Trypsin activity and physiological aspects in larval rearing of European sea bass (Dicentrarchus labrax) using live prey and compound diets
Abstract
The aim of this study was to evaluate the potential of a compound diet as a live prey substitute for feeding European sea bass larvae (Dicentrarchus labrax L.). The effect of a commercial diet (Nippai ML feed) and live prey (Artemia nauplii) on tryptic enzyme activity, protein content, growth (standard length) and survival rates of sea bass larvae were tested during a 27-day rearing experiment. Sea bass larvae were divided into two groups. The live food group (control group) was fed exclusively on newly hatched Artemia nauplii (Inve AF grade), the test group was fed exclusively with the compound diet from day 15 onwards. As trypsin has been demonstrated to be a useful indicator for evaluating digestibility of food and the nutritional condition of fish larvae, individual tryptic enzyme activity was determined in both feeding groups. Larvae older than 14 days after hatching and fed on live food showed a significantly higher tryptic enzyme activity than larvae fed the compound diet. A similar relationship between tryptic activity and standard length in both test groups was detected only in small larvae (standard length < 7 mm). The usefulness of proteolytic enzyme activity measurements in larval fish research, as well as its use in aquaculture nutrition research, was confirmed. Protein content, increase in length and survival rates of the sea bass larvae were additionally determined in order to evaluate an influence on the diet. The protein content of larvae fed the Artemia nauplii was higher and the growth of larvae fed the compound diet was reduced. Larval mortality was not affected by the diet given.
Introduction
The cultivation and management of live food (e.g. Brachionus placatilis rotifers and/or Artemia nauplii) remains costly and often provides a varying nutritional quality in the diet. As any attempt to reduce dependence on live prey in the early rearing stages of marine fish is of major bio-technical and economic interest in mass production systems, several types of micro-particulated dry diets have been developed and tested as total and/or partial live prey substitutes in early weaning trials ( Lauff 1984 ; Munilla-Moran 1990 ; Tandler 1991 ; Person le Ruyet 1993 ; Holt 1993; Barnabe 1993 ; Cahu 1997) . Total replacement of live prey with a formulated diet has so far met with very limited success in marine fish larval rearing ( Person Le Ruyet et al. 1993 ) because of induced lower survival and reduced growth performance. The lack of water stability in most micro-particulate diets also caused rapid leaching, bacterial contamination and increased water pollution through soluble and particulate organic constituents ( Jones 1993) .
The successful development of a formulated diet for live prey replacement must consider the important physiological changes in the larval digestive tract during the first weeks of life. In sea bass, the lack of a morphological and functioning stomach during the first 2 weeks of life may be one reason for the difficulties in readily digesting compound diets since the availability of digestive enzyme is limited. It has been suggested that live prey may well supply the necessary enzymes to enhance the digestive capabilities in young fish larvae ( Lauff & Hofer 1984; Ueberschär 1988) .
This study focuses on the proteolytic tryptic enzyme activity in the digestion of live prey and of a compound diet in sea bass larvae. Tryptic enzyme activity in fish larvae is an appropriate indicator of digestive capacity and can be used to measure larval condition in response to changing environmental conditions such as the quality and quantity of ingested food. Low or moderate enzyme activity indicates inadequate feeding or starving conditions. Trypsin has been demonstrated as a useful indicator for the evaluation of digestive processes and the nutritional condition of field-caught marine fish larvae (e.g. Ueberschär 1988, Ueberschär 1988 ; Hjelmeland 1984 , ). Individual tryptic enzyme activity was measured in this study to determine the nutritional condition as influenced by different foods; the results thereof were used to determine whether the digestive potential of the young sea bass larvae was sufficient for successful feeding with the compound diet, Nippai ML. Additional, protein content, length growth and survival rates of the sea bass larvae were determined and correlated with the feed.
Materials and methods
Experimental and rearing conditions
European sea bass (Dicentrarchus labrax L.) yolk sac larvae were obtained from a commercial hatchery in France and transported in plastic bags topped with pure oxygen at 14 °C over a 10 h period. Upon arrival at the laboratory, larvae were slowly acclimated by gradually replacing the bag water with water from the rearing system. The larvae were divided among six conical 65 L tanks (initial stocking density of 145 larvae L−1) attached to a closed sea-water recirculating system ( Fig. 1). Water quality (temperature, pH, oxygen, total ammonium, nitrate and nitrite) was monitored daily. Water temperature was slowly raised from 15.0°C (day 3) to 22.0°C (day 20); the pH remained close to 8. Initial salinity was 34 p.p.t. then lowered to 24 p.p.t. during the first 7 days after hatching to improve the opportunity for swim bladder inflation. The oxygen level was maintained above 7 mg dm−3 and maximum water exchange rates in the tanks were 50% per hour (flow rate: 0.5 L min−1). The light cycle was maintained at 12 h day−1, with a maximum intensity of 1500 lux at the water surface.
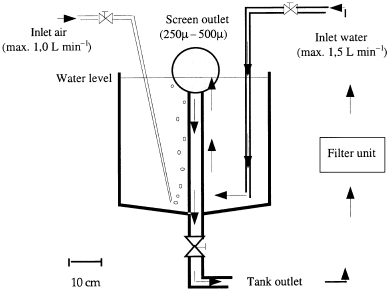
(D)iagram of the rearing units. Each of the six 65 L tanks was stocked at an initial density of 145 sea bass larvae L−1. Arrows indicate direction of flow; hatched lines show water flow through the recirculation system
Mortality was estimated daily by siphoning the tank bottoms and counting the dead larvae, expressed in percentages for each tank.
Diets and feeding practices
The experimental set-up of six tanks was divided into two groups, each with three batches of larvae. The live food group (control group) was fed exclusively on newly hatched Artemia nauplii (Inve AF grade) up to day 27 (end of the experiment).
Using a binocular microscope, Artemia nauplii concentrations in the tanks were monitored each day before and after feeding by counting the number of prey organisms in three 10 mL aliquots and the Artemia nauplii concentration was adjusted twice daily from 1 nauplii mL−1 (day 10) up to 4 nauplii mL−1 at the end of experiment (day 27).
The tank group receiving the test diet was fed on newly hatched Artemia nauplii until day 12 (1 nauplii mL−1). Thereafter, an alternating diet of Artemia nauplii (1.5 nauplii mL−1) and the compound diet was offered between day 13 and 14, followed exclusively by the compound feed until day 27. Fish were fed in excess (up to 600 mg dry feed per tank) 12 h per day. Using the recommendations of the producer, dry feed particles (100–280 μm diameter) were given from day 13 to 26; from day 18 onward, additional particles (250–430 μm) were offered. Prior to feeding, the dry diet was mixed with water for 2 min then dispersed among the tanks. Food ingestion was monitored twice daily by binocular microscope observation of the digestive tract of caught larvae.
Sampling and chemical analyses
Larvae samples
Samples of 10 to 15 larvae per tank were taken at 1- to 3-day intervals and immediately shock frozen (−72 °C) to prevent protein autolysis. Prior to analysis, the standard length (SL) was measured (precision 0.1 mm), the gut content was noted and damaged larvae were discarded.
Determination of tryptic enzyme activity
Tryptic enzyme activity was assayed by the highly sensitive fluorometric method ( Ueberschär 1988) using N-α-benzoyl- l-arginin-methyl-coumarinylamide (MCA) as the fluorescent substrate for each larvae. Larvae were individually homogenized in 250 μL ice-cold TRIS-HCL buffer (0.1 mol, pH 8). The total body homogenate was transferred into Eppendorf-cups and centrifuged at 4 °C and 4110 × g for 60 min.
The supernatants of the homogenates were then used to determine tryptic enzyme activity. Enzyme activity is expressed as the amount of hydrolysed substrate per unit time (hydrolysed MCA larva −1 min−1).
Protein content
Soluble protein content was subsequently measured in the homogenate of each sea bass larva remaining from the tryptic enzyme determination. Procedures followed the photometric method of Bradford (1976) using a protein pssay dye reagent (Bio-Rad, Art. no. 5000–0006). Measurement results were used to evaluate the influence of the test feed and control diet on the larval protein content. The relationship between mean larval protein content and its individual tryptic activity was also examined.
Statistical analyses
Results are given as means (SEM) (sample sizes varied from 14 to 46). To evaluate the significance of the data set differences, these were compared by one way analysis of variance ( anova) followed by a least square difference multiple range test when significant differences occurred at the 5% threshold.
Results
Digestibility and dry food particle performance
Observations of the larval digestive tract and results of the tryptic enzyme measurements indicated that the compound diet was well accepted throughout the experiment. The compound diet particles displayed good water stability, although, their limited buoyancy resulted in a shorter time in the water column when compared with the live prey, and the tank bottoms of the compound diet group had to be cleaned with care to prevent bacterial build-up and water pollution. As known from common feeding practices, the live prey were also readily ingested throughout the experiment.
Mortality and growth
In all six trials the daily mortalities were highest during the first 2 weeks. Mortality rates varied from 29 to 48%. A relationship between the diets that the larvae were fed and larval mortality was not observed. Larvae that were fed exclusively on live prey grew faster and reached a higher mean total length ( Fig. 2) at the 5% threshold than did larvae that were fed on the compound diet.
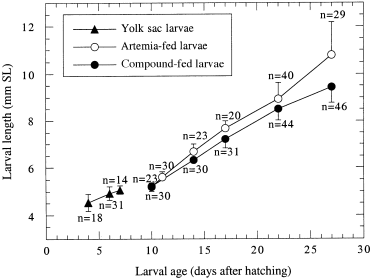
)rowth of sea bass larvae (Dicentrarchus Labrax) raised either on live food (Artemia salina nauplii) or on a combination of live food for 2 days followed thereafter by a compound diet. Data points are the means of 14 to 46 individually measured larvae. The means of the Artemia-fed larvae are significantly different from those of the larvae fed the compound diet (analyses of variance, P > 0.05). Vertical bars represent standard error of the mean
The mean SL of larvae that were fed the compound diet reached 95% (day 14), 94% (day 17), 95% (day 22) and 87% (day 27) of the mean SL of the larvae that were fed Artemia (control group).
Tryptic enzyme activity
Unfed yolk sac larvae showed an increase in the tryptic enzyme activity until day 7 ( Fig. 3), which demonstrated the existence of a larval proteolytic digestive potential allowing digestion of the first exogenous feed.
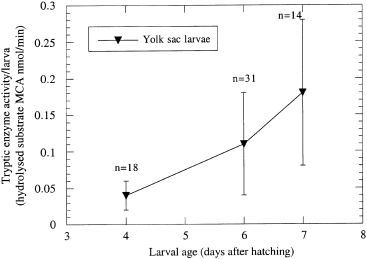
)ryptic enzyme activity of unfed sea bass (Dicentrarchus labrax) yolk sac larvae related to larval age. Data points represent the means of 14 to 31 individually measured larvae. Vertical bars represent standard error of the mean
However, enzyme activity varied greatly between individuals of the same size, in particular after the start of feeding. An initial increase in tryptic enzyme activity with larval age was observed in both feeding groups ( Fig. 4) which was followed by a period of little change between days 17 and 22. Larvae that were older than 14 days after hatching and fed on live food showed a significantly higher tryptic enzyme activity than larvae that were fed the compound diet. However, this effect disappeared in older larvae. At day 27, tryptic enzyme activity was similar in both groups (5.25 nmol min−1 in the control group and 5.11 nmol min−1 in the test group). These results indicate a significant difference in enzyme activity between both groups over a considerable period of time (analyses of variance, P > 0.05), with the Artemia-fed group showing superior results at least during the first 2 to 3 weeks after hatching. The fact that the test diet group later reached similar enzyme activity levels signifies an improved metabolic capability in older larvae of both feeding groups.
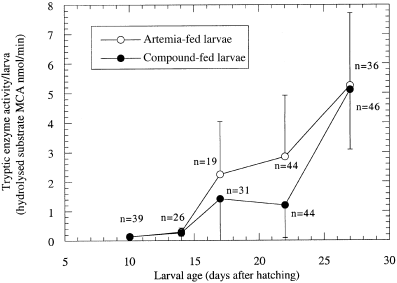
)ryptic enzyme activity of sea bass larvae (Dicentrarchus labrax) raised either on live food (Artemia salina nauplii) or on a combination of live food for two days followed thereafter by a compound diet. Data points are the means of 14 to 46 individually measured larvae. The means of the Artemia-fed larvae are significantly different from those of the larvae fed the compound diet (analyses of variance, P > 0.05). Vertical bars represent standard error of the mean
Figure 5 compares the tryptic enzyme activities for larvae of the same standard length within the size range of 4.5–13.5 mm SL. A similar relationship between tryptic activity and standard length in both test groups was found only in small larvae (SL > 7 mm). Variability greatly increased in larger larvae, with no obvious relationship of enzyme activity to feed type. This is not surprising, as actual enzyme activity is expected to relate more to individual size and instant metabolism rather than to larval age.
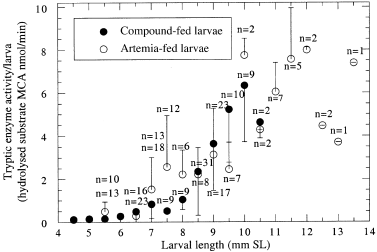
)ryptic enzyme activity of sea bass larvae (Dicentrarchus labrax) raised either on live food (Artemia salina) or on a combination of live food for 2 days followed therafter by a compound diet in relation to larval SL. Data points are the means of 1 to 23 individually measured larvae of same standard length classes. The means of the Artemia-fed larvae are significantly different from larvae of the same size which were fed the compound diet (analyses of variance, P > 0.05). Vertical bars represent standard error of the mean
Protein content
The protein content of larvae is presented in terms of both age after hatching, as well as in SL of larvae regardless of age; from day 10 (first exogenous feeding) onward, both test groups showed an increase in protein content. Maximum rate of protein increase in larvae was observed on day 22 after hatching; however, the mean protein content of larvae that were fed exclusively on live prey was significantly higher at the 5% threshold level in comparison with larvae fed dry feed ( Fig. 6). An increase in protein content occurred more rapidly in the group that were fed live food; by comparison, in the dry food group the mean protein content of the larvae was 89% (day 10), 82% (day 17), 67% (day 22) and 53% (day 27).

)rotein content of sea bass larvae (Dicentrarchus labrax) raised either on live food (Artemia salina) or on a combination of live food for 2 days followed thereafter by a compound diet. Data points are the means of 14 to 46 individually measured larvae. The means of the Artemia-fed larvae are significantly different from those of the larvae fed the compound diet (analyses of variance, P > 0.05). Vertical bars represent standard error of the mean
The high variability of the data already indicated the notable differences in individual growth rates. Therefore, data were also analysed in relation to larval size to ascertain whether nutritional differences were reflected in different protein contents of larvae at the same SL. Results showed that larvae of the same size contained the same mean protein content, indicating that the protein content was therefore not affected by the quality of feeds offered; the differences were mainly due to differences in growth rates ( Fig. 7).
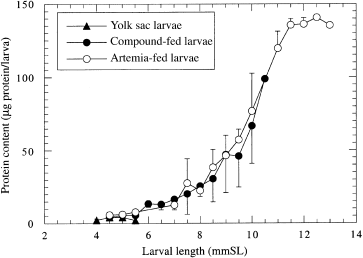
)rotein content of sea bass larvae (Dicentrarchus labrax) Raised either on live food (Artemia salina) or on a combination of live food for 2 days followed thereafter by a compound diet. Data points are the means of 14 to 46 individually measured larvae per length class. The means of the Artemia-fed larvae are significantly different from those of the larvae fed the compound diet (analyses of variance, P > 0.05). Vertical bars represent standard error of the mean
Discussion
In an attempt to limit the dependence on live food during larval fish rearing, a dry feed was tested as a suitable alternative nutrient source.
In general, factors influencing the usefulness of prepared diets are: water stability of the dry feed particles, high acceptability of the particles, their digestibility and nutritional composition.
For excellent performance in the water column, compound diet particles need a high water stability in combination with a long-lasting buoyancy and visual detection to improve diet attractiveness. The lack of a morphological and functioning stomach in early larval stages seems to be a major limitation to the utilization of dry feed during the first 2 or 3 weeks of life. The dry feed should be easily digested by the larva’s digestive tract, which is not yet completely developed. In addition, dry feed particles must not only meet the nutritional needs of the larva, but the nutrients in the micro-pellets must also be digestible. This requires a detailed understanding of the digestive physiology.
Reduced growth and lower protein content in larvae that were fed compound diets have been reported by other authors, particularly when using micro-encapsulated feeds as partial or total replacement of live prey ( Gatesoupe 1977 ; Bengston 1993; Jones et al. 1993 ; Person Le Ruyet et al. 1993 ). In comparison with sea bass larvae that were fed commercial compound diets, those fed on live prey achieved much faster initial development, although the mean protein content of comparable length larvae remained unaffected, even in different feeding groups.
Tandler & Kolkovski (1991) suggested that poorer growth can be explained by a reduced food intake of dry feeds since these may be less attractive than live prey, mainly caused by lack of stimuli which are evinced by live, mobile organisms. in this experiment, mobility of the dry food particles was temporarily enhanced by pipetting particles into the water column of the rearing tanks. Additionally, the feed was offered in excess; it is therefore unlikely that food availability in the compound diet feeding was a limiting factor.
In larval pancreatic tissue most of the trypsin is present as an enzymatically inactive trypsinogen, whereas most of the trypsin in the intestinal tract is enzymatically active ( Ueberschär 1992) . The fluorescence technique used in this study measured only the active form (trypsin). However, the ratio of proteolytic enzyme quantity (expressed as the amount of hydrolysed substrate MCA larva−1 min−1) was significantly reduced when feeding the compound diet. It can be suggested that secretion of pancreatic enzymes (trypsinogen) was minimized and/or delayed but ceased in the compound diet feeding trial. Consequently, the trypsin patterns observed in the two feeding groups can be attributed to the different nutritional values of the two diets.
The observed increase in tryptic enzyme activity in yolk sac larvae just prior to first feeding is also reported in recent literature (sea bass, Dicentrarchus labrax, in Alliot 1977 ; Herring, Clupea harengus, in Pedersen 1988 , Ueberschär 1988) . It can be assumed that this initial increase is a general feature of fish larvae that hatch at embryonic stages with large yolk sacs.
Assuming that adequate amounts of Artemia nauplii and dry food were fed in this experiment, the observed differences in the enzyme activity, protein content and growth rates of sea bass larvae can be attributed to certain superior nutritional qualities of live prey when compared with prepared diets. Person Le Ruyet et al. (1993) pointed out the nutritional deficiency of artifical dry diets when noting the components in live prey that stimulate larval enzyme secretion ‘active factors’. This also demonstrates the limited understanding to date of what comprises an adequate nutritional diet in the early life history stages of fish. If digestive enzymes are among the factors that can be extracted from live food organisms, then a dietary supplement of commercial digestive enzymes may enhance food assimilation. Rearing experiments with carp (Cyprinus carpio) larvae showed enhanced growth and food conversion rates when adding proteolytic enzymes to the dry feed ( Dabrowski 1978 , 1979).
The gastro-intestinal hormone cholecystokinine (CCK) is also known to be an important factor in stimulating pancreatic tissue growth. In future studies on stimulation of pancreatic enzyme secretion, one may wish to test the effect of CCK when added to diets.
The analytical procedure described here does not distinguish between exogenous and endogenous trypsin. However, the differences in tryptic activity of both feeding groups therefore cannot be directly attributed to an exogenous enzyme contribution from the live prey organisms. Trypsin and tryptic-like enzymes (Chrymotrypsin, Aminopeptidase), however, occur in many marine organisms ingested by fish larvae, including cultured Artemia nauplii ( Hirche 1979; Lauff & Hofer 1984). As reported in recent literature, only a minor part of the total enzyme activity has its source in the ingested food organisms ( Ueberschär 1988) . The major portion is synthesized by the larvae themselves as an immediate response to food ingestion ( Fänge 1979 ; Hjelmeland 1988 ; Pedersen & Hjelmeland 1988; Pedersen 1992) .
Conclusions
Digestive enzyme activity can be used as an indicator of larval food acceptance and to some extent serve as an indicator for the digestive capacity in relation to the type of feed offered. Taking the latter indicator function, it is clear that the compound diet does not appear to meet the larval nutritional requirements fully. Live prey such as Artemia, although a common species not entirely close to the spectrum of natural prey, performs much better. The excretion from live prey autolysis may be a stimulation to the secretion of trypsinogen from the pancreas into the intestine and could activate zymogens present in the gut.
It was demonstrated in this study that tryptic enzyme activity is a strong indication of short-term variations in feed quality which makes this methodology of tryptic enzyme analysis a useful tool in studies of nutritional requirements in the early life history stages of fish.
Digestive protease trypsin is a suitable short-term indicator reflecting the nutrition condition of larva. The method applied in this study for individual tryptic enzyme activity follows the highly sensitive fluorescence technique described by Ueberschär (1988) and is applicable to aquaculture nutrition research, in particular as a tool for testing the suitability of larval feeds for early weaning and/or first feeding.




