exopods are present on the three maxillipeds (here thoracopods 2 and 3). The value of this character might be questioned, because only vestigial exopods occur in Tanaidacea (Sieg 1984).
Walking mechanisms:
The following three characters were introduced and discussed in detail by Hessler (1982b). In some cases different limbs in the same species show different conditions. We tried to simplify these characters. For a phylogenetic analysis that focuses on the peracarid taxa it would be valuable to study these characters in more detail.
[31] Thorax – coxa articulation: (0) allows promotion/remotion; (1) allows abduction/adduction. According to Hessler (1982b) character state (0) characterizes the Leptostraca, the decapod taxa, the Anaspidacea, Thermosbaenacea, the Cumacea and the Isopoda (Phreatoicida, Asellota), character state (1) the Mysida, Lophogastrida, Spelaeogriphacea and Amphipoda. We interpret the statement of Bowman et al. (1985) that the pereiopodal coxae in Mictacea are partly immobilized as evidence that scoring character state (1) is appropriate for this taxon. Both character states occur in Tanaidacea (we scored the Tanaidacea polymorphic 0/1).
[32] Coxa – basis articulation: (0) dicondylic, allows abduction/adduction; (1) monocondylic, motion in different directions is possible. Hessler (1982b) described a dicondylic articulation for the Decapoda, Anaspidacea, Lophogastrida, and Mysida. The articulation is monocondylic in Isopoda, Spelaeogriphacea, Cumacea, Thermosbaenacea, and in Mictacea (Bowman et al. 1985). Hessler (1982b) questioned the homology of the monocondylic articulation in Thermosbaenacea and peracarids (which was suggested by Sieg 1984). In Amphipoda and Tanaidacea, some thoracopods possess a dicondylic, others a monocondylic, articulation [for the amphipods only the eighth thoracopods of Gammarus pulex (Linnaeus 1758)]. We scored these two taxa as polymorphic (0/1).
[33] Intrabasal articulation: (0) absent; (1) present. Hessler (1982b) described an intrabasal articulation for Mysida, Lophogastrida, and Anaspidacea. In the Lophogastrida and Mysida, the intrabasal articulation allows a promotion and remotion of the thoracopods. This kind of movement is not possible in the taxa using the thorax-coxa articulation (see above). The function of the intrabasal articulation in the Anaspidacea is unknown.
[34] Coxal plates on thoracopods: (0) absent; (1) present. Only in Amphipoda and Isopoda do the coxae of the thoracopods possess plate-like extensions. In the phylogenetic analyses of Wägele (1989) as well as of Brusca and Wilson (1991) the coxal plates of the Isopoda is a derived character evolved within this taxon. We decided to score the Isopoda (0/1).
[35] Thoracopods 4–5: (0) achelate; (1) chelate
[36] Thoracopod 6: (0) achelate; (1) chelate. Thoracopods 4–6 are achelate in most Malacostraca. The Stomatopoda (here scored 0) possess subchelae on the first (maxilliped) to fifth thoracopods, which can be clearly distinguished from true chelae. The thoracopods 4–6 are chelate in the Dendrobranchiata and Stenopodidea. Both character states are present in the Reptantia. Based on the proposed phylogenetic relationships within the Reptantia (Scholtz and Richter 1995), we suggest that the character states in the Achelata (no chelae), Thalassinida (various character states), Anomala (only fourth thoracopods with chela), and Brachyura (only fourth thoracopods with chela) are derived. For the ground pattern of the Reptantia we propose a chelate character state for thoracopods 4–6 which is present in Polychelida, Homarida, and Astacida. Thus, the Reptantia is scored (1) for both characters. Within the Caridea only the thoracopods 4 and 5 are chelate (with the exception of Pseudochelesenigma Chace and Brown 1978 where chelae are present additionally on the thoracopods 6–8; Christoffersen 1988). In Procarisascensionis (Caridea) all thoracopods are achelate. We decided to score the Caridea (0/1) for the first character and (0) for the second character.
[37] Pleopods: (0) biramous; (1) uniramous. The first four pleopods in the Leptostraca are biramous, the last two pairs reduced and uniramous (scored 0). In the Anaspidacea, the endopods are reduced to small lappets or to complete absence (scored 0). The Bathynellacea, Mictacea, and Thermosbaenacea possess uniramous pleopods (scored 1).
[38] Pleopods: Appendices internae on the endopods: (0) absent; (1) present. Within the Decapoda, appendices internae occur in the Reptantia and the Caridea, but not in the Dendrobrachiata and the Stenopodidea (Balss 1940–44). Appendices internae also occur in the Leptostraca, Stomatopoda, Amphionidacea, and Euphausiacea.
[39] First and/or second pleopods modified for sperm transfer in males: (0) no or only minor modifications; (1) stomatopod petasma, including modifications of the exopod of the second pleopod; (2) endopod of the first pleopod completely modified for sperm transfer, modifications different in the second endopod. The first and sometimes the second pleopods of many Malacostraca are modified for sperm transfer. Different terms are used (petasma, gonopods, etc.), but the terminology implies nothing about the homology or convergence of this character. We agree with Gruner (1993: 720) that the term ‘petasma’ can be used for all these modifications. Although the conditions are sometimes quite different in the two pairs of pleopods we deal with both together. Schram and Hof (1998) scored the petasma as present in Euphausiacea, Dendrobranchiata, Caridea, Reptantia (actually scored as 0/1), Anaspidacea, and Isopoda, but absent in the Stomatopoda. However, according to Wägele (1989) and Brusca and Wilson (1991) the gonopods in the Isopoda evolved within this taxon, and they are not part of the isopod ground pattern. A petasma is probably also not part of the ground pattern of the Euphausiacea, because it is missing in Bentheuphausia amblyops, the putative sister group of the other euphausiaceans. In addition, the structure of the euphausiacean petasma is very different from the petasma in the other taxa. In the Dendrobranchiata, the endopod of the first pleopod is modified for the sperm transfer, in the second pleopod one to two appendices masculinae appear. Also, in the Reptantia the endopod of the first and second pleopods can be modified for the sperm transfer. Only minor modifications of the first and second pleopods occur in the Caridea; there is an appendix masculina in many species on the second pleopod (Caridea scored as 0). The endopod of the first pleopod seems to be missing in the Stenopodidea (scored as inapplicable). In the Anaspidacea, the endopods of the first and second pleopod are modified for sperm transfer, but the endopods of all pleopods are lappet-like. Therefore, the petasma in the Anaspidacea is not much more than the extended lappet-like endopods of the posterior pleopods. The endopod of the first pleopod and the exopod of the second pleopod show minor modifications in the Stomatopoda. The stomatopod petasma is only used for moving the genital papillae (Gruner 1993). We scored the Stomatopoda with a separate character state (1). Dendrobranchiata, Reptantia, and Anaspidacea were scored (2), although a homology at least between the petasma of the Anaspidacea and Decapoda has been questioned (Richter 1995).
[40] Last pleopods: (0) small, far from the telson, not forming a tail fan; (1) modified to broad uropods, forming a tail fan together with the telson; (2) oriented posteriorly close to telson but not forming a tail fan. Only in the Leptostraca are the sixth pleopods small, uniramous and far (separated by the seventh pleomere) from the telson. In Stomatopoda, Anaspidacea, Euphausiacea, Amphionidacea, Dendrobranchiata, Caridea, Stenopodidea, and Reptantia, the sixth pleopods are modified to uropods and form a tail fan together with the telson. This is also true for the Lophogastrida, Mysida, Spelaeogriphacea, Mictacea, and Thermosbaenacea. The last pleopods are oriented posteriorly in Bathynellacea, Amphipoda, Cumacea, and Tanaidacea. They do not form a tail fan together with the telson. Within the Isopoda, taxa occur with and without broad uropods forming a tail fan. There is a dispute about which kind of terminal pleopods is part of the isopod ground pattern (e.g. Wägele 1989, 1994; Brusca and Wilson 1991; Wilson 1996). We scored the Isopoda (1/2).
[41] Pleopods: (0) without respiratory function; (1) with gills. The exopods possess a respiratory function in the Isopoda. In the Stomatopoda, specialized gills originate from the exopods. Although, the structures are quite different in detail, a basic homology cannot be excluded a priori. Both taxa were scored (1), all other taxa (0).
[42] Telson form: (0) round, segment like; (1) flattened. This character can be seen in a functional correlation with a tail fan formed by the uropods and telson. However, uropods and telson are different structures. Therefore, it is justified to deal with both characters separately. Only in Leptostraca and in Bathynellacea is the telson more or less round in transverse sections.
[43] Telson: (0) with furca; (1) without furca. The Leptostraca and the Bathynellacea (Schminke 1981) possess a telson with a furca, which is missing at least in the adults of the other groups. The suggestion of Bowman (1971) that the furcal rami of the Leptostraca are the homologues of the uropods in other Malacostraca has been shown to be incorrect (Scholtz 1995).
[44] Globuli cell clusters in the deutocerebrum associated with the olfactory lobe: (0) one; (1) two. Two globuli cell clusters associated with the olfactory lobe in the deutocerebrum can be found in Caridea, Stenopodidea, and Reptantia (Sandeman et al. 1993; Sandeman and Scholtz 1995). Dendrobranchiata, Stomatopoda, Euphausiacea, Isopoda, Amphipoda, and Anaspidacea possess only one cluster (Chaigneau 1994; Sandeman and Scholtz 1995). For other groups this is not unambiguously described but from the figures in Hanström (1928, 1933) it can be tentatively concluded that one globuli cell cluster is also present in Leptostraca, Mysida, Lophogastrida and Tanaidacea.
[45] Compound eyes: (0) present; (1) absent. The simplest and most problematic character related to compound eyes is their presence or absence, because in taxa where the majority of species also possess compound eyes some species do not. This shows that the probability of loss of compound eyes in comparably high. Nevertheless, we decided to score (1) only taxa where all species lack the compound eyes. Compound eyes are absent in the Bathynellacea. In the Thermosbaenacea, Mictacea, and Spelaeogriphacea no ommatidia exist, although in some cases eye stalks are present (here scored as 1). Mayrat (1981) questioned the homology of the lateral eyes in Amphipoda and Cumacea with compound eyes. However, at least in the Amphipoda the ommatidial structure is very similar to that of the other taxa, therefore a homology of the eyes is obvious. The Cumacea studied possess at least light-sensitive elements (Meyer-Rochow 1989). Both taxa were scored (0).
[46] Eye-stalks: (0) present; (1) absent = sessile eyes. Only in the Amphipoda and Isopoda do all representatives (with eyes) possess sessile eyes. Sessile eyes are also known in a few Anaspidacea (but here scored as 0). The character in Cumacea is more complicated, but fossil cumaceans possess eye stalks (personal communication F. Schram) (here scored as 0). Within the Mictacea (here scored 0) movable eye-stalks are present in Mictocaris but absent in Hirsutia (Bowman et al. 1985). The Bathynellacea is scored as inapplicable.
[47] Number of optic neuropiles: (0) two; (1) three. In the Leptostraca two optical neuropiles were described (Elofsson and Dahl 1970; Scholtz 1992), in all other malacostracan taxa there are three optical neuropiles.
Ultrastructure of the ommatidia:
The following five characters were discussed in detail by Richter (1999). Hanström (1934) described the eyes of ‘Amphion A’ and ‘Amphion B’. We interpret these two ‘species’ as representatives of Amphionides reynaudii or another unknown amphionidacean species.
[48] Ultrastructure of ommatidia: (0) crystalline cone tetrapartite; (1) crystalline cone bipartite
[49] Ultrastructure of ommatidia: (0) bipartite cone completely round in transverse sections, cone without any extensions; (1) cone with two lateral extensions (in transverse sections button-like), formed by one cone cell each. A tetrapartite cone is known from the Leptostraca, Stomatopoda, Amphionidacea, Dendrobranchiata, Caridea, and Reptantia. A bipartite cone is known in Euphausiacea, Anaspidacea, Lophogastrida, Mysida, Amphipoda, Tanaidacea, and Isopoda. This character is inapplicable for all taxa without compound eyes.
We decided to score only taxa with bipartite cones as applicable for character [49] although in transverse sections round cones are also common in the taxa with tetrapartite cones. Only representatives of the Lophogastrida and Mysida possess these cone extensions.
[50] Ultrastructure of ommatidia: (0) crystalline cones with four cone cell processes; (1) only the two accessory cone cell processes are present; the processes of the two main cone cells are missing; (2) all cone cell processes missing. In the Leptostraca, Stomatopoda, Amphionidacea, Dendrobranchiata, Caridea, and Reptantia all four cone cell processes exist, only the two processes of the accessory cone cells occur in the Euphausiacea, Anaspidacea, Lophogastrida, Mysida, and Isopoda. In the Amphipoda all cone cell processes are missing. In the Tanaidacea too only two processes occur. However, these processes were interpreted as the processes of the main cone cells (Andersson et al. 1978). We scored the Tanaidacea (?).
[51] Ultrastructure of ommatidia: (0) all four cone cell nuclei lying in one plane on top of the cone; (1) nuclei of the accessory cone cells distally displaced. Character state (0) is true for all taxa with tetrapartite cones and for the Anaspidacea, character state (1) occurs in all other taxa with representatives with a bipartite cone.
[52] Ultrastructure of ommatidia: (0) no clear zone between crystalline cone and rhabdom (apposition eye); (1) clear zone formed by retinular cells and/or distal pigment cells, cone and rhabdom not in direct contact (superposition eye). In the Leptostraca, Stomatopoda, Amphipoda, Tanaidacea, and Isopoda a clear zone is missing; these taxa possess an apposition eye. An apposition eye is also known from some Reptantia, but according to the proposed phylogenetic relationships within the Reptantia (Scholtz and Richter 1995) a clear zone (and a superposition eye) is part of the ground pattern of the Reptantia. Anaspidacea, Euphausiacea, Dendrobranchiata, Caridea, Lophogastrida, and Mysida possess a clear zone and therefore a superposition eye.
[53] Nauplius eye s. str. (0) present; (1) absent
[54] Dorsal frontal organ: (0) present; (1) absent
[55] Ventral frontal organ: (0) present; (1) absent. These three characters were described and discussed in detail by Elofsson (1963, 1965, 1992). All three organs are present in the Stomatopoda, Dendrobranchiata, Caridea, and Reptantia (at least in the ground pattern). In the Anaspidacea, the dorsal frontal organ is vestigial, the ventral frontal organ is absent, the nauplius eye s.str. is present. The nauplius eye and the ventral frontal organ are present, the dorsal frontal organ is missing in the Euphausiacea. Within the peracarid taxa studied, only Mysida and Lophogastrida possess ventral frontal organs. Dorsal frontal organs and the nauplius eyes s.str. are missing in all peracarid taxa. All three organs are missing in the Leptostraca. The character states of the three characters are unknown in Bathynellacea, Amphionidacea, Stenopodidea, Thermosbaenacea, Mictacea, and Spelaeogriphacea.
[56] Statocyst in the basal segment of the first antenna: (0) absent; (1) present. A statocyst is described for the Anaspidacea, Dendrobranchiata, Caridea, and Reptantia. In Hansenomysis sp. a statocyst also seems to occur (therefore the Mysida were scored 1) (see Siewing 1956).
[57] Antennal gland: (0) present; (1) absent
[58] Maxillary gland: (0) present; (1) absent. Only in the Leptostraca are both organs present and function to the same extent as excretory organs (Cannon 1960). Both organs are present in the Lophogastrida, but the antennal gland is the main excretory organ (Siewing 1953). Only the antennal gland is present in Mysida, Amphipoda, Euphausiacea, Dendrobranchiata, Caridea, and Reptantia (Siewing 1953; Gruner 1993). In the Stomatopoda, Cumacea, Tanaidacea, Isopoda, Anaspidacea, and Bathynellacea only the maxillary gland is present (Siewing 1953; Gruner 1993). Both glands are absent in Thermosbaenacea (Siewing 1958). The conditions in Mictacea and Spelaeogriphacea are unknown (Boxshall 1999; Hessler 1999).
[59] Pleon musculature: (0) simple; (1) precaridoid; (2) caridoid. According to Hessler (1964) a typical caridoid musculature is found in Anaspidacea, Euphausiacea, Dendrobranchiata, Caridea, Reptantia (again in the ground pattern), Lophogastrida and Mysida. Hessler (1964) emphasized that the Stomatopoda has a musculature which is similar in some respects. We scored the Stomatopoda with a separate character state (1). In the Amphipoda, Isopoda, Tanaidacea, and Thermosbaenacea at least the longitudinal musculature is simplified. We scored these taxa (0) although we very much doubt that it is possible to homologize the ‘simple’ pleon musculature of these taxa with the one in the Leptostraca (also scored 0).
[60] Tail fan escape reaction: (0) absent; (1) present. This character is in close functional correlation to the previous one. However, not only the musculature is involved, as a particular innervation is also important (Silvey and Wilson 1979; Edwards et al. 1999). A detailed study by Heitler et al. (2000) shows that the Stomatopoda (scored 0) do not possess the same tail flexion system as, for example, the different decapod taxa. Fryer (1965) reports the existence of a tail flip in the thermosbaenacean Monodella argenatarii, Stella 1951. According to Brusca and Wilson (1991) this escape behaviour is absent in Amphipoda, Tanaidacea, Mictacea, Spelaeogriphacea, Isopoda. However, Bowman and Iliffe (1985) described a similar behaviour in Mictacea. We decided to score the Mictacea (?).
Heart and circulatory system:
The circulatory system in the Malacostraca was described for example by Claus (1884a,b) and by Siewing (1953, 1955, 1956, 1959) in detail. Siewing (1956) and later Watling (1983, 1999) discussed the phylogenetic implications of the circulatory system. However, not all of the homology decisions made by these authors seem plausible.
[61] Number of pairs of ostia: (0) more than 5; (1) 5; (2) 3; (3) 2; (4) 1; (5) 0. This character is problematic because the position of the ostia is not the same in every case. Therefore, one could argue that a correct scoring would be every ‘segment × with ostia absent or present’. However, it cannot be excluded that the position of a pair of ostia can change. In every case the value of this character is limited. The Leptostraca has seven pairs of ostia and the Stomatopoda has 13 pairs. We decided to score this high number of ostia as one character state (0). It should be noted that five pairs of ostia exist in several species of the Caridea (Balss 1940–44; Pillai 1965); and this seems also to be true for the Dendrobranchiata (Bell and Lightner 1988). In the Reptantia three pairs occur, the number of ostia in the Stenopodidea seems to be unknown (Balss 1940–1944). Three pairs of ostia occur in the Lophogastrida and Amphipoda, two pairs in the Mysida, Tanaidacea, Isopoda and Euphausiacea, one pair in the Anaspidacea, Cumacea and Thermosbaenacea. In the Bathynellacea ostia seem to be absent (5).
[62] Extension of the heart: (0) in whole thorax (head in part) and pleon; (1) thorax; (2) only posterior part of the thorax and pleon. This character is, in particular, of interest in the Isopoda, Leptostraca, Stomatopoda, and Anaspidacea. These are the only taxa, where an extension of the heart into the pleon is described. A functional correlation to the pleonal gills in the case of the Isopoda and Stomatopoda seems to be reasonable (Brusca and Wilson 1991), although the heart is also extended in the Leptostraca and Anaspidacea where no pleopodal gills exist. The conditions in Anaspides tasmaniae which possesses an extended heart but only one pair of ostia show that these two characters are independent. Nylund et al. (1987) described in the Isopoda a completely different ultrastructure of the heart musculature. We think it is justified to score the Isopoda with a separate character state, which implies that at least a part of the isopod heart is an apomorphy of this taxon. On the other hand, we follow Siewing (1956) when he states that the extension of the heart into the pleon and the whole thorax in Stomatopoda, Anaspidacea, and Leptostraca represents the plesiomorphic condition.
[63] General form of the heart: (0) long, extended; (1) bulbous; (2) simple short. A bulbous heart is described in the Thermosbaenacea, Cumacea, Euphausiacea, Dendrobranchiata, Caridea, and Reptantia. In all other taxa the heart is more or less long and extended. The heart of the Bathynellacea is relatively short but not bulbous (Siewing 1959), thus we scored it as (2).
[64] Arteria subneuralis/supraneuralis: (0) absent; (1) present. An arteria subneuralis which mainly supplies the ventral nervous system occurs in Stomatopoda and Isopoda. In Euphausiacea, Dendrobranchiata, Caridea, Reptantia, Lophogastrida, and Mysida the main function of the arteria subneuralis is to supply the thoracopods. An arteria supraneuralis occurs in the Anaspidacea, which we believe is homologous to the arteria subneuralis (scored 1; see also below). Also in this case, the thoracopods are supplied.
[65] Aorta descendens (sternal artery) as the only connection between heart and arteria subneuralis (or supraneuralis): (0) absent; (1) present. In the Stomatopoda and Isopoda some lateral arteries supply the arteria subneuralis. In the other taxa an unpaired (in exceptional cases paired, e.g. Balss 1940–44; Belman and Childress 1976) sternal artery (aorta descendens) is present. This character is scored as inapplicable for all taxa without arteria subneuralis or arteria supraneuralis, respectively.
[66] Aorta descendens: (0) the undivided sternal artery passes through the ventral nervous system; (1) sternal artery branches off into three branches dorsal of the ventral nervous system, all branches pass separately through the nerve cord. Euphausiacea, Mysida, Lophogastrida, and even the Anaspidacea share the following similarities which are not present in the Decapoda. The sternal artery branches off into three branches dorsal of the ventral nervous system, all branches pass separately through the nerve cord. One branch supplies the seventh and eighth thoracopods, a second the sixth thoracopod, and a third the anterior thoracopods. The only difference in the Anaspidacea is that the anteriormost branch runs as the arteria supraneuralis (this is not scored here). This character is scored as inapplicable for all taxa without an aorta descendens.
Foregut:
There is no doubt that a phylogenetic analysis of the Malacostraca should include characters of the foregut, and the previous studies of Siewing (1956) were based in part on a detailed comparison of the malacostracan foreguts. The main problem is to homologize the various structures of the foregut between the representatives of the Malacostraca. In several recent analyses the foreguts of different taxa were described and comparatively discussed (e.g. Wallis and Macmillan 1998). However, no analysis discussed in detail all structures through all malacostracans. The most recent comparison has been carried out by Kobusch (1998). For the decapod taxa we considered Patwardhan (1935) and the review by Balss (1940–44).
[67] Lateralia and inferolateralia anteriores in the cardiac chamber: (0) absent; (1) present
[68] Superomedianum (unpaired): (0) absent; (1) present. The symmetrical lateralia are more or less prominent infoldings of the cardia, the superomedianum is an unpaired infolding located at the transition from the cardia to the pyloric chamber. Lateralia seem to be present in all taxa with the exception of the Leptostraca (Siewing 1956).
A superomedianum seems to be present in the Leptostraca (‘dorsale Hakenplatte’Siewing 1956, although the homology has been questioned by Scheloske 1976), but seems to be absent in the Stomatopoda (Kunze 1981). It is also absent in the Isopoda and Amphipoda (see Kobusch 1998 for original references).
[69] Inferomedianum anterius (midventral cardic ridge): (0) absent; (1) present
[70] Inferomedianum posterius (midventral pyloric ridge): (0) absent; (1) present
[71] Atrium between the inferomediana connecting the cardiac primary filter grooves with the pyloric filter grooves: (0) absent; (1) present
[72] Number of secondary filter grooves in the inferomedianum posterius: (0) numerous; (1) eight to six; (2) three; (3) two; (4) one. The inferomediana are midventral ridges of the ‘cardia’ and the ‘pylorus’, respectively. They are connected by the atrium which functions as a link for moving fine food particles. Both inferomediana and the atrium are absent in the Leptostraca. An inferomedianum anterior and an atrium also seem to be absent in the Thermosbaenacea (Fryer 1965). In the Anaspidacea only one inferomedianum is present. According to Wallis and Macmillan (1998) it represents the anterior inferomedianum, and there is no separate inferomedianum arising in the pylorus. There is also no atrium in the Anaspidacea. Within the Euphausiacea, at least Bentheuphausia amblyops possesses an inferomedianum posterius; in other species it is more reduced (Suh and Nemoto 1988). Kobusch (1998) summarized the number of secondary filter grooves throughout the Malacostraca. Taxa without an inferomedianum posterius are scored as inapplicable.
[73] Formation of the midgut: (0) by entoderm; (1) at the border between stomodaeum and proctodaeum
[74] Entoderm: (0) unpaired entoderm plates; (1) paired entoderm plates
[75] Dorsal caeca: (0) absent; (1) present. The short midgut of many malacostracan taxa is formed by the entoderm which is generated either in a one-phased or two-phased gastrulation. In Isopoda, Tanaidacea, and Thermosbaenacea, however, the midgut is formed at the border between ectodermal stomodaeum and proctodaeum (see Zilch 1974); but only in the Isopoda the gut tube seems to be entirely ectodermally derived (Brusca and Wilson 1991). Siewing (1953) mentioned a syncytial midgut as a potential synapomorphy of Cumacea, Tanaidacea and Isopoda. However, according to Wägele (1989) this ‘syncytial midgut’ is nothing more than the proctodaeum that displaced the midgut.
The entoderm-plates generate the midgut-gland and/or the midgut in malacostracans. According to Zilch (1974) unpaired entoderm plates can be found in Leptostraca, Stomatopoda, Anaspidacea, Euphausiacea, Dendrobranchiata, Caridea, and Reptantia; paired entoderm plates occur in Mysida, Amphipoda, Thermosbaenacea, Tanaidacea, and Isopoda.
Dorsal caeca are – similar to the midgut glands – extensions of the dorsal part of the midgut. Dorsal caeca seem to be missing in Leptostraca (Siewing 1956). There are no dorsal caeca in Cumacea, Tanaidacea, and Isopoda (Siewing 1953; Wägele 1989).
Sperm characters:
The following five characters deal with the morphology of the spermatozoa and spertmatophores. We follow Jamieson (1991), Medina (1995) and Medina et al. (1998). Sperm morphology was also included in the data matrix of Schram and Hof (1998).
[76] Sperm acrosome: (0) present; (1) absent
[77] Sperm nuclear membrane: (0) present; (1) absent, chromatine diffuse
[78] Sperm microtubular arms or spikes: (0) absent; (1) present
[79] Cross striated perforatorium (possibly centriolar root homologue): (0) absent; (1) present
[80] Spermatophore: (0) none; (1) present. Most representatives of the Euphausiacea possess spermatophores. The only known exception is Bentheuphausia amblyops. This species is the putative sister group to all other Euphausiacea. Therefore, we scored the Euphausiacea (0/1). A spermatophore is missing in the Stomatopoda and in the peracarid taxa. One remarkable exception has been described within the Mysida (Wittmann 1982).
[81] Brood care: (0) none; (1) brood care with thoracopods without feeding by the mother; (2) brood care attaching the eggs to the pleopods; (3) brood care using a dorsal brood pouch; (4) brood care using a marsupium formed by oöstegites; (5) brood care using elongated first pleopod. The brood care using a marsupium is already implied in character [27]. We included this character state to get a complete list of brood care modes in the Malacostraca. Most Dendrobranchiata and Euphausiacea release their eggs into the water column and show no brood care. However, among the Dendrobranchiata the representatives of Lucifer and among the Euphausiacea the representatives of Nematoscelis, Nyctiphanes, Stylocheiron, and Pseudeuphausia carry their eggs for a short period (Zimmer and Gruner 1956; Gruner 1993; Lindley 1997). Lucifer spp. attach the eggs to posterior thoracopods whereas the mentioned taxa of the Euphausiacea form brood sacs. In both cases the mode of brood care has probably evolved within the groups.
[82] Development: (0) hatching at a larval stage; (1) direct development. The most general difference in the development in the Malacostraca is the ‘direct development’ versus a ‘development with a free larval stage’. We find both in the Reptantia and Caridea. There is no serious problem claiming the larval development as being part of the ground pattern of both groups (both taxa scored 0). Direct development in these taxa has evolved several times independently, e.g. in correlation with the transition into freshwater life (e.g. Scholtz 1999).
[83] Free living nauplius larva: (0) present; (1) absent. This character is only applicable for taxa with free larvae. Only in the Euphausiacea and the Dendrobranchiata the larvae hatch as a nauplius. In Stomatopoda, Bathynellacea, Amphionidacea, Caridea, Stenopodidea, and Reptantia they hatch as a zoea or zoea-like larva (with the exception of the species with direct development, see character 82).
[84] Development of appendages: (0) advanced development of anterior head appendages; (1) continuous anteroposterior decrease in the degree of appendage formation. This character refers to the free living nauplius larvae and to the so-called egg-nauplius of malacostracans with a hatching stage later than the nauplius (zoea larva or direct development). Nauplius and egg-nauplius are characterized by an advanced morphogenesis of the segments of the first and second antennae and of the mandibles. There is a distinct gap in the degree of differentiation between these naupliar segments and the postnaupliar segments (Dohle and Scholtz 1988). In the Mysida, only the two antennae show an advanced development but not the mandible (Manton 1928; Scholtz 1984). Nevertheless, we scored the Mysida (0). In some groups such as Amphipoda (Weygoldt 1958; Scholtz 1990; Meschenmoser 1996), Isopoda (Strömberg 1967; Meschenmoser 1996), Tanaidacea (Scholl 1963; Dohle 1972; Meschenmoser 1996) and Cumacea (Dohle 1970, 1976; Meschenmoser 1996) this gap does not exist and there is a continuous antero-posterior degree of differentiation (Dohle and Scholtz 1988; Scholtz 2000).
[85] Cleavage: (0) superficial cleavage; (1) mixed cleavage; (2) total cleavage. The superficial cleavage is characterized by the absence of membranes between the early cleavage products and by a blastoderm stage with a central yolk mass. The total cleavage is a holoblastic cleavage with true blastomeres and a hollow blastula stage. Mixed cleavage combines aspects of both cleavage types (Fioroni 1970; Scholtz 1998). Only Dendrobranchiata, Euphausiacea, Amphipoda, and Anaspidacea (and some parasitic isopods) show a total cleavage. The cleavage is mixed in the only thermosbaenacean species studied, Thermosbaena mirabilis Monod 1924 (see Zilch 1974). In the Stenopodidea, the cleavage seems to be superficial (Korschelt 1944); Caridea and Reptantia show a variety of superficial and mixed cleavage modes (see Anderson 1973); both taxa were scored (0/1).
[86] Number of ectoteloblasts: (0) 19; (1) variable; (2) none. Ectoteloblasts are large cells in the growth zone of the embryonic germ band giving rise to the material of most post-naupliar segments. The invariant number of 19 ectoteloblasts has been reported for most malacostracan groups (Dohle 1972; Scholtz 1984; Scholtz 2000). The only exceptions found are the Isopoda, Cumacea, Mysida, and Tanaidacea with a variable number of between 14 and more than 20 ectoteloblasts (Dohle 1972; Scholtz 1984). Within the Reptantia the freshwater crayfish show a variable number of about 40 ectoteloblasts (Scholtz 1993). However, this is clearly derived and the reptantian ground pattern shows 19 ectoteloblasts (Scholtz and Richter 1995). Amphipoda do not possess ectoteloblasts at all (Bergh 1894; Scholtz 1990).
[87] Arrangement of ectoteloblasts: (0) forming a ring around the caudal papilla (giving rise to embryonic ventral and dorsal material); (1) forming a transverse row (only the ventral side of the embryo is formed by ectoteloblasts and the dorsal side is closed much later in development). If present, the ectoteloblasts are either arranged in a complete circle surrounding the caudal papilla or they form a transverse row. In the first case, they give rise to embryonic ventral and, from a certain stage on, dorsal material, whereas in the latter case, only the ventral side of the embryo is formed and the dorsal side is closed much later in development. Also, only the ventral side is formed by the growth zone in the Amphipoda (Weygoldt 1958; Scholtz 1990). Thus, although ectoteloblasts are absent in the Amphipoda we scored this group (1) for this character.
[88] Early embryo (nauplius larva): (0) ventrally folded; (1) with a dorsal fold. This character has been proposed by Siewing (1951, 1953). In most malacostracan embryos, a transverse ventral groove forms in the thoracic region and the posterior embryonic parts including the growth zone are folded towards the anterior and ventral forming a caudal papilla (Dohle 1972; Scholtz 1984). Even in the free-living nauplius of Dendrobranchiata and Euphausiacea the growth zone is bent downwards. In Cumacea (Dohle 1970, 1976), Isopoda (Strömberg 1967), Mictacea (Bowman et al. 1985), and Tanaidacea (Scholl 1963) this ventral groove is not formed but the germ band grows straight around the egg surface. An indention occurs only on the dorsal side in the extra-embryonic region. Nothing is known with this respect from the Spelaeogriphacea.
[89] Yolk distribution in the embryo: (0) posterior part of the embryo contains no yolk; (1) posterior part of the embryo contains yolk. This character is independent from the occurrence of a ventrally or dorsally folded embryo. All dorsally folded embryos contain yolk in their hind region. Although the embryos in the Mysida, Amphipoda and Thermosbaenacea are folded ventrally the posterior region is filled with yolk (Zilch 1974; Scholtz 1984). This is not the case in the other malacostracans where the embryonic development is known.
[90] Number of thoracic appendages in hatchling: (0) eight; (1) seven; (2) six. This character is scored as unordered because there is no reason to assume that the condition in the Thermosbaenacea where the hatchlings possess only six thoracopods is derived from the ‘manca’ stage with seven thoracopods in Mictacea (Bowman et al. 1985), Cumacea, Tanaidacea, and Isopoda (Siewing 1956). Again, nothing is known about the hatchlings of the Spelaeogriphacea. This character is only applicable for taxa with direct development.
[91] Embryonic dorsal organ: (0) present; (1) absent. The embryonic dorsal organs we are referring to are not the sense or osmoregulatory organs of some crustaceans which are also called dorsal organs (Fioroni 1980; Martin and Laverack 1992; Meschenmoser 1996). Irrespective of the function which is unknown in detail (Meschenmoser 1996), from position and cell types found in the dorsal organs we suggest their homology within the malacostracans.
[92] Embryonic dorsal organ: (0) simple layer; (1) cup shaped. Within the dorsal organs of malacostracans two different types can be discerned. They are made up of either a simple layer of cells in Leptostraca, Anaspidacea, Caridea, and Reptantia or cup-shaped in Mysida, Cumacea, Amphipoda, Isopoda, and Thermosbaenacea (Meschenmoser 1996). This character is inapplicable for taxa without a dorsal organ.
[93] Transient paired lateral organs: (0) absent; (1) present. The embryonic transient lateral organs apparently fulfil a function similar to the embryonic dorsal organs (Meschenmoser 1996). Proposed phylogenetic relationships within the Isopoda (Wägele 1989; Brusca and Wilson 1991) imply that the saddle-like embryonic organ of oniscids is derived from a set of lateral organs and a dorsal organ found in other Isopoda (Meschenmoser 1996). Meschenmoser (1996) expresses some doubt concerning the homology of the lateral organs of the Mysida, on the one hand, and Isopoda and Tanaidacea, on the other hand, because they appear in a different region. We treat them as the same organs in our study.
All characters are listed in our data matrix (Appendix).
Monophyly of the Malacostraca
For the polarization of the character states we took not only the remaining crustacean taxa but also the Insecta into account.
At least five independent characters support the monophyly of the Malacostraca.
1 Constant number of segments: eight thoracomeres and seven pleomeres (e.g. Calman 1909)
The representatives of the Leptostraca have seven pleomeres whereas other Malacostraca have six. Recently, Olesen and Walossek (2000) found an additional anlage of a putative eighth pleon segment in one Nebalia species. Also in several eumalacostracans an anlage of at least a seventh pleomere has been described (e.g. Manton 1928 for a representative of the Mysida). Using a molecular marker for the segmentation gene engrailed, Scholtz (1995) found anlagen of three additional segments in the posterior region of crayfish embryos. This can be seen as support for an idea put forward by Lauterbach (1975) who suggested that the seventh pleomere of the Leptostraca without appendages represents the vestigium of the entomostracan abdomen. This view is also supported by recent Hox gene data which suggest that the thorax of branchiopods corresponds to the thorax and pleon in malacostracans (Abzhanov and Kaufman 2000).
2 Defined position of the genital openings in the sixth thoracomere in females, in the eighth thoracomere in males
In the malacostracan ground pattern the genital openings are on the coxae in both sexes (e.g. Calman 1909).
3 Differentiation of the posterior part of the foregut to a proventriculus with oesophagus, stomach chamber, and funnel region
Kobusch (1998) mentioned subunits of the eumalacostracan foregut, which are also found in the Leptostraca. Such a proventriculus is unknown in the other Crustacea.
4 Major part of the post naupliar germ band derived from a ring of exactly 19 ectoteloblasts (e.g. Dohle 1972)
The growth zone of malacostracan embryos shows specialized large cells called teloblasts. These teloblasts produce the ectodermal and mesodermal material for most post-naupliar segments. In many malacostracan taxa the teloblasts are arranged in a ring surrounding the caudal papilla. In the Leptostraca, Stomatopoda, Anaspidacea, Dendrobranchiata, Caridea, Reptantia, Thermosbaenacea, and Euphausiacea there is a ring of 19 ectoteloblasts and an inner ring of eight mesoteloblasts in a specific arrangement. Some exceptions to this pattern are found in the freshwater crayfish within the Reptantia and in the Peracarida (Dohle 1972; Scholtz 1984, 2000). A pattern like this has not been found in any other crustacean group. Only in the Cirripedia have some (less than 10) ectoteloblasts which never form a ring been reported (Anderson 1973). Anderson (1973) also describes the occurrence of eight mesoteloblasts in cirripedes but their relative position is somewhat different.
5 Biramous first antenna
Most malacostracan taxa possess a biramous first antenna (see character 11). In Leptostraca and Bathynellacea, one ramus is represented by a scale-/knob-like structure. Only in the groundpattern of the Isopoda is the first antenna uniramous. The triramous first antenna in Stomatopoda is an autapomorphy of this taxon. Outside the Malacostraca the first antenna is uniramous (Insecta, Myriapoda, ‘Entomostraca’), with the exception of the Remipedia. Whether the condition in the Remipedia represents a homology or a convergence with that of the Malacostraca is unclear.
Elofsson and Dahl (1970) suggested as a further apomorphy of the Malacostraca the presence of a chiasma between the two optic neuropiles Laminaganglionaris and Medullaexterna (see also Dahl 1987; Wägele 1992). However, such a chiasma is also present in insects, scutigeromorph myriapods and chelicerates (Hanström 1928). Under the assumption of homology of this chiasma, the absence of the chiasma in ‘entomostracan crustaceans’ has to be interpreted as apomorphic.
Results of the cladistic analysis
Our first analysis, with all characters – except character [14] – scored as unordered and equally weighted, resulted in, respectively, 6 (‘amb-’) and 10 (‘amb=’) most parsimonious cladograms of 237 steps. Six of the 93 characters are uninformative in a cladistic sense. The CI is 0.55 (exclusive of the uninformative characters: 0.53), the RI 0.64 (exclusive of the uninformative characters: 0.64). The strict consensus tree is given in Fig. 5. The Peracarida (including the Thermosbaenacea), the Syncarida and the Decapoda are supported as monophyletic. The Stomatopoda is the sister group to the remaining eumalacostracan taxa, which implies that the Caridoida is also supported. The Eucarida is not monophyletic, the Euphausiacea is the sister group to the Peracarida (including the Thermosbaenacea), and the Syncarida is the sister group to both. The Decapoda s.l. (including the Amphionidacea) is the sister group to the remaining Caridoida. The differences between the cladograms concern the Peracarida and the Decapoda. In all cladograms Amphipoda, Spelaeogriphacea, Cumacea, Tanaidacea, Mictacea, and Isopoda form a clade. The Mysidacea (Mysida + Lophogastrida) is monophyletic. The position of the Thermosbaenacea varies. It is either the sister group of the clade Amphipoda and Spelaeogriphacea, Cumacea, Tanaidacea, Mictacea, Isopoda (Fig. 6, with different relationships within the sister taxon of the Amphipoda) or of the entire Peracarida (Fig. 7). Within the Decapoda the differences concern the Pleocyemata (Caridea, Stenopodidea, Reptantia). The Reptantia is either the sister group to the Caridea (Fig. 6) or to the Stenopodidea (Fig. 7).
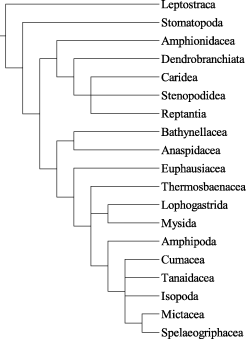
Strict consensus tree of 6 (‘amb–’) most parsimonious cladograms with a length of 237 steps. The strict consensus of the 10 (‘amb =’) tress is identical
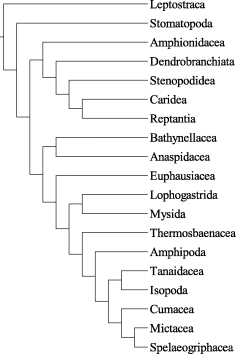
Phylogenetic relationships of the Malacostraca. One of the most parsimonious trees of the initial analysis showing one of two alternative relationships within the Decapoda and one of several alternatives within the Peracarida
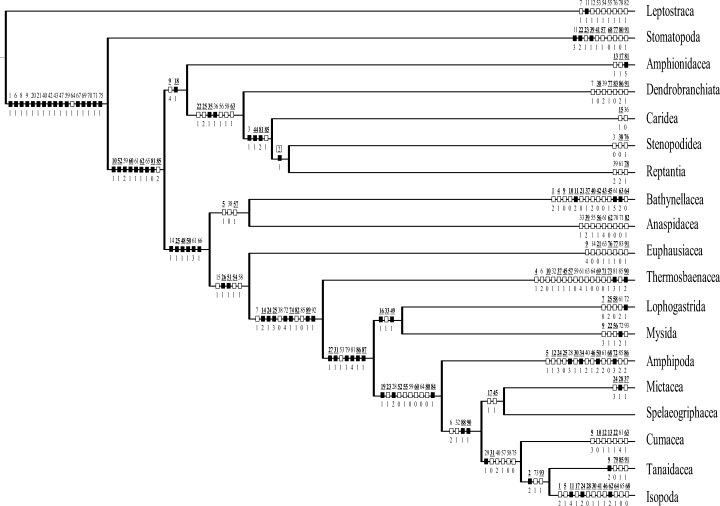
Phylogenetic relationships of the Malacostraca as favoured by the authors. One of the most parsimonious trees of the initial analysis. All character states under preferred optimizations are labelled on the cladogram (unambiguous apomorphies are depicted in bold and are underlined). Black hashmarks = non-homoplastic character states; white hashmarks = homoplastic character states. This cladogram is identical to one of the two with a maximum of total fit under implied weighting (PEE-WEE, constant of concavity k=3, 4, 5, 6)
Four analyses under implied weighting with PEE-WEE (constant of concavity k=3, 4, 5, 6) resulted in the same two trees each (which differ only with regard to the sister group of the Reptantia) that are identical with two of the six, respectively, 10 most parsimonious trees of the initial analysis. We favour these two trees as phylogenetic hypotheses for the Malacostraca (Fig. 7 shows one of them). Using the more extreme assumptions of a constant of concavity k=1, 2 which imply a stronger downweighting of homoplastic characters than the other constants resulted, respectively, in two and one cladograms. The major difference in comparison to the other cladograms is the position of the Bathynellacea. Using lower k-values, the Bathynellacea is the sister group of all other Eumalacostraca (including the Stomatopoda). This implies that the monophyly of the Syncarida (Bathynellacea + Anaspidacea) supported by the other analyses is mainly based on homoplastic characters.
The basal split between Leptostraca and Eumalacostraca is not supported by any unambiguous (in a cladistic sense, i.e. independent from the mode of optimization) character changes. However, this is merely due to the usage of only one operational out-group (in the sense of Nixon and Carpenter 1993). Technically one cannot distinguish between autapomorphies and plesiomorphies of the operational out-group, respectively, of the in-group. However, polarity decisions can be made using out-group comparison in the ‘traditional sense’.
Based on out-group comparison we suggest the following characters as support for a monophyletic Eumalacostraca (for additional characters see Fig. 7):
Character complex 1: Six pleon segments [1], last pleopods transformed into uropods [40], the pleopods form a tailfan together with the flattened telson [42], precaridoid pleon musculature [59] and loss of the furca [43]. The character states of this character complex are not found in any other crustacean or arthropod.
Character complex 2: Proventriculus consists of lateralia [67], inferomedianum anterius [69], inferomedianum posterius [70], atrium [71] and at least one dorsal caecum [75]. These character states are not known from the Leptostraca, but the complete absence of a proventriculus in other Crustacea makes the interpretation difficult.
Character 3: Univalved carapace [6]. The polarization of this character depends on the homology of the carapace in Crustacea. Accepting that the bivalved carapace in Diplostraca and Ostracoda is homologous to the one in the Leptostraca, the univalved carapace could be suggested as an apomorphy of the Eumalacostraca.
Character 4: Stenopodous limbs [20]. The polarization of this character depends on the homology of the phyllopodous limbs in Crustacea, in particular between the Branchiopoda and Leptostraca. Otherwise it cannot be excluded that the phyllopodous limbs in the Leptostraca represent an autapomorphy of this taxon (see Spears and Abele 1999).
Character 5: Three optic neuropiles [47]. A third optic neuropile is missing in all other Crustacea. Under the assumption that this structure in insects is convergent, a third optic neuropile could be used as support for the Eumalacostraca.
Character 6: First thoracopod transformed into a maxilliped [21]. Some other crustaceans also possess a maxilliped (e.g. Copepoda, Remipedia, Mystacocarida) but the homology of this character is doubtful and should be tested in a comprehensive analysis of crustaceans.
It is obvious that some of these characters can be questioned. Nevertheless, the total support for the Eumalacostraca is high and there is no or only minor support for other possible relationships, such as a sister-group relationship between Stomatopoda and Leptostraca.
Discussion
Major phylogenetic relationships within the Malacostraca
Our analysis supports Calman’s (1909) classification and Siewing’s (1956) phylogeny in some major points. The Stomatopoda is the sister group to the remaining taxa, the Peracarida is monophyletic and the Syncarida found some support in most of our analyses. There is no indication for a position of the Stomatopoda close to the Eucarida in our analyses (but see Schram and Hof 1998; Wills 1998). Instead, the Caridoida is well supported (see also Hessler 1982a). The caridoid pleon musculature, the tailflip escape reaction, a clear zone in the compound eyes, as well as the aorta descendens of the circulatory system are valuable apomorphies of this taxon. The simple pleon musculature and the absence of a tailflip escape reaction in some peracarid taxa has to be interpreted as derived. Concerning the clear zone in the compound eyes, a convergence between the Decapoda and the remaining taxa based on the different eye physiology cannot be excluded (Richter, 2001). Further apomorphies which support the Caridoida in our analysis are more problematic. In our opinion, it seems to be more probable that the reduction of the pleonic part of the heart has happened several times than that it has reappeared in the Anaspidacea. Also, the two developmental characters – total cleavage, and loss of the brood care with the thoracopods – can be questioned. It has been shown recently that two taxa without brood care – Euphausiacea and Dendrobranchiata – can be interpreted as derived with respect to some developmental characters (Scholtz 2000). And in our analysis, the free nauplius larvae, which is often suggested as a general crustacean character (e.g. Müller 1864), seems to be an autapomorphic character of each of the two taxa.
Within the Caridoida two clades are supported – the Decapoda s.l. and another taxon which comprises the remaining taxa. For the latter taxon the name Xenommacarida was suggested (Richter 1994b, 1999; Ax 1999). This taxon is mainly based on two apomorphic characters which concern the ommatidial structure – the bipartite crystalline cones, and the loss of the two main cone cell processes. Other characters which support this clade are the reduction of the number of pairs of ostia to two (and the reverse evolution to three ostia in Lophogastrida and Amphipoda) and a sternal artery which is divided into three branches. However, a polarization of the latter character is difficult. The interpretation of an undivided sternal artery as an apomorphy of the Decapoda is similarly plausible. The intrabasal articulation is only known in Anaspidacea, Lophogastrida, and Mysida (Hessler 1982b). Our analysis also suggests the presence of two epipodites as an apomorphy of the Xenommacarida. However, in our opinion two of the five decapod epipodites are homologous to the two found in the Xenommacarida. Therefore, we suggest the two epipodites as an additional apomorphy of the Caridoida. The additional epipodites/gills in the Decapoda and the Lophogastrida should be interpreted as convergences.
Within the Xenommacarida, the Syncarida is the sister group to the remaining taxa. We found some support for syncaridan monophyly in contrast to Wills (1998) and Schram and Hof (1998) who proposed the Bathynellacea as the sister group to all other Eumalacostraca – a position which is also a result of our analysis using a particular weighting in PEE-WEE. The absence of a cephalothorax and of any maxilliped in the Bathynellacea can be interpreted as evidence for this view. However, the missing carapace and the loss of the antennal gland support syncarid monophyly. The absence of a carapace in syncarids has been discussed controversially (Calman 1909; Newman and Knight 1984; Dahl 1991; Watling 1999). According to our analysis, the interpretation of a carapace loss as a derived character is most parsimonious.
Our analysis supports almost all of Calman’s ‘divisions’– with one exception – the Eucarida. According to our analysis, the Euphausiacea is the sister group to the Peracarida and the Thermosbaenacea. This is supported by the distally displaced nuclei of the accessory cone cells in the ommatidia, by the one branch of the epipod which is carried under the thorax, the loss of the dorsal frontal organ, and more questionably, the larval lacinia mobilis (which is also found in some representatives of the Caridea). In the nineteenth century, the Schizopoda concept implied a close relationship between the Euphausiacea and the Mysidacea. The closer relationship between Mysidacea and the remaining Peracarida was not recognized at that time. A re-introduction of the Schizopoda was suggested by Land (1981) based on eye physiology. However, this special eye type – the refractive superposition eye – which in the 1980s was only known in Euphausiacea and Mysida – is now also known from Anaspidacea, some Dendrobranchiata and some Reptantia (Nilsson 1990).
The monophyly of Peracarida and Thermosbaenacea together is comparatively well supported. The question of whether the Thermosbaenacea is part of the Peracarida or not will be considered below. The major split into ‘caridoid’ malacostracans and the remaining taxa as suggested by Watling (1981, 1983, 1999) seems not to be justified. Furthermore, a position of the Isopoda outside of the Peracarida (see Nylund et al. 1987) finds no support. Some of the characters which support the monophyly of the Peracarida (and Thermosbaenacea) have been recognized as such for a long time: the lacinia mobilis in the adults, and the epipodite of the maxilliped which produces a respiratory current. The cross-striated perforatorium in the sperm and the loss of the nauplius eye also support the Peracarida but the character states are as yet unknown in the Thermosbaenacea. We see no reason to question the homology of any of these characters. In addition, some developmental characters support peracarid monophyly. These are, in addition to the unspecific character ‘direct development’, the paired entoderm plates generating the midgut gland, and the posterior part of the embryo containing yolk. Therefore, no separation of the Mysida and Lophogastrida from the remaining Peracarida, and accordingly no closer relationships of these taxa to other ‘caridoid taxa’ is supported.
One unresolved question concerns the position of the Thermosbaenacea. Former approaches favoured a sister-group relationship between Thermosbaenacea and Peracarida (Siewing 1956, 1960, 1963; Sieg 1984; Pires 1987; Richter 1994b; Ax 1999; Kobusch 1999), between Thermosbaenacea and Spelaeogriphacea–Tanaidacea–Isopoda (Fryer 1965), or between Thermosbaenacea and Mysidacea (Watling 1981) or a close relationship between Spelaeogriphacea–Cumacea–Tanaidacea and Thermosbaenacea, united as Brachycarida (Watling 1983). Later, Watling (1999) added the Mictacea to this taxon. Our analysis favours a sister-group relationship between Thermosbaenacea and Peracarida (using implied weighting), but also a position inside the Peracarida is represented in some of the most parsimonious cladograms. In particular, the embryological data favour a sister-group relationship between Peracarida and Thermosbaenacea. The latter possesses the plesiomorphic condition concerning the number of ectoteloblasts (19) and their arrangement (ring). If the Thermosbaenacea were placed within the Peracarida, the variable number of ectoteloblasts and their arrangement in a transverse row would be convergent in the Mysidacea and in the remaining Peracarida. On the other hand, it is similarly questionable to interpret the conditions in the Thermosbaenacea as reversals. Moreover, the different kind of brood care of the Thermosbaenacea suggests a position outside the Peracarida. There is no reason to derive the thermosbaenacean dorsal brood pouch from the peracaridan ventral marsupium as did Fryer (1965), or to propose a convergent evolution of oöstegites in Mysidacea and the remaining Peracarida (Watling 1999). Ax (1999) suggested the name Neocarida for a monophylum with Peracarida and Thermosbaenacea as sister-groups.
Within the Peracarida, Schram (1981, 1984, 1986) proposed a taxon Hemicaridea comprising the Cumacea, Tanaidacea, and Spelaeogriphacea and a taxon Edriophthalma (actually introduced by Leach in 1814) comprising Amphipoda and Isopoda. Recently, Schram and Hof (1998) argued again for such a sister-group relationship. However, characters such as sessile eyes, coxal plates (according to Wägele (1989) and Brusca and Wilson (1991) coxal plates evolved within Isopoda), uniramous first antenna (many amphipods possess a biramous first antenna), absence of exopods of the thoracopods (the exopods are only vestigial in the Tanaidacea) are questionable, and previously Siewing (1951) suggested that similarities between Amphipoda and Isopoda are convergences. On the other hand, a close relationship between Cumacea, Tanaidacea and Isopoda (Mictacea and Spelaeogriphacea not considered), i.e. Hessler’s (1983)‘mancoids’, is supported by the dorsally folded embryo, the manca stage and the similar formation of the midgut. In our analysis a sister-group relationship between Amphipoda and Isopoda is not supported. Our results are summarized in Fig. 8.
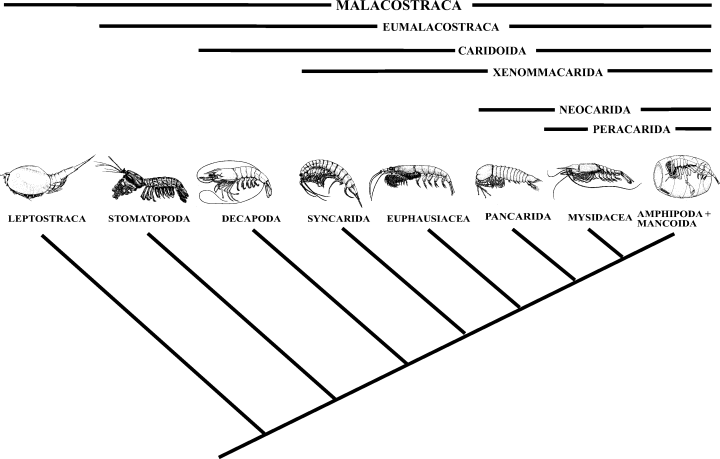
Summary of our results. We suggest that the Decapoda should also comprise the Amphionidacea. The Decapoda s.str. comprises the sister groups Dendrobranchiata + Pleocyemata; the Syncarida comprises the sister groups Bathynellacea + Anaspidacea. No new taxon names are introduced here. Already published names are used for the convenience of the reader. Drawings of the representatives of the taxa according to Cannon (1960) and Gruner (1993)
Although we favour particular relationships, we have to concede that other suggestions also seem justified. Too many contradictory characters have to be considered and at least at the moment there is no single ‘most parsimonious’ or ‘most plausible’ explanation for the interpretation of the malacostracan disparity. A recent analysis of malacostracan phylogeny based on 28S rDNA data supports the view that Euphausiacea and Decapoda are not sister taxa (Jarman et al. 2000).
Footnotes
Acknowledgements
We are grateful to Dr J.W. Wägele, Dr J. Olesen, Dr F. Schram, Dr R. Meier, Dr J. Dunlop and Dr H. Enghoff for critically reading and improving this manuscript. This does not imply that they agree with all of our conclusions. We also thank F. Alwes for help with the references list. Parts of this investigations were supported by grants of the Deutsche Forschungsgemeinschaft (Scho 442/5–1) and of the Training and Mobility of Researchers (TMR), Access to Large Scale Facilities (LSF) program which allowed studies at the Kristineberg Marine Station, Sweden.
Appendix
Character matrix for the Malacostraca.





