Vitamin C Plasma Concentrations and Leg Weakness in the Forelegs of Growing Pigs
Abstract
Four litters (41 pigs) of cross-bred pigs were studied from 6 to 26 weeks of age. Blood samples were collected at 6, 13, 21 and 26 weeks of age and analysed for contents of vitamin C, calcium (Ca), inorganic phosphorus (P) and alkaline phosphatase (ALP). The pigs were examined clinically for foreleg weakness at the ages of 21 and 26 weeks. At the age of 26 weeks the pigs were slaughtered and the right forelegs were examined macroscopically and selected samples were collected for radiological, histological and ultrastructural examination. The prevalence of foreleg lesions was high, with lesions of dyschondroplasia of the distal growth plate of the ulna in 30 pigs, synovitis of the elbow joint in 24 pigs and osteochondritis dissecans of the elbow joint in 25 pigs. At the ages of 21 and 26 weeks, five pigs had evidently crooked forelegs and 14 pigs (age 21 weeks) and 25 pigs (age 26 weeks) had mildly deformed forelegs. The serum levels of Ca, P and ALP were within normal values for growing–finishing pigs. The range of vitamin C concentrations in plasma showed a wide difference (7.1–49.8 μmol/l) but was not associated with deformed forelegs. The serum concentrations of Ca, P and ALP and the plasma concentration of vitamin C differed significantly (P=0.05) between age groups and there was a significant (P=0.001) positive correlation between the levels of vitamin C in plasma and the serum levels of ALP at 6 weeks of age. The aim of the present study was to determine if there was any association between the plasma levels of vitamin C and the extent of crooked or deviated forelegs in growing–finishing pigs. We could not find a vitamin C deficiency during the study and no association between low levels of vitamin C in plasma and the presence of deformed forelegs of these 40 pigs.
Introduction
Leg weakness, present in both hindlegs and forelegs, is an important problem in fast-growing slaughter pigs (Jørgensen, 1995) and the breeding stock (Dewey et al., 1992). Osteochondrosis (OC)/osteochondritis dissecans (OCD) and dyschondroplasia are the most important causes of leg weakness in grower and breeder pigs (Reiland, 1978a; Dewey et al., 1993). However, no clear association has been found between OC/dyschondroplasia and leg weakness in pigs (Thurley, 1969; Grøndalen, 1974a; Pointillart and Gueguen, 1978; McCaw and Mitten, 1980; Jørgensen, 1995; Jørgensen et al., 1995; for review see Hill, 1998). The prevalence of OC and/or dyschondroplasia in pigs at slaughter may reach 100% (Grøndalen, 1974b; Reiland, 1978b; Nakano et al., 1979). Leg weakness can be manifested as crooked and/or bent forelegs (Grøndalen, 1974c; Reiland, 1978c; Nielsen and Vinther, 1982). Deformed forelegs have been associated with OC/OCD (Jørgensen et al., 1995), ulnar dyschondroplasia (Reiland, 1978c), Mycoplasma hyosynoviae arthritis (Lawrisuk et al., 1987), genetic predisposition (Draper et al., 1988; Rothschild and Christian, 1988; Jørgensen and Vestergaard, 1990), contraction of the flexor tendons and vitamin C deficiency (Nielsen and Vinther, 1982; Cleveland et al., 1987; Draper et al., 1988).
Vitamin C, or ascorbic acid (AA), is essential in collagen formation (Kipp and Schwartz, 1989) and vitamin C deficiency results in scurvy, characterized by an abnormal extracellular matrix (ECM) of the connective tissues (Franceschi et al., 1994) and a disrupted endochondral ossification (Wegger and Palludan, 1994; Kipp et al., 1996; Schrenzel et al., 1996).
Pigs are assumed to synthesize adequate ascorbic acid to meet their metabolic needs, hence, scurvy has not been described in commercial pigs. However, a genetic loss of the ability to synthesize vitamin C has been found in a Danish mutant strain of pigs named Osteogenic Disorder pigs (OD-pigs) (Jensen et al., 1983). Skeletal lesions typical of scurvy were described in OD-pigs deprived of dietary vitamin C (Palludan and Wegger, 1988; Wegger and Palludan, 1994). On the other hand, no beneficial effects on bone metabolism (Pointillart et al., 1997), osteochondrotic (Nakano et al., 1983; Grøndalen and Hanssen, 1984)/ulnar dyschondroplastic lesions (Walker et al., 1966) and leg weakness (Strittmatter, 1977) have been observed in vitamin C-supplemented commercial pigs.
The commercial pig synthesizes vitamin C throughout its life (Braude et al., 1950; Lund et al., 1980; Pointillart et al., 1997). However, this does not imply that sufficient amounts are synthesized under different conditions, such as early weaning or at a high environmental temperature (Riker et al., 1967; Mahan et al., 1994; de Rodas et al., 1998).
The purpose of the present study was to evaluate plasma levels of vitamin C in growing–finishing commercial pigs and to identify any relationships between the plasma levels of vitamin C and the extent of deformed forelegs.
Materials and Methods
Animals and sampling
Four litters (19 females and 22 castrated males) of cross-breed (Norwegian Landrace–Duroc × Swedish Landrace–Yorkshire) pigs, weaned at 5 weeks of age were included in the study. Each litter was housed in a concrete-floored pen with minimal straw as bedding. The pigs were kept in the same pen from birth to slaughter. The pigs were fed twice daily with equal rations and food. The declared food composition is shown in Table 1.
Venous blood samples were collected at 6, 13, 21 and 26 weeks of age. Plasma was immediately stabilized with 5% metaphosphoric acid (MPO) and kept at −20°C until analysis for vitamin C. Serum samples were frozen until analysed for calcium (Ca) (methylthymol blue reaction, UNI-kit II; Roche Basel, Switzerland), inorganic phosphorus (P) (phosphomolybdate test, unimate 7; Roche) and alkaline phosphatase (ALP) (kinetic colourimetric test, unimate; Roche). Food samples obtained when the animals were 13, 21 and 26 weeks old were minced and stabilized with 5% MPO and kept at −20°C for vitamin C analysis by high-pressure liquid chromatography (Mœland et al., 1999). Homogenized food samples were analysed for Ca and P concentrations, using inductively coupled plasma spectrometry (Jobin Yvon JY 50P, Instruments S.A., division Jobin Yvon, Longjumeau, France), on dried residues from wet ashed samples (Foss-Tecator AB, Höganäs, Sweden).
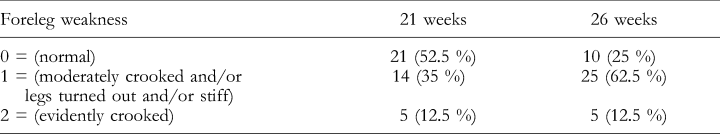
A clinical evaluation of the forelegs was made by means of a subjective scoring system (normal=0; moderately crooked and/or legs turned out and/or stiff=1; evidently crooked=2), when the animals were 21 and 26 weeks of age.
All animals were slaughtered by stunning and bleeding at the age of 26 weeks. At slaughter, the live body weight was estimated by multiplying the carcass weight by a factor (1.34), and the daily live weight gain (from birth to slaughter) was calculated using this estimated live body weight.
Morphological examination
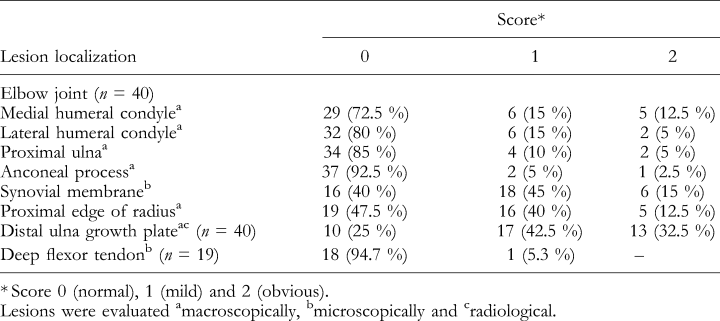
Immediately after slaughter the right foreleg was removed and stripped of muscles. The deep digital flexor tendon was examined grossly and small samples, 1 cm proximal to the tendon bifurcation, were immersed in 10% neutral formalin and 2% glutaraldehyde for histological (light microscopy) and ultrastructural studies (transmission electron microscopy). The shoulder, elbow and antebrachiocarpal joints were opened and the cartilage was examined grossly. Lesions in the elbow joint were scored macroscopically as 0 (normal), 1 (mild lesions) and 2 (obvious lesions). Score 2 included articular cartilage flaps (dissecans) and ulcers of distal humeral condyles and proximal ulna as well as fragmented anconeal process and severe thinning of the articular cartilage and bony depression of the craniomedial edge of the proximal radius.
Ulna and radius were radiographed in the dorso-ventral and latero-medial positions with a radiographic unit (Orbix, Siemens-Elema, Stockholm, Sweden) using a high-resolution film-screen system (Fuji Um Mammo, Fuji Ltd, Stockholm, Sweden). A focus-to-film distance of 90 cm was used, and the exposure data were 50 kV and 6.4 mA. Lesions in the distal growth plate of the ulna and radius were scored radiographically as 0 (normal), 1 (mild lesions), or 2 (obvious lesions). The lesions were classified as obvious (score 2), when microfractures, haemorrhages and myelofibrosis were also seen. A saggital section of the proximal ulna was examined for the presence of fragmented anconeal process (Bittegeko and Arnberg, 1996) and macroscopically scored as 0 (normal), 1 (mild lesions), or 2 (obvious lesions). Frontal sections of the distal growth plate of the ulna and radius were scored macroscopically as 0 (normal), 1 (mild lesions), or 2 (obvious lesions).
Selected samples from the articular cartilage, tendon, the synovial membrane of the elbow joint and the distal ulna, including the growth plate and metaphyseal bone, from seven, 19, 40 and 10 pigs, respectively, were fixed in 10% aqueous solution of buffered formaldehyde, embedded in paraffin, cut into approximately 6-μm sections and stained with haematoxylin & eosin (H & E) and Alcian-Periodic Acid Schiff, for light microscopy. The tendon and the distal growth plate of ulna, corresponding to material sampled for light microscopy, were cut into sections 1 × 1 × 3 mm and processed for the transmission electron microscope. The samples were prefixed in a 2% glutaraldehyde solution in 0.15 M sodium cacodylate buffer (pH 7.4) with 3 mM CaCl2, at room temperature for 10 h, rinsed in 0.1 M sodium cacodylate buffer (pH 7.4) with 0.1 M sucrose and 3 mM CaCl2, post-fixed in 1% osmium tetroxide in a 0.2-M phosphate cacodylate buffer for 2 h, dehydrated in ascending concentrations of ethanol and acetone and embedded in agar 100 resin (Agar Scientific Ltd, Essex UK). Semi-thin sections (1 μm) were cut with glass knives and stained with toluidine blue and ultrathin sections were cut with a diamond knife and placed on 200-mesh copper grids. The cartilage sections were counterstained with uranyl acetate and lead citrate, tendon sections being stained with phosphotungstic acid and uranyl acetate, and then examined in a Philips EM 420 electron microscope at 60 kV.
The synovial membrane from the elbow joint and the deep digital flexor tendon were histologically evaluated and scored as 0 (normal), 1 (mild lesions), or 2 (obvious lesions). The synovitis was classified as mild (score 1), when a mild vascularization and a few lymphocytes were found in the subintimal tissue. Obvious synovitis (score 2) was characterized by vascularization, fibrosis and a marked cellular infiltration, mostly perivascular, in the subintimal tissue of the synovial membrane.
Vitamin C assay
Vitamin C was determined in plasma by a fluorometric method (Deutsch and Weeks, 1965; Vuilleumier and Keck, 1989) with some modifications. The assay included enzymatic oxidation of ascorbic acid with ascorbate oxidase (E.C. 1.10.3.3.; Merck Darmstadt, Germany) to dehydroascorbic acid which then reacted with 1-ortho-phenylenediamine to the fluorescent compound quinoxaline (3-(1,2-dihydroxyethyl)furo[3,4-b]quinoxaline-1-one). The formed quinoxaline was measured on a spectrofluorometer (RF-1501, Shimadzu, Japan; λex 337 nm, λem 430 nm). Samples were thawed immediately before use and centrifuged at 700 g for 5 min. Ascorbate oxidase (3.4 units/10 ml), 500 μl in acetic acid-buffered sodium acetate (pH=6.2) 2.0 M and 100 μl of 1,2-phenylendiamine 5.5 mM in distilled water, were added to 500 μl of supernatant. The samples were mixed on a shaker, incubated for 5 min at 25°C and the fluorescence was measured within 45 min. Ascorbate standards were prepared in 5% MPO (Riedel de-Häen, Germany) and subjected to the same procedures as the samples. All solutions containing ascorbate were protected from light and analytical solutions were prepared fresh every day. Samples were analysed in duplicates and expressed as μmol ascorbic acid/l. All chemicals were of pro analysis quality and supplied by Sigma Chemicals (St. Louis, MO) unless otherwise stated.
Statistical analysis
The statistical analyses were carried out using the Statistical Analysis System (SAS Institute Inc., Cary, NC). The relationships between plasma levels of vitamin C and the serum concentrations of Ca, P and ALP and the correlations of the variations in plasma levels of vitamin C within animals at the age of 6, 13, 21 and 26 weeks were assessed by partial correlations, after correcting for the effects of sex and litter and their interactions (general linear model, GLM, procedure). The variation in plasma vitamin C levels (models 1 and 2) and in serum concentrations of Ca, P and ALP (model 1) were analysed with the MIXED procedure. Model 1 included the fixed effects of sex, age, litter and their interactions, the random effects of pig nested within the combination of sex and litter were also included in the model. Model 2 was used for separate analyses for each age group. In this model, the effect of sex, foreleg lesions and clinical leg weakness was included. The effect of litter was regarded as a random effect. The variation of the growth rate (from birth to slaughter at 26 weeks of age) was also analysed with the MIXED procedure, where the model included the effect of sex and the regression of the plasma levels of vitamin C at the age of 26 weeks.
Associations between lesions of the forelegs and between lesions and clinical leg weakness of the forelegs as well as between lesions of the forelegs and sex and between lesions of the forelegs and litter were performed using Fisher’s exact test (two-tailed).
The results are presented as least square means ± standard error of the mean (LSM ± SEM) unless otherwise indicated. A P-value (= 0.05) was considered significant.
Results
Animals
One animal died of an undetermined cause before 21 weeks of age. The live body weight at slaughter was 101.8 ± 13.8 kg and the daily weight gain (from birth to slaughter) was 0.55 ± 0.07 kg (mean ± SD).
At the ages of 21 and 26 weeks, five pigs (12.5%) were evidently crooked (Fig. 1) in the forelegs (score 2) and 14 (35%) and 25 pigs (62.5%) had mildly deformed forelegs, respectively (Table 2).
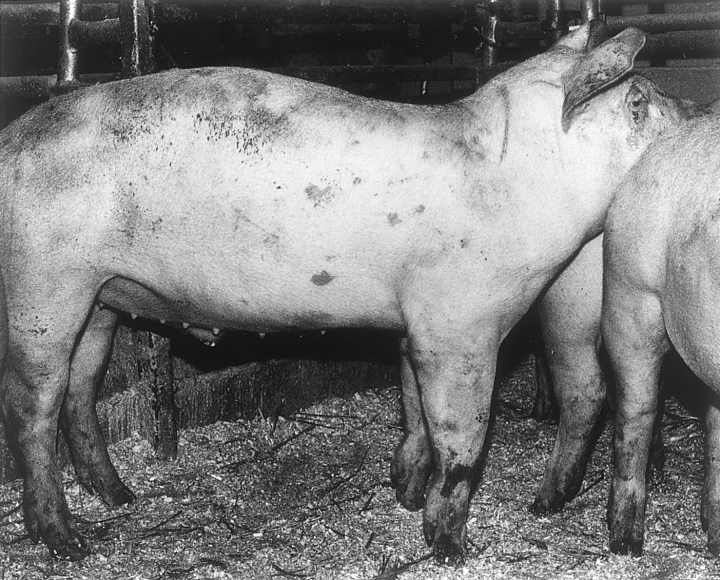
A finishing pig with evidently crooked forelegs (score 2).
Morphological examination
The number of pigs with lesions in the forelegs at the age of 26 weeks is shown in Table 3. Most lesions were observed in the growth plate of the distal ulna (30 pigs), the synovial membrane of the elbow joint (24 pigs), or the proximal edge of the radius (21 pigs). Fewer lesions were found in the articular cartilage of the medial (11 pigs), lateral (eight pigs) humeral condyles and the proximal ulna (six pigs).
No macroscopic changes were observed in the articular cartilage of the shoulder and antebrachiocarpal joints. Fragmentation of the anconeal process of the proximal ulna (score 2) was only found in one pig and no macroscopic changes were observed in the deep digital flexor tendons. A few areas of fibroblast proliferation could be seen in the tendon from one pig. At ultrastructural examination, regular, intact collagen fibres were present in all the samples.
OCD in the elbow joint, seen in 25 pigs, was characterized by invaginations, grooves and flaps of the articular cartilage of the distal humeral condyles and the proximal ulna. Thinning of the articular cartilage and/or bony depression of the craniomedial edge of the proximal radius (Fig. 2) were evident in five pigs and mild in 16 pigs. Microscopically, the lesions of the proximal radius were characterized by loss of articular cartilage replaced by a thin layer of connective tissue covering the subchondral bone.

The proximal part of the right radius and ulna. There is an obvious thinning of the articular cartilage with depression in the bone (arrow) of the craniomedial edge of the proximal radius (score 2).
Synovitis of the elbow joint was found in 24 pigs. The synovitis of the elbow joint showed a significant association with the articular cartilage lesions in the craniomedial edge of the proximal radius (P=0.05) and the OCD lesions in the lateral humeral condyle (P=0.001).
A thickened and irregular growth plate in the distal ulna was seen in 30 (75%) of the pigs at 26 weeks of age (Fig. 3). A significant correlation (Spearman rank correlation, r=0.97, P=0.001, data not shown) was found between the severity of radiological and macroscopically identified lesions of the distal growth plate of ulna, hence these parameters were combined into one. These lesions were microscopically characterized by an accumulation of hypertrophic-like chondrocytes projecting into the metaphyseal bone. Obvious lesions were present in 13 pigs (32.5%). The hypertrophic-like chondrocytes were ultrastructurally grouped in clusters with an extensive dilated endoplasmatic reticulum. Swollen mitochondria and lipid droplets were occasionally seen. Matrix calcification was not observed in the zone with accumulation of chondrocytes. No radiological or macroscopic lesions were present in the distal growth plate of the radius.
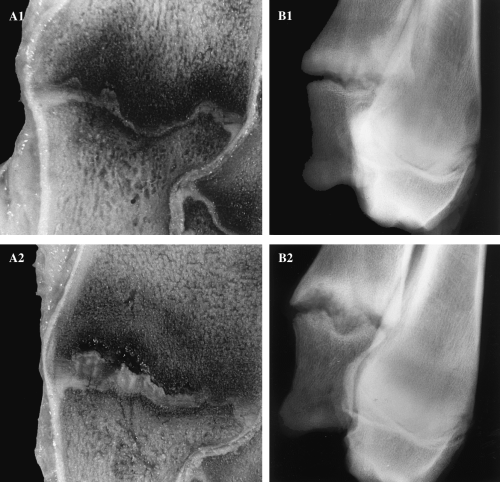
Frontal sections (A) of the distal growth plates of ulna of 26-week-old pigs and latero-medial radiographs (B) of the distal ulna and radius of the same specimen. (A1) Mild lesions of the growth plate in distal ulna with focal areas of thick cartilage. (A2) Obvious lesion with extensive cartilage retentions with haemorrhages projecting into the metaphyseal bone. (B1) A mild lesion with irregular growth plate in the distal ulna. (B2) Obvious lesion in the growth plate of distal ulna with a thickened, irregular appearance.
No significant associations were found between the morphological lesions and the clinical leg weakness. However, a certain association (P=0.056) between synovitis of the elbow joint and the clinical leg weakness of the forelegs was observed. Lesions of the proximal and distal ulna and the medial humeral condyle were significantly (P=0.001) associated with the litter. Lesions of the lateral humeral condyle and the proximal edge of radius were significantly associated (P=0.01) with both the sex (female) and litter.
Blood (serum and plasma) parameters
The serum concentrations of Ca, P and ALP and the plasma levels of vitamin C are shown in Table 4. Serum concentrations of Ca (P=0.05) and P (P=0.001) differed significantly between age groups. The serum ALP concentration decreased from 6 to 26 weeks of age (P=0.001).

The vitamin C concentration in plasma increased (P=0.001) from 6 to 13 weeks of age, decreased significantly 2 months later (P=0.001) and was lower, but not significantly so, by the end of the study (Table 4). The range of vitamin C content showed a large variation (7.1–49.8 μmol/l).
A significant positive correlation (r=0.68, P=0.001) was observed between levels of vitamin C in plasma and serum levels of alkaline phosphatase at the age of 6 weeks, but there were no positive correlations between levels of plasma vitamin C and serum levels of Ca and P.
Various factors that could influence the level of vitamin C in plasma were analysed. The age of animals (P=0.001), and the interaction between age and litter (P=0.001) had a significant effect on the plasma levels of vitamin C. However, neither sex nor litter of the animals, nor interaction between sex and litter nor between sex and age, influenced the levels of vitamin C in plasma. A significant positive correlation (r=0.45, P=0.05) of the plasma levels of vitamin C within animals was observed between the 21- and 26-week-old groups. Also, most of the lesions and the clinical signs of leg weakness did not influence the plasma levels of vitamin C and the plasma levels of vitamin C and the sex did not influence the growth rate. A few significant differences were found in the levels of vitamin C at the age of 13 weeks between normal animals (score 0) and animals with mild lesions (score 1) in the distal growth plate of the ulna (P=0.05), and obvious lesions (score 2) in the proximal radius (P=0.05) at the age of 26 weeks and between the levels of vitamin C at the age of 26 weeks and mild lesions (score 1) in the proximal ulna (P=0.01) at the age of 26 weeks.
Food parameters
The mean concentrations of vitamin C, Ca and P in the food samples are shown in Table 5. The levels of vitamin C in all samples were below the minimal detected values for the analysis used.
Discussion
Vitamin C deficiency will result in failure of the development of connective tissues (Kipp et al., 1996), and dietary vitamin C supplements for growing–finishing pigs have been suggested as beneficial in reducing the development of crooked or bent forelegs (Nielsen and Vinther, 1982). The symptoms of crooked and/or bent forelegs may imply contracted flexor tendons and weak ligaments, which might indicate impaired development of the growing loaded connective tissues.
The prevalence of leg weakness in the four litters in the present study was high (75% at the age of 26 weeks), but the vitamin C concentrations in plasma did not indicate deficiency. In the present study, the plasma concentrations of vitamin C were not associated with the clinical symptoms of leg weakness or clearly associated with the pathological lesions in the elbow joints and the distal ulnar growth plate. The deep digital flexor tendons did not show any macroscopic changes, and the tendons from 18 of 19 pigs did not show any pathological lesions under light microscopy or electron microscopy (one tendon showed mild fibroblast proliferation).
A considerable age variation in plasma vitamin C concentrations in pigs has been reported, with highest levels during lactation, lower during weaning and gradually increasing until 24 weeks of age (Hanssen et al., 1979; Lund et al., 1980; Wegger and Palludan, 1984; Pointillart et al., 1997). Lower levels are, however, observed in adult breeding animals (Lund et al., 1980; Wegger and Palludan, 1984). In the present study, a higher mean concentration of plasma vitamin C was observed in the 13-week-old animals with a lower concentration in the 21- and 26-week-old pigs. This could be attributed to an altered balance between synthesis and utilization of vitamin C (Wegger and Palludan, 1984). In any stress situation or period of excitement, such as lactation, training, high environmental temperature, subclinical disease or lameness, a high vitamin C requirement and a consequent diminished plasma concentration of vitamin C can be expected (Riker et al., 1967; Teare et al., 1979; Lund et al., 1980; Wegger and Palludan, 1984; Sigurdsson, 1997). The pigs in this study were exposed to a high environmental temperature due to problems with the ventilation during early spring, which may have contributed to the variations in the vitamin C plasma levels. It has also been suggested that the requirements of vitamin C synthesis are not met during the fast growth of the pigs, and that the level of plasma amino acid is affected by the amount of dietary energy (Brown et al., 1970, 1975; Dvorak, 1974). However, we did not find a significant association between the plasma vitamin C levels and growth rate. Different stress situations are common among growing–finishing pigs, such as crowding and respiratory problems (Hyun et al., 1998). These conditions can cause decreased levels of vitamin C, which may explain the slightly lower values found in the pigs at the ages of 21 and 26 weeks compared with the 13-week-old pigs.
The mean plasma vitamin C concentrations in the 6-, 13-, 21- and 26-week-old animals found in our study are similar to those in other studies (Jensen et al., 1983; Kristensen et al., 1986; Schwager and Schulze, 1998), but higher (Brown et al., 1970; Cromwell et al., 1970; Dvorak, 1974; Nakano et al., 1983; Pointillart et al., 1997) and lower (Lund et al., 1980; Wegger and Palludan, 1984) plasma levels have been described in pigs of the same age. This discrepancy in reported plasma vitamin C values can be attributed to differences in the analytical techniques (Wegger and Palludan, 1984), inappropriate preservation of the samples (Margolis et al., 1990) and an individual genetic variation in the vitamin C synthesizing capacity of the pig (Wegger and Palludan, 1984). In our study, special attention was given to the stabilization, storing and processing of the plasma samples in order to avoid vitamin C oxidation. A wide difference in the range of plasma vitamin C concentration was found in the present study (7.1–49.8 μmol/l). Similar differences have also been reported in growing pigs (Braude et al., 1950; Lund et al., 1980; Wegger and Palludan, 1984), horses (Sigurdsson, 1997) and birds (Hanssen et al., 1979). This variation was considered to be attributed to an individual hereditary difference in the requirements for vitamin C (Lund et al., 1980; Wegger and Palludan, 1984), subclinical diseases and stress (Sigurdsson, 1997). A variation in the vitamin C provided by the diet can also explain the different levels of vitamin C reported in plasma of growing–finishing pigs. The amount of vitamin C in the food in the present study was considered negligible (< 0.1 mg/l).
A positive correlation between levels of ALP and vitamin C at 6 weeks of age was observed in the present study. Serum levels of ALP decreased with age, but were within the normal range for growing–finishing pigs (Copland, 1976; Pricoszovits and Schuh, 1995; Carter et al., 1996). A significant age variation was also observed in the serum levels of Ca and P in the present study. However, these values were also normal for growing–finishing pigs (Pointillart et al., 1997; Egeli et al., 1998; Eklou-Kalonji et al., 1999).
Seventy-five per cent (30 pigs) of the pigs in the present study had lesions of dyschondroplasia in the growth plate of the distal ulna. A similar high prevalence of dyschondroplasia in the distal ulna of production pigs has been reported by others (Thurley, 1969; Reiland et al., 1978; Nakano et al., 1984; Jørgensen et al., 1995). These lesions of the distal ulna growth plate have been considered as part of the osteochondrosis syndrome (Reiland et al., 1978; Nakano et al., 1984; Jørgensen et al., 1995; Uhlhorn et al., 1995). However, these dyschondroplastic lesions have also been considered similar to growth plate lesions found in scurvy (Holmes, 1962; Walker et al., 1966; Hanssen et al., 1979; Grøndalen and Hanssen, 1984).
The lesions in the growth plate of pigs with osteogenic disorder (OD pigs) with scurvy were characterized by an impaired hypertrophic zone with columnization of chondrocytes with an irregular size together with a diminished amount and differentiation of osteoblasts, consequent osteopenia and massive subperiosteal haemorrhages (Jensen et al., 1983; Wegger and Palludan, 1986, 1994; Palludan and Wegger, 1988). These changes are the classical lesions found in primates with scurvy (Tsunenari et al., 1991; Schrenzel et al., 1996; Kipp et al., 1996). On the other hand, focal areas of an excessive accumulation of uncalcified hypertrophic-like chondrocytes projecting into the metaphyseal bone are described in early dyschondroplastic lesions in the growth plates of commercial pigs (Reiland, 1978c; Farnum et al., 1984; Wardale and Duance, 1994). Hence, an alteration of the hypertrophic zone in the growth plate, with a subsequent impaired endochondral ossification, has been observed both in scurvy and dyschondroplasia. However, the alteration differs between the syndromes and the changes in scurvy are generalized. The lesions of dyschondroplasia are localized and not accompanied by increased subperiostal haemorrhages, dedifferentiation of osteoblasts and osteopenia (Farnum et al., 1984; Tsunenari et al., 1991; Wardale and Duance, 1994; Wegger and Palludan, 1994). The skeletal lesions in OD-pigs develop when the plasma vitamin C levels are less than 5.7 μmol/l (Jensen et al., 1983; Wegger and Palludan, 1986, 1994; Schwager and Schulze, 1998). In the present study, we did not find any deficient mean values of vitamin C in the plasma at any time.
The lesions of OCD and dyschondroplasia in the right forelegs were not associated with the clinical leg weakness of the pigs in the present study. The lack of correlation may be due to the difficulties in objectively examining production pigs clinically and it could also be due to the age of the pigs (26 weeks). The subsequent osteoarthritis that follows an OCD lesion would probably correlate with the leg weakness. No clear relationship between OC/dyschondroplasia and clinical leg weakness of the forelegs was found in production pigs (Thurley, 1969; Grøndalen, 1974a; Jørgensen et al., 1995), but a correlation between OCD in the elbow joint and clinical leg weakness of the forelegs has been reported (Jørgensen et al., 1995). The synovitis of the elbow joint was associated with the OCD of the distal lateral humeral condyle (P=0.001) but showed a weaker association to the clinical leg weakness (P=0.056). Synovitis can produce pain with symptoms of lameness from the affected joint (Caron, 1996). The high prevalence of thinning of the articular cartilage and/or bony depression of the craniomedial edge of the proximal radius present in the pigs has also been reported by others (Reiland, 1978c; Nakano et al., 1984; Jørgensen, 1995; Jørgensen et al., 1995). These changes have been associated with OCD of the distal humeral condyles (Nakano et al., 1984; Jørgensen, 1995), but we could not find this association. The morphology of the lesions of the craniomedial edge of the proximal radius are similar to a synovial fossa, but the localization is not compatible with a synovial fossa (Doige and Horowitz, 1975; Wegener et al., 1993). The significant association (P=0.05) with these changes and the synovitis of the elbow joint may suggest that they are part of an early osteoarthritis.
The aim of the present study was to evaluate the plasma levels of vitamin C in growing–finishing pigs and determine whether this was associated with crooked or bent forelegs. We did not find a vitamin C deficiency or any association between low plasma levels of vitamin C and deformed forelegs in the 40 pigs investigated. There were no associations between morphological lesions of the elbow joint and the distal ulnar growth plate, the growth rate and the plasma levels of vitamin C. The deep digital flexor tendon of these pigs did not show any pathological lesions. We conclude that a deficiency of plasma vitamin C levels in production pigs is not involved in the pathogenesis of the crooked and/or deviated forelegs observed in these pigs.
Acknowledgements
This work was supported by grants from the Swedish Foundation for International Co-operation in Research and Higher Education (STINT). The authors would like to thank Ulla Hammarström and Tapio Nikkilä for excellent laboratory work, Nils Lundeheim and Padet Tummaruk for valuable help and discussion concerning the statistical analysis, and the owner, Mikael Hugosson, and all the workers at the Alviksgården pig herd for making access to the animals possible.