Changes in bacterial abundance and community structure in cage fish-culture caused by water turbulence during feeding
Abstract
Water and sediment quality conditions in and around a cage culture unit located in Wismar Bay (Western Baltic Sea, Germany) were investigated during summer 1994 using bacterial group indicators (Enterobacteriaceae, saprophytic bacteria and Vibrio, determined on selective media). Fish were fed once daily with extruded commercial pellets. Over a period of 8 days samples were taken before and after feeding at different sampling points close to or at the cages. The results showed that a drastic (up to 10 times) increase in different bacterial groups occurred inside the cages 10 min after feeding as well as occasionally in the surrounding water. Additional electron-microscopic observations of the bacterial biofilm on the cage nets revealed that a decrease (in comparison with the original number) in total bacteria on the net materials of 9.5 up to 42.8% occurred after feeding. It was concluded that water mixing caused by frenzied swimming just before and during feeding caused a release of bacteria which were growing vigorously on the surfaces of the cage system. Since the pathogenity and hydrophobicity of bacteria are closely related, this process of redistribution of bacteria into the water column could constitute an important stress factor for fish, which could probably also have a negative effect on fish production.
Introduction
Fish cultured in hatcheries, farms or cages, can easily be stressed by environmental factors and management practices. The reduction of stress factors has been recognized as one of the most important tasks to ensure high growth efficiency by reducing the danger of infectious disease outbreaks ( Bullock 1971 ; Roberts 1993). Most fish pathogenic bacteria are facultative (some can even be considered saprophytic), and most of them are capable of independent existence outside of the fish, whether in water ( Roberts 1993) or in sediment ( Enger 1992) .
Studies on salmonids in land-based aquaculture systems have demonstrated that fish typically behave in a frenzied manner during feeding, and that the amount of bacteria in the water subsequently increases after feeding ( Niemi 1985). In cage culture, feeding stress is also evident and therefore it can be expected that a temporary increase in bacterial counts may also occur under these conditions. A variety of sources responsible for the observed changes have been discussed, including the food ( Kerry 1995) , faecal pellets, mixing of the surface layer of water, and mucus sloughed from the surface of the fish ( Cipriano 1992) . On the other hand, other investigations have shown that additional bacterial inputs are not supplied with the food ( Niemi 1985), or by the faecal pellets ( Enger 1992). However, the role of the enriched surface layer of water, of mucus from the fish, and the release of bacteria from the nets remains uncertain.
In the present study the nature and sources responsible for bacterial increases after feeding were investigated. In particular, the extent of bacterial detachment from nets during feeding was determined by four different bacterial indicators.
Materials and methods
Effect of feeding and swimming on the concentration of bacteria in the water
Bacterial abundance was determined at an Oncorhynchus mykiss (Walbaum 1792) cage farm located in the Wismar Bay (Western Baltic Sea, Germany) for 8 days in August 1994. The fish were hand-fed once a day with extruded pellets. The fish were highly active immediately before and during feeding.
Bacteria were quantified from water samples before feeding and 10 min thereafter at four sampling points within the cages at a depth of 1.5 m, and at a station 10 m beyond the cages. At each sampling point and station, 500 mL samples were taken with a sterilized microbiological sampler. The samples were diluted with sterile seawater from the site, giving final dilution ratios of 1/10, 1/100, 1/1000 and 1/10000. Two replicates were made; only the dilutions providing 30–300 bacterial colonies were considered for further analysis. Numbers of colony forming units (CFU/mL) were determined on four different media: Endo-C, selective for Enterobacteriaceae; ZoBell and TSA (CASO), selective for aerobic heterotrophic bacteria; and TCBS, selective for Vibrio. Data were statistically analysed using the Mann–Whitney test, with a significance level of P < 0.05 ( Zar 1996).
Effect of feeding and swimming on the detachment of bacteria from nets
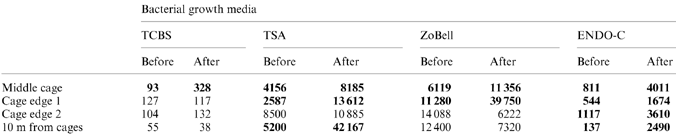
The bacterial detachment from nets was quantified in an Oncorhynchus mykiss cage farm in the Kiel Fjord (Western Baltic Sea, Germany) in July and August 1996. For this study, previously used 400 cm2 pieces of clean nylon net with 15 mm between knots were used. The net pieces were washed, rinsed and autoclaved; the absence of bacteria was confirmed by scanning electron microscopy (SEM). The fine structure of the net fibres is shown in Fig. 1.
Scanning electron micrograph of rope from a net showing the individual fibres (arrows) which were investigated for their bacterial biofilm development. Bar = 500 μm
The pieces of experimental net were affixed to the nets of the cages at 1.5 m depth in four positions, where they remained exposed for at least 18 days. Each day, pieces, 4 cm in length, from the experimental nets were removed just before and 10 min after feeding. Care was taken to remove the pieces carefully to retain the biofilm. Samples were fixed with 2% formalin in 0.2 μm filtered fjord water. After dehydration and critical point drying they were examined using a Zeiss DSM 940 scanning electron microscope. For each sample, at least 35 fields (each 285.23 μm2) were examined and the number of bacteria counted. Total bacterial counts obtained for the four net positions in the cage were pooled and averaged over periods of 1–8, 9–15, and 16–18 days of exposure. It has to be noted that the most reliable bacterial counts were obtained from the second period; bacterial distribution was very patchy during the first period, and colonization by other organisms (e.g. Bryozoa) hindered the determination of bacteria during the third period. The final results were expressed as the numbers of total bacteria per field and percentage change after feeding compared with before feeding. The significance of the changes was assessed by the Mann–Whitney test, with a significance level of P < 0.05 ( Zar 1996).
Results
Effect of feeding and swimming on the concentration of selected bacterial groups in the water
Ten minutes after feeding, the increase of bacterial counts on ENDO-C was significant at all sampling points ( Table 1). On TSA and Zobell Media – both suitable for saprophytes – the increase after feeding was significant for samples from the middle of the cage and from the cage edge (in one of two cases). Surprisingly, the bacteria growing on TCBS Medium were generally very few and no significant increase was observed after feeding with one exception.
Effect of feeding and swimming on the detachment of total bacteria from nets
A decrease in the quantity of total bacteria (determined by electron microscopy) on the nets after feeding was detected in all three periods of observation, but was significant in only one of three cases ( Table 2). The release of bacteria from the nets into the water during feeding was estimated to be between 2.5 × 109 and 3.9 × 1010 cells m−2 net material. The total wet surface of the net material per cage was calculated to be 171 m2. Thus, the release of bacteria per cage net corresponded to a detachment of 4.3 × 1011 to 6.7 × 1012 cells (average about 3.6 × 1012 cells per cage net). Changes in the bacterial colonization of net fibres were documented by SEM photographs ( 1-(a) ).
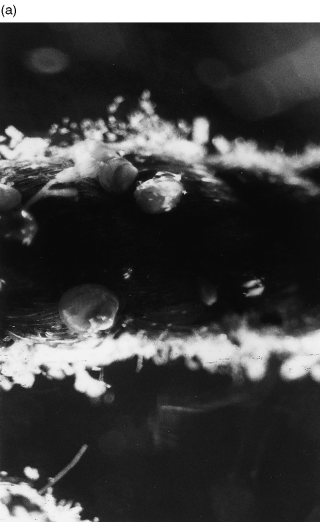

Fig. 2 Photograph of rope before feeding showing fouling. Magnification =× 25. (b) Scanning electron micrograph of fibre from a net before feeding, showing mat of polysaccharides and evidence of heavy bacterial growth. Bar = 5 μm
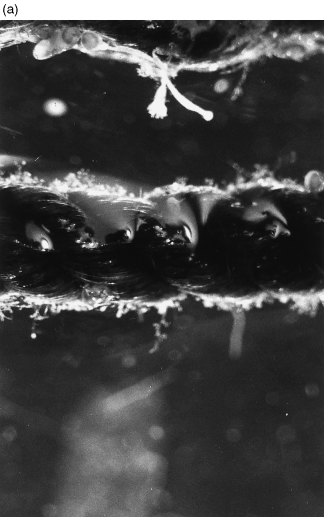
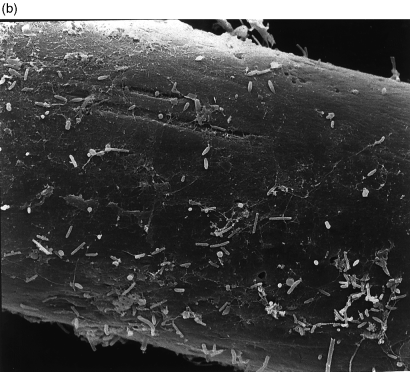
Fig. 3 Photograph of rope after feeding showing reduced amount of fouling. Magnification =× 20. (b) Scanning electron micrograph of fibre from a net after feeding, showing no mat of polysaccharides, note that individual bacteria remained. Bar = 5 μm
For comparison, the total bacteria count of the water inside the cages was also estimated; the difference before and after feeding was about 30 × 103 ml−1, corresponding to 3 × 1010 m−3. Extrapolating this to a cage volume of 56 m3 results in an increase of 1.68 × 1012 bacteria per cage after feeding. The number of bacteria released from the net materials was roughly twice as high; however, it has to be taken into consideration that the bacteria released from the nets were, to a certain extent, also distributed outside the cage.
Discussion
The effect of fish behaviour during feeding on the bacterial abundance in fish cages was studied. A selective increase of bacterial concentrations – indicated by plate counts on four different media – was observed post-feeding compared with the pre-feeding situation. This difference was significant in ten out of 16 cases ( Table 1). A remarkable result was that only the bacterial counts on TCBS-Agar did not increase significantly after feeding (with one exception). This indicates that the increase of bacteria after feeding most probably did not originate from the faecal pellets produced by fish, because one of the main bacterial components of the intestines of coastal fish is Vibrios ( Colwell 1962; Okozumi 1969 ; Yoshimizu 1976 ; Brown 1997). Strong increases of Enterobacteria growing on ENDO-C and of saprophytes growing on TSA after feeding were also occasionally observed at the station located 10 m from the cages ( Table 1). This finding suggests that there is a considerable export of bacteria and/or organic materials from the cages into the surrounding water.
This study has shown that the amount of bacteria attached to the biofilm on net materials can significantly decrease after feeding, most probably due to the turbulence caused by frenzied swimming ( Table 2). The detached bacteria obviously contributed to the observed increase of total bacteria inside the cages. This is documented by the relatively good correspondence of the number of detached bacteria (3.6 × 1012 cells per cage net) to the increase of bacteria in the cage-water (1.68 × 1012 bacteria per cage). On the other hand, very high total bacterial numbers in Cyprinus faeces, ranging from 105 to 1010 per g faeces (reported by Sugita 1988) , could also have contributed to the increase of total bacteria inside the cages. However, it has to be considered that the faecal pellets of trout (the organism under observation) are very solid, which hinders a rapid disruption and considerable release of bacteria into the water column ( Brown 1997).
Input of bacteria through food is considered irrelevant, since trout eat the pellets before they dissolve. Contrarily, pellets may harvest bacteria from the water–air interface, since they are rapidly coated by hydrophobic bacteria which are the main bacterial component of the water–air interface ( Enger 1992) . A contribution of fish slime to the observed increase of total bacteria after feeding cannot be ruled out. Austin (1983) found that fish slime provides an excellent habitat for marine bacteria, which possibly contributed to the large numbers of bacteria present in his fish-holding tanks. Also the contribution of the bacterioneuston to the observed increase of bacteria in the cage-water after water mixing is not clear. In this lipid-rich water–air border layer predominantly hydrophobic bacteria can be enriched by factors of 10 up to 10 000 ( Enger 1992). However, the present investigations revealed that the detachment of bacteria from the net materials could well explain the observed increase in total bacteria after fish-feeding.
The present results suggest that bacteria associated with biofilms on nets are released by frenzied swimming of fish during feeding and may be the most important reason for the observed increase in bacterial counts after feeding. However, significant differences in bacterial detachment were found in only one of the three experiments ( Table 2). This can possibly be attributed to handling problems with the experimental net materials. It should be taken into consideration that an irregular detachment of bacteria from the net pieces during sampling and by the subsequent formaldehyde fixation and ‘critical point’ drying time cannot be avoided. Net samples taken during the calm water phase before feeding would be more affected by these procedures than those taken after feeding when the loose parts of the biofilm were already detached by water turbulence. Therefore, it can be assumed that the amount of released bacteria was underestimated.
From the data obtained in the Kiel Fjord, it was calculated that the numbers of released bacteria reached a total between 3 × 1012 and 4.7 × 1013 cells for the investigated fish farm (seven cages with a total water volume of 383 m3). Such quantities of bacteria detached from nets during feeding seem not to be dramatic, since the average number of bacteria in fjord water is about 2 × 1012 m−3. However, problems arise when considering that the number of detached bacteria is probably seriously underestimated and that the release occurs during a few seconds. It has also to be taken into consideration that the released bacteria are highly hydrophobic; a quality which is frequently combined with virulence ( Duff 1937; Udey 1978) . It can be expected that feed pellets become rapidly coated by a biofilm, including potentially pathogenic bacteria from the net surfaces ( Enger & Thorsen 1992). Furthermore, the exposed areas caused by scale loss on a fish's body could also be inhabited by potential pathogens that are pulse-released from the nets. These processes imply very serious health risks for cultured fish.
Therefore, since relaxed movements of fish could avoid bacterial release from the nets and stress during feeding, the use of automatic feeders is recommended for improving fish health and growth in cage culture.