Haematology of the turbot, Psetta maxima (L.): ultrastructural, cytochemical and morphological properties of peripheral blood leucocytes
Abstract
Optimum procedures for fish handling and sample processing for use when employing haematological parameters as health indicators in turbot, Psetta maxima (L.), have been established. We found thrombocytes to be the most abundant blood cell, representing approximately 52% of circulating leucocytes (lymphocytes, 40.8%; granulocytes, 5.6%; monocytes, 1.6%; total number of leucocytes=1.3 × 105 ml−1; packed cell volume=22.7%). The light- and electron-microscopical characteristics of these cell types are described, together with their cytochemical properties using Sudan Black B, Periodic Acid Schiff, Non-specific Esterase, and Acid and Alkaline Phosphatase. Turbot thrombocytes showed a high degree of shape alterations when observed in live preparations using phase contrast microscopy, while ultrastructural observations following the in vitro uptake of carbon particles supported an active process of phagocytosis by the thrombocytes, rather than passive entrapment. The lymphocytes of turbot are structurally similar to mammalian lymphocytes with the highest nuclear:cytoplasmic ratio of all the leucocytes observed. Small lymphocytes predominated, large lymphocytes forming less than 1% of the total white blood cell population. The most frequent granulocyte type was a neutrophil-like cell with an eccentric nucleus, only rarely seen in segmented form. In vitro uptake of carbon particles by granulocytes was not observed under the conditions of the experiment, although turbot granulocytes are capable of phagocytosis under different circumstances. These are discussed, along with other physiological and technical factors which can influence the blood parameter findings in fish.
Introduction
It is generally accepted that the peripheral blood leucocytes of fish show distinct morphological similarities to those of birds and mammals, and comprise thrombocytes, lymphocytes, granulocytes and monocytes. The most notable differences in fish relate to thrombocytes, which are nucleated and much larger than their mammalian counterparts, and to granulocytes which vary in the appearance of their granules, their proportion of the total leucocyte population and the nomenclature used to describe them (Ellis 1977; Rowley et al. 1988; Hine 1992). The function of fish leucocytes, not simply their morphology, needs to be established before mammalian terminology can be employed. Therefore, different authors have used a variety of immunochemical and cytochemical tests to demonstrate specific substances and enzymes within the cellular structure of fish leucocytes or specific membrane markers on their cell surface. Most of these studies have concentrated on particular species and/or specific cell types.
The experiment set out to optimize some of the routine techniques available for haematological studies on the turbot, Psetta maxima (formerly Scophthalmus maximus) (L.). This is an important farmed fish, and therefore, of interest in relation to disease control. It is also one of the flatfish species used in environmental studies such as those on the effects of marine pollution (e.g. Hutchinson et al. 1999). Once simple haematological parameters similar to those used in veterinary and clinical medicine have been established for a particular fish species, these can be used as health indicators for various purposes (e.g. see Hrubec et al. 1997; Schütt et al. 1997). The simplicity of blood sampling makes such tests valuable for use with turbot, but as for other fish species, baseline information is needed. Therefore, the present study documents the morphological, ultrastructural, cytochemical and morphometric properties of turbot peripheral blood leucocytes in apparently healthy, aquarium-held fish. A brief description of the morphological criteria used for identification was given by Burrows and Fletcher (1987). The present paper provides a more detailed report which includes histochemistry and electron microscopy.
Materials and methods
Fish and fish handling
Turbot were originally obtained from a commercial fish farm (Hunterston, UK), then held in 500-L polythene tanks containing recirculating sea water at 13 ± 1 °C. Water quality was tested biweekly and maintained at a salinity of 36‰, pH 7.8 with ammonia levels < 0.025 mg L−1. The fish were fed on Trout No. 7 pellets (Ewos-Baker, West Lothian, UK) supplemented with fresh fish and mussels, Mytilus edulis L. For blood sampling, fish weighing between 300 and 500 g were removed from their tanks by hand. One hand was placed beneath the head and used to hold the undermost pectoral fin close to the body, whilst the other hand was positioned in the tail region, enabling the fish to be raised gently from the bottom. Holding the fin in this way and maintaining the fish in its normal position ensured that individuals did not struggle, eliminating the stress of net capture and use of anaesthetics. Fish were transferred to a wet paper towel on the bench where a damp tissue was placed over the eyes. Under these conditions, the turbot would remain for several minutes without obvious signs of distress, enabling blood samples to be taken before being returned to the water. Blood was withdrawn from the renal portal vein of the unanaesthetized fish using a 21-G needle attached to a disposable sterile syringe. The needle was inserted on the ventral side of the lateral line, close to the caudal peduncle and between neural spines. For terminal bleeding, a site closer to the body cavity was used. By avoiding excessive pressure when drawing blood up through the needle and removing the needle before expressing blood from the syringe, cell lysis was minimized.
Preparation of blood samples
Blood (1.8 ml) was drawn into a 2-ml syringe containing 0.2 ml of 3.2% trisodium citrate (TSC) in distilled water. Blood and TSC were mixed gently by removing the needle, withdrawing the plunger along the barrel to introduce some air and inverting the syringe several times. Providing that the fish was caught and handled carefully and rapidly, coagulation of blood samples did not occur. Trisodium citrate was the anticoagulant of choice, causing the least damage to delicate turbot leucocytes in comparison with collection in lithium heparin-coated tubes (15iu, Sarstedt, Leicester, UK) and ethylenediamine-tetra acetic acid (EDTA, 1.5 mg ml−1 blood). Smears were prepared by spreading a 10-μl drop of uncoagulated blood on an alcohol-cleaned microscope slide with the single even, slow, ‘push’ stroke of another slide. Smears were dried completely in a warm air stream, followed by immediate fixation for 5 min in methanol. When smears were prepared for comparison from blood without the use of anticoagulants, rapidity of drying became even more critical if a high degree of cell lysis was to be avoided.
Histochemical staining
The following histochemical staining techniques were used: Sudan Black B (Sheehan and Storey 1947); Periodic Acid Schiff (PAS) (Pearse 1968; counter stained in Harris’s haematoxylin); Acid Phosphatase (Hayhoe and Flemans 1979); Alkaline Phosphatase (Hayhoe and Flemans 1979); and Non-Specific Esterase (Yam 1971). May-Grunwald/Giemsa (MGG) stain was used for routine staining (Burrows 1995).
Cell counts, cell measurements and live cell observations
Haematocrit values (packed cell volumes) were obtained by introducing freshly collected blood samples into heparinized microcapillary tubes (1.1–1.2 mm in diameter, Gelman-Hawksley Ltd, Sussex, UK) and spinning in a haematocrit centrifuge at 200 g for 10 min before reading in a Hawksley haematocrit reader. Triplicate values were obtained for each of 20 fish. Total leucocyte counts were determined in triplicate after thoroughly mixing the blood sample in TSC (0.016% in 1.1% saline) using a Neubauer Improved Haemocytometer and examining at × 400 magnification. Differential white cell numbers were based on counting a minimum of 200 leucocytes in MGG-stained blood smears from 20 fish. Cell dimensions were measured at × 400 magnification using an eyepiece graticule and nuclear:cytoplasmic (N:C) diameter ratios calculated for each leucocyte type.
Direct observations of live cell structure and behaviour were carried out using phase contrast microscopy. Small samples of anticoagulated blood were placed on microscope slides, mounted with coverslips, sealed with vaseline and observed over a 30-min period.
Electron microscopy
For electron microscopy, blood was collected into heparinized tubes, then spun in a low speed microcentrifuge (100 g, 30 min) to minimize cell damage. Plasma was removed and the pelleted cells fixed using 3% glutaraldehyde (EM grade, Agar Aids, Essex, UK) in 2% sodium cacodylate buffer (pH 7.2). The fixative was removed and the samples were postfixed for 2.5 h in 2 ml 2% osmium tetroxide at 4 °C. Tissues were then dehydrated through a series of alcohols and embedded in Spurr resin (TAAB, Reading, UK). Ultra-thin sections of resin embedded material were cut on a Sorvall Porter-Blum MT2-B ultramicrotome using glass knives cut on an LKB 7800N glass knife maker. Spreading of the sections was enhanced by treating with chloroform. Those sections showing gold or silver interference patterns (60–100 nm thickness) were positioned on copper grids (200 mesh size, Polaron Ltd, London, UK) and stained with lead citrate and saturated uranyl acetate. Sections were visualized and photographed using a Phillips 300 transmission electron microscope. To study phagocytic activity, anticoagulated blood (TSC) was spun down (100 g, 30 min) to produce a buffy layer of leucocytes. Plasma was removed and leucocytes resuspended in plaice Ringer (Cobb et al. 1973). The leucocyte suspension (0.5 ml) was placed in an Eppendorf tube with an equal volume of a 1:20 dilution of Pelikan ink, particle diameter 0.02–0.05 μm (11/1431a; Gunter Wagner, Hanover, Germany) to detect phagocytosis. Tubes were incubated at 12 or 22 °C and turned every half hour to resuspend the cells. The cells were then pelleted, the supernatant carefully removed and fixative added without disturbing the pellet. This was then processed for electron microscopy as above.
Results
Haematological studies
Four major types of turbot leucocytes were identified on the basis of their cell size, nuclear:cytoplasmic ratio, shape, structure, histochemical staining and intensity of staining, and ultrastructure. These were: thrombocytes, lymphocytes, granulocytes and monocytes. Summaries of the haematological results are given in Tables 1 and 2.
Thrombocytes
Light microscopy and histochemistry.
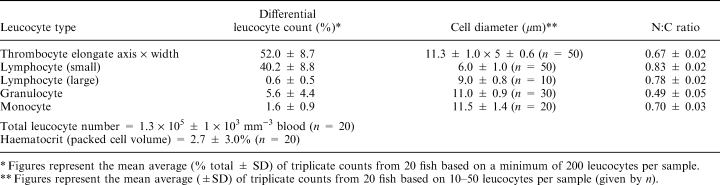
The most abundant leucocytes in blood smears and live cell preparations were thrombocytes. The relatively large nucleus, surrounded by a distinct rim of pale blue cytoplasm in MGG-stained preparations, existed in several forms: round, oval, or indented and appearing almost bilobed on occasions. The thrombocyte showed some similarities to the lymphocyte, although the nucleus of the former stained more intensely. Although rounded forms were encountered, the cytoplasm was frequently drawn out, tapering at one or both ends of the cell; it was this form which appeared more mobile in live preparations (Fig. 1), being able to radiate cytoplasmic pseudopodia. Live cells were capable of a high degree of shape alteration which may account for the variability in thrombocyte forms encountered in blood smears. When clotting was observed in live cell preparations using phase contrast microscopy, long threads were seen to spread out from the cell pole. An increasingly tangled network, assumed to be fibrin, was slowly created in which other cells became trapped. Histochemical staining showed numerous fine and course granules of PAS-positive material dispersed throughout the cytoplasm of thrombocytes. All morphological forms showed positivity with PAS. Larger, scattered granules of acid phosphatase activity localized within the cytoplasm were also noted. The other histochemical stains used gave negative results.
0 μm)
Electron microscopy and phagocytosis.
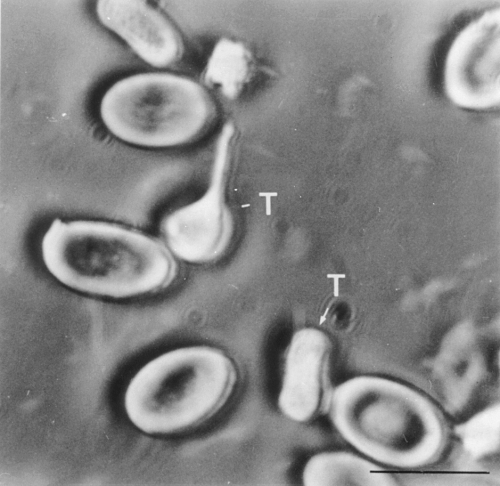
Thrombocytes were the most abundant cell type within leucocyte pellets. They exhibited an elongate, fusiform shape when cut longitudinally and were circular in transverse section. The nucleus was sharply indented and centrally positioned with very dense chromatin at its periphery (Fig. 2a). The most prominent cytoplasmic features were large, electron-lucent vesicles (Fig. 2b) caused by numerous deep infoldings of the plasmalemma. Connections between these vesicles and the cell surface were seen in some sections. Cytoplasmic vacuolation was used to distinguish between transversely sectioned thrombocytes and the lymphocytes which they resembled. A single electron-dense granule was frequently observed within the cytoplasm (Fig. 2b). A few clusters of free ribosomes and several mitochondria were normally present, and some elements of rough endoplasmic reticulum were seen. Occasionally, well-developed and prominent Golgi complexes surrounded by numerous small vesicles occurred close to the cell nucleus (Fig. 2c). Bundles of microtubules were seen below the plasmalemma, often concentrated at the cell poles, in thrombocytes sectioned longitudinally. In transverse section, they appeared to encircle the cell. Two types of small granule were seen, one electron lucent, assumed to be glycogen, and the other electron dense, assumed to be lysosomal. Following exposure to carbon in vitro, many thrombocytes were found to contain carbon particles within much enlarged cytoplasmic vacuoles (Fig. 3a) at both 12 and 22 °C. Pseudopodial-like projections were evident, extending to encircle aggregations of carbon (Fig. 3b). Carbon uptake occurred to a greater extent at the higher temperature. Such was the amount of carbon taken up by some cells that the nucleus appeared displaced towards the periphery of the cell. Engulfed cellular remains from disrupted cells were also seen within cytoplasmic vacuoles of this cell type.
5 100). (c) Section through a thrombocyte illustrating the well-developed Golgi complex (Gc) close to the nucleus (× 9740)
(a) Electron micrograph showing the internalization of colloidal carbon (C) by a thrombocyte at 12 °C (× 10 050). (b) Pseudopodal processes of thrombocyte-encircling carbon particles at 22 °C. The arrow indicates a phagosome nearing completion of formation (× 9740)
Lymphocytes
Light microscopy and histochemistry.
Lymphocytes were characterized by a large nucleus which was usually indented. The nucleus stained less intensely than that of the thrombocyte. It occupied almost the entire cell, resulting in a high N:C ratio of 0.83. A narrow rim of basophilic agranular cytoplasm surrounded the nucleus. Small and large lymphocytes were identified and classified by their diameters and relative amounts of cytoplasm; the latter were only rarely encountered, constituting just 1% of the lymphocyte population. The high N:C ratio was reduced slightly in larger lymphocytes which contained a greater proportion of cytoplasm (Table 1). The majority of lymphocytes stained positively for acid phosphatase with particulate staining appearing in the cytoplasm. The histochemical tests employed to detect lipid, glycogen, alkaline phosphatase and non-specific esterase activity were all negative.
Electron microscopy and phagocytosis.
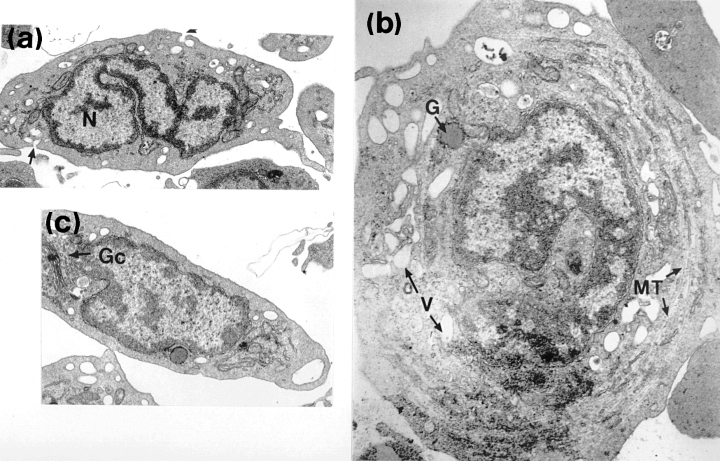
Lymphocytes contained dense, radially arranged nuclear chromatin enclosing the interchromatin material and a nucleolus was occasionally seen. The centrally positioned, round to oval nucleus was often indented. These frequently observed cells resembled transversely sectioned thrombocytes. The nucleus of small lymphocytes was surrounded by a narrow band of agranular cytoplasm, often exhibiting an irregular outline and being characterized by occasional microvilli (Fig. 4a,b). Numerous free ribosomes were scattered throughout the cytoplasm. Large, elongated mitochondria with prominent tubular cristae occurred frequently, often accumulating in one region of the cell. Larger lymphocytes (Fig. 4a) were characterized by their greater amounts of cytoplasm and larger mitochrondria. Distinct profiles of rough endoplasmic reticulum (visualized as basophilic areas at the light microscopy level), along with occasional Golgi bodies, made up the few cytoplasmic organelles seen. No phagocytosed carbon was observed within the cytoplasm of in vitro exposed lymphocytes.
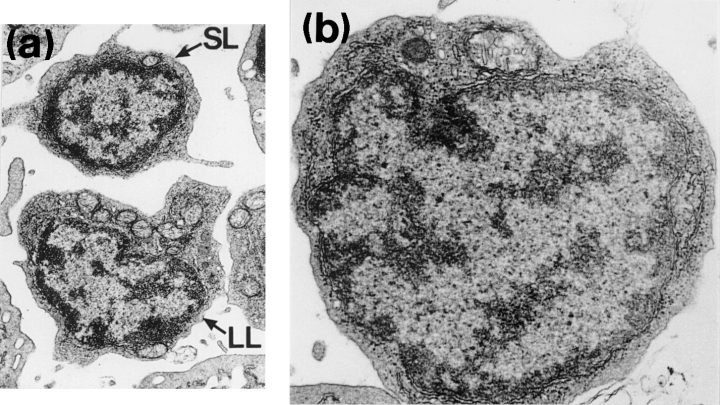
850). Larger lymphocytes (LL) contain more mitochondria than small lymphocytes (SL). (b) Electron micrograph of a lymphocyte (× 12 500)
Granulocytes
Light microscopy and histochemistry.
Spherical granulocytes contained an eccentrically positioned, rounded nucleus occupying between one-quarter and one-third of the cell. The nucleus ranged from round or oval to deeply indented. Occasionally, a segmental form was seen with a maximum of two lobes. The cytoplasmic granules of these large round cells were unstained with MGG, but PAS staining resulted in a strong, dense, granular positivity throughout the cytoplasm. The most intense reaction was observed in granulocytes with the lowest N:C ratios. Pre-digestion with salivary amylase resulted in these cell types giving negative staining with PAS, indicating the presence of glycogen. Discrete, scattered granules with strong sudanophilia were observed following staining with Sudan Black B. Acid and Alkaline Phosphatase activities were also detected.
Electron microscopy and phagocytosis.
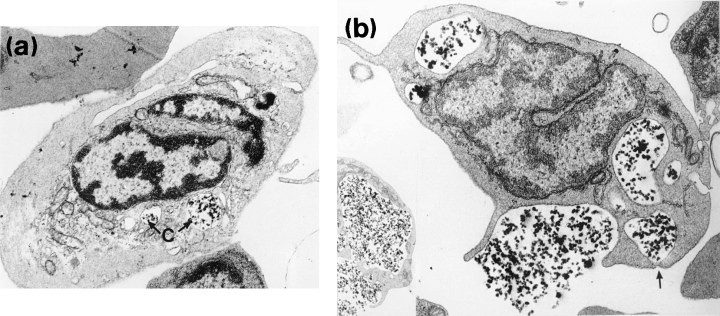
The eccentrically positioned nucleus was characterized by a moderately electron-dense peripheral layer of chromatin and dense areas of interchromatin. Polymorphic forms were rarely seen. The relatively smooth cytoplasmic margin showed few surface projections. The most notable features of the granulocyte were the presence of numerous elongate cytoplasmic granules. Membrane bound, rod-shaped granules were rounded or elliptical in section, ranging in size from 0.5 × 0.2 to 1.0 × 0.3 μm. They contained an electron-dense matrix of varying intensity, separated from the limiting membrane by an electron lucent zone seen as a clear halo (Fig. 5a). Some granules were seen to contain fibrillar inclusions. Numerous aggregates of irregularly shaped granules 20–30 nm in diameter were also seen within the cytoplasm. Many parallel arrays of rough endoplasmic reticulum were observed localized close to the nucleus or in the nuclear cleft (Fig. 5a,b). Rounded or elongate mitochondria scattered throughout the cytoplasm were fewer in number than observed in lymphocytes. Golgi bodies comprising a few flattened sacs were only occasionally encountered. The cytoplasm of putatively less mature cells, with their non-lobate centrally positioned nucleus, contained a high proportion of rough endoplasmic reticulum and only a few small granules. More mature cells possessed a lobate nucleus, with smaller amounts of rough endoplasmic reticulum. However, their granules were larger in size and more numerous. No phagocytosed carbon was seen within the cytoplasm of the in vitro exposed cells.
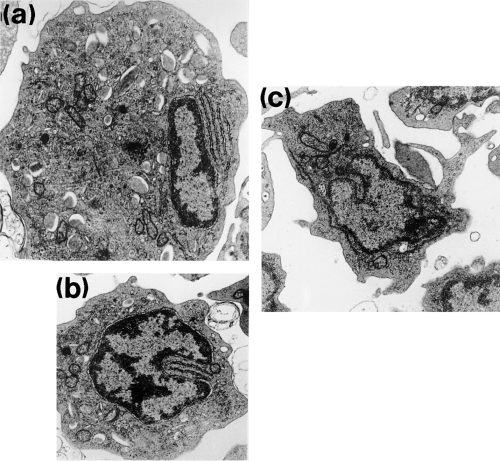
0). (c) Electron micrograph of a monocyte showing extrusion of pseudopodia (× 4600)
Monocytes
Light microscopy and histochemistry.
Monocytes were the largest and most variably shaped of the peripheral blood leucocytes. The large spherical or indented nucleus of this rarely encountered leucocyte type occupied about half the cell, occasionally exhibiting the classical ‘horse shoe’ shape. The relatively abundant basophilic cytoplasm was often vacuolated. Monocytes adhered to glass surfaces with a tendency to spread during in vitro observations. Alkaline Phosphatase and a diffuse Acid Phosphatase activity of monocytes was detected. Scanty, scattered Non-Specific Esterase positivity was also observed. Monocytes were not stained by the Sudan Black B or the Periodic Acid Schiff stains.
Electron microscopy and phagocytosis.
Dense chromatin was localized around the periphery of the pale-staining nucleus. Abundant agranular cytoplasm was drawn into irregular and lengthy pseudopodia and microvilli extending from the cell surface (Fig. 5c). Cytoplasm rich in large mitochondria with distinct cristae was often a predominant feature. Occasional electron lucent vesicles appeared to contain cellular debris and amorphous material. Long profiles of rough endoplasmic reticulum and numerous scattered lysosomes were also evident. Some carbon particles were observed within the cytoplasm of this cell type after exposure to carbon in vitro.
Discussion
Haematological indices can be used as an effective method for monitoring physiological and pathological changes in fish. However, the findings may sometimes vary between one study and another unless technical details for the species are well defined. In the present study, total erythrocyte and leucocyte counts for the turbot fell within the ranges found for other fish species, while the haematocrit values of 22.7% fell just below the 25–50% range reported for other fish. Since results can be affected by erythrocyte swelling which occurs in anaesthetized or stressed fish (Ross et al. 1986), our fish were sampled without the use of an anaesthetic. Ellis (1976) found leucocyte numbers in the plaice, Pleuronectes platessa, to be very variable. Large variations in red and white cell numbers have also been reported in other fish species, often associated with known stress factors including handling (Ellsaesser and Clem 1986). In the present study, stress during capture and handling was minimized, as it was found to cause rapid clotting of the blood samples and serious distortion of the leucocytes. Haemolysis of the cells was avoided by ensuring that only thin blood smears, produced and dried as soon as possible, were used. Initially, the occurrence of damaged cells in turbot blood film preparations was a problem. Such cells, which appear as amorphous purple areas, were named ‘smudge cells’ by Williams and Warner (1976). In the present study, smudge cells were numerous in badly prepared thick smears, in blood sampled from stressed fish, and when certain anticoagulants were used, most notably heparin and EDTA; TSC was found to be the most effective anticoagulant. Inter-species variation in the effectiveness of different anticoagulants in fish was noted by Mainwaring and Rowley (1985), who suggested that this may be a result of their differences in osmolarity and pH.
The variability of fish thrombocyte shape seen in the present study is supported by the findings of many authors, including Ellis (1977) who categorized these different forms into four specific types: spiked, spindle, ovoid and lone nucleus. Observations of live thrombocytes here and in the plaice (Wardle 1971) have suggested that these cells are capable of a high degree of movement, including cell extension and shortening, twisting and waving of spindle filaments, resulting in many different shapes being seen. The lone nucleus was rarely observed in well-prepared blood films, but has commonly been reported in stressed fish. This probably reflects their function in the blood clotting mechanisms since increased clotting rates as a result of stress would result in a high proportion of lone (denuded) nuclei. The findings that the different shapes of the thrombocytes are not artifactual support those of Volynkin (1983), who suggested that classification of thrombocytes into separate types is artificial after describing thrombocyte shapes transitional between all forms. Ultrastructural findings of pseudopodia, organelle free areas and the presence of microtubules suggest that turbot thrombocytes are capable of movement. The presence of large numbers of microtubules below the plasma membrane, occasionally running the length of the cell, has been equated with motility. In mature cells, microtubules aggregate into a marginal band.
In the present study, thrombocytes represented 52% of the circulating leucocytes in the turbot. This is a considerably higher proportion than that reported by Quentel and Obach (1992) studying adult turbot and by Santarém and Figueras (1995), who used younger (body weight=8–10 g) turbot. A possible explanation of this discrepancy may lie in the marked fragility of this cell type. Difficulties in differentiating between thrombocytes and lymphocytes may also account for some of the differences. Indeed, problems in distinguishing between rounded thrombocytes and small lymphocytes have frequently been noted (Ellis 1977) and an enormous variation in thrombocyte number has been reported in the literature for different species of fish (Lewis 1996). The variation in counts may also be a result of clotting or thrombocyte nuclei being denuded of cytoplasm during smear preparation. In the differential cell counts reported here, rounded thrombocytes were distinguished from lymphocytes by the more basophilic, undulating cytoplasm of the lymphocyte when compared to the very pale, discrete staining cytoplasm of the thrombocyte. Histochemical differences, the presence of microtubules and N:C ratios also helped to differentiate between these cell types. The PAS positivity in fine and course granules scattered throughout the cytoplasm of turbot thrombocytes indicates the presence of polysaccharides such as glycogen. The acid phosphatase activity also reported here could suggest the presence of lysosomes and is compatible with a phagocytic capacity.
Evidence of in vitro and in vivo phagocytosis exists for thrombocytes of various fish species, although the possibility has been raised by Ellis (1976) and other workers (reviewed by Rowley et al. 1988) that the presence of foreign material within the cell may be caused by mechanical entrapment rather than active phagocytosis. The demonstration of phagocytosis by turbot thrombocytes in the present study seems to support the findings of Ferguson (1976) for the plaice where foreign particles were observed to be within large vesicles. The rapid rate at which carbon appeared within turbot thrombocytes following in vitro exposure and the observation of extended pseudopodia at the EM level suggests that the uptake of carbon in our experiments was indeed an active process. Furthermore, recent evidence using monoclonal antibodies directed against putative fibrinogen receptors on rainbow trout thrombocytes (Hill and Rowley 1998) strongly indicates that fish thrombocytes internalize bacteria via an active process and not simply by passive sequestration into an open canicular system.
The lymphocytes of the turbot seem to be structurally similar to mammalian lymphocytes with the highest N:C ratio of all the leucocyte types observed. Their size ranged in diameter from 6 to 9 μm with N:C ratios of 0.83 and 0.78, respectively. Their cytoplasm was basophilic, reflecting the presence of a large number of ribosomes. Ultrastructural observations confirmed the presence of large quantities of rough endoplasmic reticulum. The lymphocytes displayed no phagocytic activity, a result supported by findings for many fish species including the plaice (Ellis 1977). The monocytes of turbot exhibited pseudopodia, abundant slightly basophilic cytoplasm, an indented nucleus and rER, Golgi complex, vesicles, cytoplasmic granules, and histochemical staining characteristics which resembled those of the monocytes of other teleost species. Phagosomes contained internalized cellular material. The presence of the hydrolytic enzymes, non-specific esterase and acid phosphatase, seen here in turbot monocytes, are also indicative of a phagocytic capability, and may help to differentiate between monocytes and large lymphocytes in identification studies.
Turbot granulocytes measured approximately 11 μm in diameter and represented 5.6% of the leucocyte population. This is compatible with the findings of Santarém and Figueras (1995) for younger turbot and similar to the findings of Ellis (1976) for plaice, but at variance with the findings of Quentel and Obach (1992), who identified 30.2% of adult turbot leucocytes as granulocytes. Such a large discrepancy with the results for turbot reported here possibly reflects an undetected stimulated immune state in their fish. This could result in an increase in granulocyte numbers similar to that produced in turbot by injection of O-antigen bacterins (Santarém and Figueras 1995). Granulocytes represent a smaller proportion of leucocytes in fish than in mammals, where they are the most abundant leucocyte type. This difference may be related to the phagocytic role of the thrombocyte in fish, a function normally associated with the granulocytes in mammals. The granulocyte type found in the present study most closely resembled the mammalian neutrophil; similar findings were reported in the plaice (Ellis 1976; Ferguson 1976). Ellis (1977) stated that this granulocyte type was the most abundant type encountered in teleost species. Turbot granulocytes were characterized by nuclear morphology and by the tinctorial properties of the cytoplasmic granules which were unstained with acid or basic dyes. However, whether these cells should be regarded as polymorphonuclear leucocytes (PMNLs) is questionable because the segmentation of up to four lobes recorded for mammalian PMNLs was never observed in the present experiment, the turbot granulocyte nucleus being rounded or occasionally bilobed. Cytoplasmic PAS-positive granules have been recorded in the neutrophil-like granulocytes of many fish species, including the plaice (Ellis 1976) where, as in the present study, positivity was lost following treatment with salivary amylase. The most intense staining in turbot occurred in more mature (low N:C ratio) cells, suggesting that, as in mammals, the accumulation of glycogen proceeds with time. The intense sudanophilia of turbot granulocytes, indicative of a high lipid content, has also been reported in plaice, as has positive acid and alkaline phosphatase activity (Ellis 1976).
The granulocyte composition of different teleost species appears to be variable, as does the ability of granulocytes from different fish to phagocytose particles and/or pathogens (Hine 1992). The lack of phagocytic activity seen during our in vitro studies on turbot granulocytes may reflect suboptimal experimental conditions. The nature of the foreign particles used is also relevant. This was noted by Santarém and Figueras (1995), who assayed phagocytosis using sheep erythrocytes and reported a low level of phagocytic ability in turbot peripheral blood neutrophils which increased following immunization. The acid phosphatase activity demonstrated in turbot granulocytes in the present experiment suggests the presence of lysosomal enzymes and the possibility of phagocytosis; phagocytosis by suitably stimulated plaice neutrophils was also clearly demonstrated by MacArthur and Fletcher (1985). In his review on fish granulocytes, Hine (1992) concluded that the neutrophils of most fish species are capable of phagocytosis in vitro and in vivo, but that neutrophil subpopulations may differ in their phagocytic ability (e.g. during inflammatory responses), with variability between individual fish. Indeed, the fact that blood leucocyte types can vary in their functional status and in their proportional representation, not only according to the physiological condition and state of health of the individual fish but also between different species of fish, is now well known. Therefore, a baseline knowledge of the leucocyte types of farmed fish species such as the turbot is of considerable importance in applied studies.