Evidence that endo-1,4-β-glucanases act on cellulose in suspension-cultured poplar cells
Summary
Suspension-cultured poplar (Populus alba) cells produce two distinct endo-1,4-β-glucanases, one of which is released in the extracellular culture medium and the other localized in their walls. Two cDNA clones, PopCel1 and PopCel2, isolated from a poplar cDNA library, encode the extracellular and the wall-bound endo-1,4-β-glucanases, respectively, based upon deduced amino acid sequences. The products of these two genes contained domains conserved in endo-1,4-β-glucanase (family 9) and showed 91.5% amino acid identity. The levels of both PopCel1 and PopCel2 mRNAs increased during the lag phase of growth and decreased rapidly during the linear phase. After the levels had decreased, they were again increased by addition of sucrose to the culture medium and further enhanced by the addition of 2,4-dichlorophenoxyacetic acid (2,4-D) in the presence of sucrose. The accumulation of the mRNAs was correlated with the solubilization of cello-oligosaccharides. Cello-oligosaccharides and xyloglucan were also solubilized from the wall preparations of poplar cells incubated with enzyme preparations from the extracellular culture medium and walls. An antibody against both PopCel proteins reduced the production of cello-oligosaccharides by the extracellular enzyme by 90% and that by the wall-bound enzyme by 55%, and also prevented xyloglucan solubilization. The results show that the accumulation of poplar endo-1,4-β-glucanases is regulated indirectly by auxin in the presence of sucrose and can act on cellulose in suspension-cultured poplar cells.
Introduction
Plant cell growth is controlled by the actions of cell-wall-related enzymes on wall architecture. This is probably a complex process involving the coordinate actions of expansins ( Cosgrove, 1998), endo-1,4-β-glucanase ( Hayashi, 1987; Matsumoto et al., 1997 ), xyloglucan endotransglucosylases ( Fry, 1995; Nishitani, 1998) and cellulose synthases ( Delmer and Amor, 1995), among others. Suspension-cultured poplar cells produce a high level of endo-1,4-β-glucanase activity that is localized in the cell walls and the extracellular culture medium ( Nakamura and Hayashi, 1993b; Ohmiya et al., 1995 ). The level of mRNA encoding endo-1,4-β-glucanases in (undifferentiated) cultured cells was much higher than that in differentiated tissues, such as roots and stems ( Nakamura et al., 1995 ). The accumulation of the mRNA was further enhanced by the application of 2,4-dichlorophenoxyacetic acid (2,4-D) during normal growth, but reduced by the application of gibberellic acid, benzyladenine or abscisic acid. Only a small number of genes encoding endo-1,4-β-glucanases seem to be required for cell growth in suspension-cultured poplar cells ( Nakamura and Hayashi, 1993b; Ohmiya et al., 1995 ), although multiple forms of endo-1,4-β-glucanases were expressed as determined by the presence of mRNA throughout the period of developmental growth in intact plants. At least eight different mRNAs for endo-1,4-β-glucanases are expressed in tomato plants ( Catala and Bennett, 1998).
Two distinct endo-1,4-β-glucanases occur in growing poplar cells, one of which is released into the extracellular culture medium ( Nakamura and Hayashi, 1993b), with the other remaining in the cell walls ( Ohmiya et al., 1995 ). The latter enzyme has a strong affinity for cell walls, although both enzymes have similar molecular weights (about 50 kDa) and pI values (about 5.5). Although the levels of extracellular and wall-bound endo-1,4-β-glucanase activities increased during the lag phase of growth, the former decreased during the linear phase and the latter decreased during the stationary phase ( Nakamura and Hayashi, 1993a). The two endo-1,4-β-glucanases may be involved in the formation and loosening of the wall during its division and enlargement. Although auxin-induced endo-1,4-β-glucanases are related to the degradation and solubilization of xyloglucan during cell expansion in pea stems ( Hayashi et al., 1984 ) , poplar endo-1,4-β-glucanases do not significantly degrade xyloglucan but cleave amorphous cellulose ( Nakamura and Hayashi, 1993b; Ohmiya et al., 1995 ).
We have characterized two endo-1,4-β-glucanases in suspension-cultured poplar cells, and analysed their induction together with the solubilization of 1,4-β-glucosyl linkages in cell-wall components.
Results
Identification of cDNA clones for two endo-1,4-β-glucanases
Several cDNAs for endo-1,4-β-glucanase were identical in a suspension-cultured poplar cell cDNA library by using PopCel1 as a probe. Half of 20 cDNA clones that hybridized to the probe were related to PopCel1 and the others were slightly different from PopCel1 and named PopCel2 ( Figure 1). We confirmed that PopCel1 encoded the extracellular endo-1,4-β-glucanase and PopCel2 the wall-bound endo-1,4-β-glucanase based upon their deduced amino acid sequences ( Ohmiya et al., 1999 ).

Deduced amino acid sequences of PopCel1 and PopCel2 gene products.
Identical residues are indicated by asterisks. The arrow indicates the N-terminus, and N-terminal polypeptides which were identified using amino acid sequencing are underlined ( Ohmiya et al., 1999 ). Possible N-glycosylation sites are represented by wavy underlining. The closed circles indicate residues that are essential for the catalytic reaction of cellulases of family 9. The box shows the amino acid sequence used for the polypeptide antigen.
The open reading frames of both PopCel1 and PopCel2 encoded polypeptides of 491 amino acids as shown in Figure 1. PopCel1 exhibits 90% identity at the DNA level and 90.5% identity at the whole deduced amino acid level to PopCel2. Comparison of the deduced amino acid sequences revealed that both mature polypeptides showed 91.5% similarity, and their putative signal sequences had 66.7% similarity. Both cDNAs have a consensus sequence for a potential site of N-linked glycosylation at Asn257 and no sequence for a transmembrane helix ( Brummell et al., 1997 ; Nicol et al., 1998 ). The deduced amino acid sequence of PopCel1 indicated a slightly higher pI value (5.53) than that of PopCel2 (5.46). Although the extracellular endo-1,4-β-glucanase bound to anion exchange resins at pH 6.2, the wall-bound enzyme bound to cation exchange resins, suggesting that there was a strong basic charge on the surface of the polypeptide. In addition, the wall-bound endo-1,4-β-glucanase had a strong affinity for cell walls, i.e. it was extracted with 20 m m sodium phosphate buffer (pH 6.2) containing 0.5 m NaCl but not with the buffer alone. Although both enzymes showed similar distributions of hydrophilic and hydrophobic regions by hydropathy analysis, the predicted secondary structures of the gene products according to the program described by Chou and Fasman (1978) were significantly different ( Figure 2). The difference between their conformations may be the cause of the different protein properties and localization.

Secondary structures of PopCel1 and PopCel2 gene products.
Endo-1,4-β-glucanases (cellulases) have been classified, on the basis of primary structural homology, into 16 families by hydrophobic cluster analysis ( Henrissat et al., 1989 ). Both the PopCel1 and PopCel2 proteins belong to family 9. It seems likely that Glu474 residues in PopCel1 and PopCel2 proteins function as a proton donor and that Asp82 and Asp85 stabilize the positively charged intermediate during the hydrolytic reaction, as shown for the endo-1,4-β-glucanase from Clostridium thermocellum ( Chauvaux et al., 1992 ; Juy et al., 1992 ). These findings indicate that both poplar endo-1,4-β-glucanases have a similar catalytic domain. The poplar endo-1,4-β-glucanases do not have a putative cellulose-binding domain, although an endo-1,4-β-glucanase mRNA with a cellulose-binding domain has been identified in tomato hypocotyls ( Catala and Bennett, 1998).
Southern blot analysis
Nuclear DNA was digested with various restriction endonucleases and hybridized with labelled PopCel1 and PopCel2 probes. The Southern blot patterns of the two genes were identical as shown in Figure 3. Because the DNA sequences of PopCel1 and PopCel2 are similar each other, it is possible that they represent two alleles of a single gene.

Genomic Southern blot analysis.
Total poplar genomic DNA (20 μg per line) was digested with restriction enzymes and hybridized with DIG-labelled PopCel1 and PopCel2 probes.
Analysis of PopCel1 and PopCel2
An antibody against the 15-amino-acid sequence (163CWERPEDMDTPRNVY167) common to both PopCel1 and PopCel2 recognizes an endo-1,4-β-glucanase in the extracellular culture medium and the walls of poplar cells to give a single band at 50 kDa by Western blot analysis. The antibody lowered the extracellular endo-1,4-β-glucanase activity by 80% and the wall-bound endo-1,4-β-glucanase activity by 55% after conjugation with protein A–Sepharose ( Figure 4a), relative to non-immune serum. This result shows that the endo-1,4-β-glucanase activity in the extracellular culture medium is predominantly due to PopCel1 and about 55% of the activity in walls is due to PopCel2.
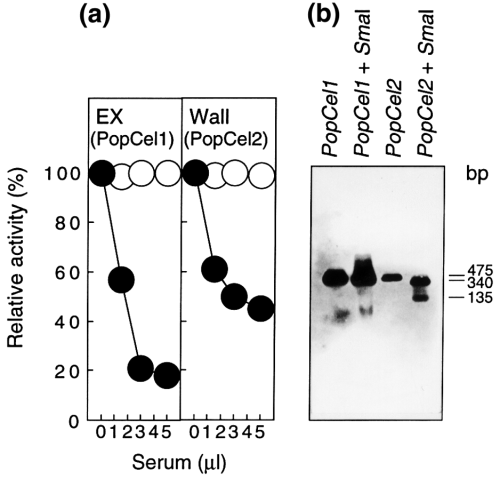
Analyses of PopCel1 and PopCel2 gene products and mRNAs.
(a) Activity levels of PopCel1 and PopCel2 as a proportion of the total endo-1,4-β-glucanase activities of poplar cells determined by immunoprecipitation with antibody. The antiserum (1.5–5 μl) was added to 100 μl of enzyme preparation (30 units) and incubated at 4°C for 1 h. Then, a suspension (50 μl) of protein A–Sepharose in PBS were added and the mixture was incubated at 4°C for 1 h and centrifuged. Then, the enzymatic activity in the supernatant (100 μl) was determined using the specific antiserum (●) and non-immune serum (○). (b) Analysis of PopCel1 and PopCel2 mRNAs by RT–PCR Southern blot analysis.
Since the DNA sequences of PopCel1 and PopCel2 resemble each other, hybridization with labelled cDNAs or even with labelled RNA as a probe did not distinguish the two mRNAs. Therefore, the levels of the mRNAs in poplar cell were measured by RT–PCR analysis using specific primers for each cDNA ( Hentzen et al., 1996 ). The amounts of first-strand cDNA in the PCR reaction mixture were adjusted by the levels of amplified DNA for poplar actin, and the levels were estimated from the linear portion of the amplification curve. After amplification for 18 cycles, DNA bands were invisible by ethidium bromide staining but were visualized by Southern hybridization ( Figure 4b). Each PCR reaction contained first-strand cDNA equivalent to 40–85 ng of total RNA. This shows that the RT–PCR analysis of mRNA is not only a highly sensitive assay for small numbers of differentiated cells ( Shimizu et al., 1997 ) and low-copy-number RNA species ( Chelly et al., 1988 ), but is also useful to distinguish between similar RNA sequences. The amplified PopCel2 DNA (475 bp) could be distinguished from PopCel1 DNA by digestion with SmaI ( Figure 4b).
Expression pattern
The levels of both PopCel1 and PopCel2 mRNAs increased within 2 days after the transfer of cells to fresh Murashige and Skoog basal medium containing sucrose and 2,4-D ( Figure 5). The levels were maximal at day 4 and decreased at a late stage of the linear phase. Western blot analysis showed that both endo-1,4-β-glucanases increased at day 2 and decreased during the linear growth phase for the extracellular protein and during the stationary phase for the wall-bound protein ( Figure 5). This is in agreement with earlier findings ( Nakamura and Hayashi, 1993a) that the activity of the extracellular and wall-bound endo-1,4-β-glucanases increased during the lag phase of growth and then decreased during the linear and the stationary phases, respectively.

Levels of PopCel1 and PopCel2 mRNAs and their gene products during growth of suspension-cultured poplar cells.
(●) Cell growth; (○) sucrose.
The mRNAs became abundant 12 h after the addition of sucrose to the culture medium during the linear or stationary phase, although the levels of mRNAs after being lowered were slightly increased by the addition of 2,4-D ( Figure 6). Nevertheless, the levels were further enhanced by the addition of 2,4-D together with sucrose. After the simultaneous addition of sucrose and 2,4-D to the culture medium, signals for both PopCel1 and PopCel2 mRNAs were strongly visible for 2 days. These findings indicate that the induction of endo-1,4-β-glucanases by auxin is dependent on the presence of sucrose in poplar cells. Although the levels of both mRNAs were similar in the presence of sucrose, the accumulation of PopCel1 mRNA was slightly greater than that of PopCel2 mRNA in the presence of both 2,4-D and sucrose.

Effect of sucrose and 2,4-D on PopCel1 and PopCel2 mRNA accumulation.
Sucrose and 2,4-D were added on day 8 of poplar cell culture and the levels of mRNAs were determined.
Solubilization of cello-oligosaccharides and xyloglucan
To examine whether poplar endo-1,4-β-glucanases cause the in situ degradation of 1,4-β-glucosyl linkages in the wall of poplar cells, we determined the amounts of cello-oligosaccharides and xyloglucan released into the cultured medium. It should be noted that xyloglucan oligosaccharides would be ineffective for the action of cellobiose dehydrogenase ( Tominaga et al., 1999 ), and xyloglucan oligomers (molecular weight < 5000) would not have been detected by the iodine–sodium sulphate method used in the present work. Figure 7 shows that soluble cello-oligosaccharides were produced in 2 days and became maximal at day 4, decreasing thereafter during the linear phase. The addition of sucrose and 2,4-D at day 8 stimulated a marked increase in the cello-oligosaccharide solubilization. Thus, the formation of cello-oligosaccharides was closely correlated with the accumulation of PopCel1 and PopCel2 mRNAs together with the appearance of their gene products during cell growth ( Figure 5). It should be noted that the low recovery of cello-oligosaccharides was potentially due to the action of β-glucosidase activity which might also be induced by auxin ( Evans, 1974).

Changes in cello-oligosaccharides and soluble xyloglucan during growth.
(●) The amounts of cello-oligosaccharides and soluble xyloglucan during growth in each column; (○) the amounts after the addition of 3% sucrose and 10 μm 2,4-D at day 8 (arrow).
Soluble xyloglucan was formed gradually during the linear phase and increased during the stationary phase ( Figure 7). When both sucrose and 2,4-D were applied to the culture medium during the stationary phase, the formation of soluble xyloglucan was increased slightly. Thus, the solubilization of xyloglucan appeared to be correlated with cello-oligosaccharide formation during the linear phase, but not during the stationary phase.
Cello-oligosaccharides and xyloglucan were solubilized when the wall preparations of poplar cells were incubated with the enzyme preparations derived from extracellular culture medium or cell walls ( Table 1). The level of solubilization was higher for cello-oligosaccharides than for xyloglucan. An antibody against a conserved endo-1,4-β-glucanase sequence reduced the production of cello-oligosaccharides and xyloglucan by extracellular and wall enzymes similarly, although it reduced the cello-oligosaccharide production by the enzyme preparation from cell walls by 55% . This is in agreement with the result of a previous experiment ( Figure 4a) indicating that the antibody lowered the wall-bound endo-1,4-β-glucanase activity by 55%. The production of cello-oligosaccharides also corresponded to the solubilization of xyloglucan, which might result in cellulose hydrolysis. In addition, cello-oligosaccharides and xyloglucan were markedly solubilized by fungal endo- and exo-cellulases which do not show any activity for xyloglucan degradation, when xyloglucan is used as a single substrate ( Amano et al., 1996 ; Shiroishi et al., 1997 ) .
| Enzyme treatment | Cello-oligosaccharides (ng cellobiose-equivalents) | Xyloglucan (ng) |
|---|---|---|
| Extracellular enzyme a | ||
| + Non-immune serum | 600 | 160 |
| + Antibody | 60 | 72 |
| Wall-bound enzyme a | ||
| + Non-immune serum | 1280 | 220 |
| + Antibody | 580 | 102 |
| Endo-cellulase b | 2080 | 1120 |
| Exo-cellulase b | 4165 | 1410 |
- a 300 units of enzyme preparation were used. b 200 units of enzyme due to an increase in reducing power was used.
The time required for cello-oligosaccharide and xyloglucan solubilization
The activities of extracellular and wall-bound endo-1,4-β-glucanases and the accumulation of PopCel1 and PopCel2 mRNAs in auxin-starved poplar cells were examined at intervals after the addition of sucrose and/or 2,4-D. After the addition of both sucrose and 2,4-D, the activity in the extracellular medium appeared at 12 h, and that in the wall increased gradually after 12 h although a certain level of the activity was present in the walls at 0 h ( Table 2). After the addition of sucrose alone, the activities of both extracellular and the wall fractions were lower than those in the presence of both sucrose and 2,4-D. The addition of 2,4-D alone did not induce the extracellular activity and decreased the activity remaining in the walls during incubation. Both PopCel1 and PopCel2 mRNAs accumulated at 12 h after the addition of both sucrose and 2,4-D, accompanying the induction of both potential gene products in extracellular and wall fractions ( Figure 8), although a small level of PopCel1 mRNA might have appeared at 6 h. This is in agreement with results ( Table 2) indicating that the activities of extracellular and wall-bound endo-1,4-β-glucanases were induced at 12 h. Thus, there is a close correlation between mRNA accumulation and the gene product formation after induction. The time dependence of the accumulation is also in agreement with the finding that the accumulation of both PopCel1 and PopCel2 mRNAs occurred at 12 h after the addition of both sucrose and 2,4-D during the stationary phase ( Figure 6). The level of the accumulation was lower after the addition of sucrose alone and was null after the addition of 2,4-D alone (data not shown).
| Time (h) | Extracellular | Wall-bound | ||||
|---|---|---|---|---|---|---|
| 2,4-D | Sucrose | Sucrose + 2,4-D | 2,4-D | Sucrose | Sucrose + 2,4-D | |
| 0 | 0 | 0 | 0 | 85 | 85 | 85 |
| 6 | 0 | 0 | 0 | 25 | 44 | 63 |
| 12 | 0 | 127 | 255 | 68 | 93 | 132 |
| 24 | 0 | 93 | 297 | 51 | 93 | 178 |
| 48 | 0 | 17 | 255 | 34 | 110 | 260 |

Induction of mRNAs and gene products of PopCel1 and PopCel2 in auxin-starved poplar cells over time.
The formation of cello-oligosaccharides and soluble xyloglucan was examined at intervals during the induction of endo-1,4-β-glucanases. As shown in Table 3, cello-oligosaccharides were detectable at 12 h and increased thereafter, although xyloglucan was scarcely detectable during the incubation period up to 24 h. The level of solubilization was much higher in auxin-starved cells after induction with sucrose and 2,4-D ( Table 3) than that during normal cell growth ( Figure 7). It is likely that the regeneration of poplar cells from arrested growth causes a drastic modification of the cell-wall network which results in significant degradation of cellulose and xyloglucan.
| Time (h) | Cello-oligosaccharides (μg cellobiose equivalents/flask) | Xyloglucan (μg/flask) |
|---|---|---|
| 0 | 0 | 0 |
| 6 | 0 | 0 |
| 12 | 150 | 0 |
| 24 | 250 | 0 |
| 48 | 310 | 200 |
To examine whether a newly synthesized protein is required for the induction of endo-1,4-β-glucanases by sucrose and 2,4-D, we examined the effects of cyclohexamide, an inhibitor of protein synthesis, on the gene expression. The results showed that cycloheximide blocked the expression of both endo-1,4-β-glucanase genes ( Figure 9) and the solubilization of cello-oligosaccharides (data not shown), indicating that protein synthesis is required for the induction by sucrose and 2,4-D.
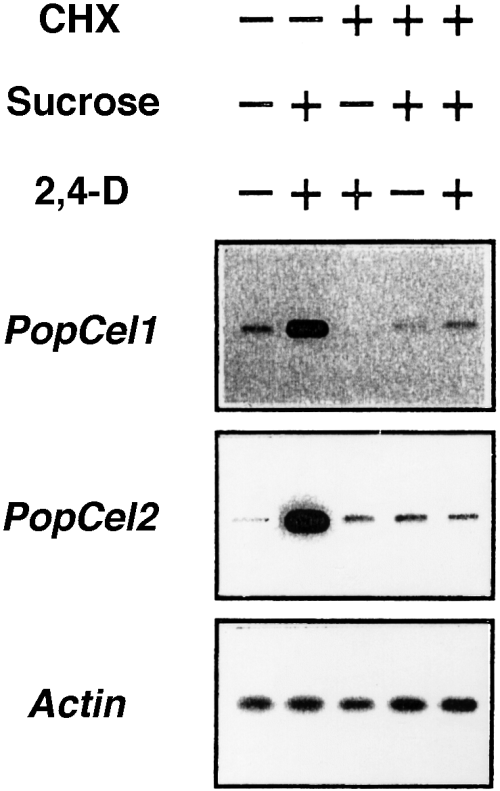
Effect of cycloheximide (CHX) on PopCel1 and PopCel2 mRNA accumulation.
Discussion
Induction of endo-1,4-β-glucanases
Poplar endo-1,4-β-glucanases were induced by sucrose, but not by auxin alone, and the effect of sucrose was enhanced by auxin ( Figure 6). If poplar cells are grown in the presence of sucrose without auxin in Murashige and Skoog's medium, their cell growth is arrested. Thus, auxin is required for the growth of poplar cells. Two endo-1,4-β-glucanases are indirectly induced by auxin in the presence of sucrose in suspension-cultured poplar cells and their expression is correlated with cell growth.
Induction of poplar endo-1,4-β-glucanases was very slow (12 h) and blocked by cycloheximide ( Table 2, 8, 9), similar to the case of barley α-amylase induction with GA-response myb gene (GAmyb) by gibberellin ( Gubler et al., 1995 ). This phenomenon should result in late expression, in which several steps including the synthesis of transcription factors may be required for induction ( Figure 9). Endo-1,4-β-glucanase was expressed 24 h after the addition of auxin in pea epicotyls ( Verma et al., 1975 ), 12 h after the addition of auxin in tomato hypocotyls ( Catala et al., 1997 ), 24 h after the addition of ethylene in pepper leaf abscission zones ( Ferrarese et al., 1995 ), and 24 h after the addition of ethylene in soybean abscisson zones ( Koehler et al., 1996 ).
Two isoforms of endo-1,4-β-glucanases
There is a possibility that PopCel1 and PopCel2 represent either alleles or tandem alleles ( Figure 3), and that the two genes are co-dominant producing twice the activity of endo-1,4-β-glucanase in poplar cells ( Table 2). Growing poplar cells produce at least two isoforms of endo-1,4-β-glucanases corresponding to PopCel1 and PopCel2 ( Figure 1). Two or more endo-1,4-β-glucanase isoforms have also been found in tomato ( Lashbrook et al., 1994 ), pepper ( Ferrarese et al., 1995 ) and pine ( Loopstra et al., 1998 ). There may be another form of endo-1,4-β-glucanase even in poplar callus tissues because the antibody reduced the cello-oligosaccharide solubilization by the wall-bound enzyme preparation by only 55%. The different localizations, soluble and bound to cell walls, may be due to differences in conformation between the two isoforms. Although the two poplar endo-1,4-β-glucanases exhibited a similar expression pattern and slightly different levels of mRNA accumulation ( 5, 6), there is no information about the difference between the two isoforms in function, expression and genomic locus in poplar cells.
Hydrolysis of cellulose microfibrils in the primary wall
A close correlation between an increase in endo-1,4-β-glucanase activity and the accumulation of PopCel1 and PopCel2 mRNAs was observed in auxin-starved cells ( Figure 8 and Table 2). The induction of endo-1,4-β-glucanases may be responsible for the formation of cello-oligosaccharides solubilized from the walls ( Table 3). The accumulation of mRNAs for PopCel1 and PopCel2 was closely associated with the formation of cello-oligosaccharides during normal growth ( Figure 7) as well as during their induction in auxin-starved cells ( Table 3). It should also be noted that the enzymes may not attack crystalline cellulose but only amorphous cellulose ( Nakamura and Hayashi, 1993b; Ohmiya et al., 1995 ). As some cello-oligosaccharides could be solubilized by incubation with poplar endo-1,4-β-glucanase preparations ( Table 1), amorphous cellulose might exist in suspension-cultured poplar cells. Thus, we conclude that cellulose is the main cell-wall substrate for the endo-1,4-β-glucanases in suspension-cultured poplar cells.
Fungal cellulases also hydrolysed cellulose microfibrils in the wall preparations of poplar cells, from which exo-cellulase solubilized cello-oligosaccharides at only a twofold higher level than endo-cellulase ( Table 1). If the cellulose microfibrils of poplar cells were highly crystalline like Avicel (microcrystalline cellulose) , the exo-cellulase may be expected to produce more than tenfold more cello-oligosaccharides than the endo-cellulase. If the microfibrils were amorphous, the endo-cellulase may be expected produce more than thousand-fold more oligosaccharides than the exo-cellulase, i.e. the ratio of Avicel to carboxymethylcellulose in terms of saccharification activity was 0.7 for exo-cellulase and 0.5 × 10−4 for endo-cellulase ( Kanda et al., 1978 ; Kanda et al., 1980 ). The result shows that the poplar cellulose does not consist of completely amorphous structures but partly superficial or para-crystalline fibres, which could then be attacked by endo-1,4-β-glucanases. Observation by electron microscopy shows that the microfibrils of elongating pea stems are much thinner than those of elongated cells ( Baba et al., 1994 ), and electron diffraction of cellulose in cultured rose cells shows superficial chains ( Chanzy et al., 1979 ). The superficiality and/or para-crystallinity of the fibres is probably due to xyloglucan association in the primary wall, because xyloglucans interfere with the ribbon assembly of cellulose microfibrils produced by Acetobacter xylinum ( Hayashi et al., 1987 ) .
Is xyloglucan turnover involved in cellulose degradation?
Do poplar endo-1,4-β-glucanases mediate the auxin-induced xyloglucan turnover, even partially by degrading cellulose microfibrils in suspension-cultured poplar cells? One might expect that endo-1,4-β-glucanases cause the reconstruction of the cell-wall network that is involved in the dynamic changes of hemicellulose xyloglucan cross-linkings ( Hayashi, 1987). Since xyloglucans appear to bind nascent 1,4-β-glucan by hydrogen bonding during cellulose biosynthesis, some xyloglucan molecules may be intercalated into paracrystalline parts of microfibrils. If xyloglucans are partially released by degrading cellulose microfibrils with poplar endo-1,4-β-glucanases, xyloglucan solubilization ensues. As shown in Table 1, xyloglucan appeared to be solubilized by incubation with poplar endo-1,4-β-glucanase preparations ( Table 1). As the antibody also prevented the solubilization of xyloglucan, it is possible that cellulose degradation causes xyloglucan solubilization.
Biodegradation and biosynthesis
It is possible that the expression of endo-1,4-β-glucanases by sucrose is involved in cellulose biosynthesis ( Figure 6), because cellulose synthesis starts at the lag growth phase of poplar cells when endo-1,4-β-glucanases are induced. There is an efficient pathway of cellulose from sucrose catalysed by sucrose synthase, which serves to channel carbon directly from sucrose to cellulose ( Amor et al., 1995 ; Nakai et al., 1999 ). When cellulose synthases transfer glucose on the exoplasmic surface of plasma membranes by catalysis, cellulases may be involved in the arrangement of microfibrils in apoplastic spaces, potentially by increasing primers during cellulose synthesis and/or by giving a flexibility to microfibrils . In pea, endo-1,4-β-glucanases enhanced β-glucan synthesis in the tissue slices of pea stems ( Wong et al., 1977 ). Arabidopsis kor mutants showed an abnormal phenotype of cell elongation probably due to the formation and assembly of cellulose microfibrils ( Nicol et al., 1998 ). In Agrobacterium tumefaciens , endo-1,4-β-glucanase is encoded in a cellulose synthase operon ( Matthysse et al., 1995 ), and, in Acetobacter xylinum, its gene is also located at the front of a cellulose synthase operon ( Standal et al., 1994 ). There is evidence that the addition of a small amount of endo-1,4-β-glucanase enhances cellulose production by A. xylinum ( Tonouchi et al., 1995 ). A concerted action of synthases and hydrolases is probably required for the extension of microfibrils at multiple loci along their lengths.
Experimental procedures
Plant materials
Suspension-cultured poplar (Populus alba L.) cells were prepared from poplar leaves or seeds in the presence of 10 μm 2,4-D. The poplar cells were cultured in 200 ml Erlenmeyer flasks containing 40 ml of Murashige and Skoog's medium ( Murashige and Skoog, 1962) with 3% (w/v) sucrose and 1 μm 2,4-D in darkness at 27°C on a rotary shaker operated continuously at 105 rev min−1 (1.3 g). Cells were transferred to fresh medium every 8–10 days. Auxin-starved cells were obtained by a two-week culture in MS medium without 2,4-D. The growth of auxin-starved cells was arrested but cells regained vigorous growth after transfer to medium that contained 1 μm 2,4-D ( Ohmiya et al., 1995 ). Cells to be treated with cycloheximide (Sigma, St Louis, MI, USA) were pre-incubated with 50 μm cycloheximide for 30 min before the addition of sucrose and 2,4-D.
Suspension-cultured poplar cells were ground in a mortar with liquid nitrogen and the resulting powder was extracted five times with 20 m m sodium phosphate buffer (pH 7.0). The wall residues were boiled, suspended in water and used as cell-wall preparations.
Antibody against PopCel gene products
The amino acid sequence of peptide antigen 163CWERPEDMDTPRNVY167 was combined with BSA and the antigen–BSA complex emulsified with Freund's adjuvant was injected into a rabbit at 2 week intervals. Serum was obtained 6 days after the last injection, and the antiserum was precipitated by ammonium sulphate at between 20 and 50% saturation. Aliquots of the antiserum were dissolved in phosphate-buffered saline (PBS) and then stored at 4°C.
Isolation of genomic DNA and Southern blot analysis
Total DNA was isolated from suspension-cultured poplar cells by the method of Taylor and Powell (1982). A 20 μg aliquot of genomic DNA was digested with the indicated restriction endonucleases. The digests were subjected to electrophoresis on 0.9% agarose gels and transferred to Hybond N+ (Amersham-Pharmacia Biotech, Uppsala, Sweden). Blots were hybridized with PopCel1 or PopCel2 labelled by random hexamer priming using digoxigenin–dUTP (DIG DNA labelling kit; Roche Diagnostics, Basel, Switzerland). Hybridization was carried out at 42°C for 16 h in 5 × SSC, 7% SDS, 50% formamide, 2% blocking reagent, 50 m m sodium phosphate (pH 7.0), 0.1% lauroylsarcosine, 5 × Denhardt's solution and 50 μg ml−1 sheared salmon sperm DNA. Blots were washed in 2 × SSC for 5 min at room temperature and then twice in 0.1 × SSC, 0.1% SDS at 68°C for 15 min. The washed membranes were developed with an immunostaining system using a DIG DNA detection kit.
RNA isolation and RT–PCR analysis
Total RNA was isolated from suspension-cultured poplar cells according to the method of Hall et al. (1978) . Poplar cells (1.2–12 g) were ground to a fine powder with liquid nitrogen in a mortar. The powder was homogenized with 3–31 ml of 0.2 m sodium borate buffer, pH 9.0, containing 30 m m EGTA, 5 m m DTT and 1% SDS. The mixture was incubated with 10 mg of proteinase K at 40°C for 2 h and centrifuged at 12 000 g for 30 min. The supernatant was chilled on ice and SDS was precipitated by the addition of 2 m KCl. After centrifugation at 20 000 g, RNA was precipitated overnight with 2 m LiCl, washed twice with cold 2 m LiCl, dissolved in 10 m m Tris buffer (pH 7.5), and precipitated with ethanol.
The first-strand cDNA was synthesized using 5 μg of total RNA at 42°C for 2 h with oligo(dT) (n = 20) and SuperScript II reverse transcriptase (Gibco BRL, Rockville, MD, USA). PCR was performed with final volumes of 20 μl containing 0.5 units of Advantage cDNA polymerase mix (Clontech, Palo Alto, CA, USA), 0.2 m m dNTPs, 3.5 m m Mg(OAc)2 and 0.4 μm gene-specific primers with the first-strand cDNA. The oligonucleotides used for forward primers were CACCACGCAATGTGTACAAAGTAACCATC (nucleotide positions 512–540) for PopCel1 and CACCACGCAATGTGTACAAAGTAACCACG (nucleotide positions 509–537) for PopCel2 which are homologous to the coding strand, and those for reverse primers were GGGTGTATATTGGGCCTGGAAACTAGAAGTT (nucleotide positions 993–1023) for PopCel1 and CAGGAGTGTATTGGGCCTGGAAACTAGAAGAG (nucleotide positions 990–1021) for PopCel2, which are complementary to each coding strand. Primer specificity was tested by amplifying 1 ng of each cDNA inserted in pBluescript II with a set of primers for 20 cycles. Since amplification of DNA was not observed in the PCR reaction containing 5 μg of total RNA instead of cDNA, there was no contaminating genomic DNA in the total RNA preparation. The PCR reaction was initially denatured at 94°C for 5 min and in the subsequent cycles at 94°C for 30 sec. Annealing and elongation cycles were both for 3 min at 68°C. PCR products were size-separated by electrophoresis in a 0.9% agarose gel and blotted onto nylon membranes (Hybond N+ Amersham-Pharmacia Biotech). Membranes were hybridized in 5 × SSC, with 1.0% blocking reagent, 0.1% lauroylsarcosine and 0.02% SDS at 42°C, to digoxigenin–dUTP-labelled probes using a DIG DNA labelling kit (Roche Diagnostics GmbH, Basel, Switzerland) ; the probes were PCR amplified from the first-strand cDNAs by gene-specific primers. Following hybridization, the membranes were washed in 2 × SSC for 5 min at room temperature and then twice in 0.1 × SSC with 0.1% SDS at 68°C for 15 min. The washed membranes were developed with an immunostaining system using a DIG DNA detection kit (Roche Diagnostics).
Endo-and exo-1,4-β-glucanases
Poplar endo-1,4-β-glucanase activity was obtained from extracellular culture medium and the extracts of wall preparations. All procedures were carried out at 4°C. The suspension culture of poplar cells was filtered through a nylon membrane (60 μm mesh) to separate the medium from cells. The extracellular enzyme was concentrated by precipitation with ammonium sulphate to 80% saturation, and aliquots were used as the enzyme source. Poplar cells were homogenized five times in a mortar with two volumes of 20 m m sodium phosphate buffer (pH 6.2). The buffer-insoluble residue (cell walls) was further homogenized twice with two volumes of phosphate buffer that contained 0.5 m NaCl. The homogenate was centrifuged at 20 000 g, and the wall-bound enzyme in the supernatant was concentrated by precipitation with ammonium sulphate to 65% saturation, and aliquots were used as the enzyme source. Fungal endo-cellulase and exo-cellulase were isolated from Polyporus tulipiferae by ion-exchange chromatography and gel filtration ( Kanda et al., 1978 ; Kanda et al., 1980 ).
Poplar endo-1,4-β-glucanase activity was assayed viscometrically at 35°C for 2 h with 0.1 ml enzyme preparation plus 0.9 ml of 10 m m sodium phosphate buffer (pH 6.2) containing 0.3% (w/v) carboxymethylcellulose in Cannon semi-microviscometers. One unit of activity was defined as the amount of enzyme which causes (during the early stages of the reaction) a 1% decrease in the relative viscosity of the mixture. Fungal endo-1,4-β-glucanase activity was assayed as saccharification activity at 35°C for 2 h with 0.1 ml enzyme preparation plus 0.9 ml of 10 m m sodium acetate buffer (pH 5.0) containing 0.3% (w/v) carboxymethylcellulose. One unit of activity was defined as the amount of enzyme required to cause an increase in reducing power equivalent to 1.0 μmol of glucose in 2 h. Fungal exo-1,4-β-glucanase activity was assayed as saccharification activity at 35°C for 2 h with 0.1 ml enzyme preparation plus 0.9 ml of 10 m m sodium acetate buffer (pH 5.0) containing 1% (w/v) Avicel. One unit of activity was defined as the amount of enzyme required to cause an increase in reducing power equivalent to 1.0 nmol of glucose in 2 h.
A large amount of immunoprecipitated endo-1,4-β-glucanase was prepared by incubation of 160 μl of enzyme preparation (300 units) and the antiserum (60 μl) and subsequent addition of a suspension (100 μl) of protein A–Sepharose in PBS. A suspension (200 μl) of the poplar wall preparation (1000 μg) was incubated at 30°C for 12 h with poplar enzymes (200 μl) in 50 m m sodium phosphate buffer (pH 6.2), with endo-cellulase in 50 m m sodium acetate buffer (pH 4.0) or with exo-cellulase in 50 m m sodium acetate buffer (pH 5.0). After incubation, the reaction mixture was boiled and centrifuged at 20 000 gto give a supernatant for determination of soluble sugars.
Determination of cello-oligosaccharides
About 0.5 ml of culture solution was boiled for 5 min and left at room temperature for 1 h in order to equilibrate the anomer configuration between α- and β-type reducing sugars. The amount of cello-oligosaccharides was determined by using cellobiose dehydrogenase from Phanerochaete chrysosporium ( Samejima and Eriksson, 1992). The reaction mixture contained 90 mU cellobiose dehydrogenase, 5 nmol cytochrome C and sample solution in 100 μl of 100 m m sodium acetate buffer, pH 4.2. After incubation for 5 min at room temperature, the absorbance was determined at 550 nm. A linear standard curve was obtained with a standard cellobiose solution and an absorbance of 0.5 corresponded to approximately 270 ng per 100 μl of reaction mixture for cellobiose ( Tominaga et al., 1999 ).
General methods
Computer sequence analysis was performed with the DNAsis software (Hitachi, Japan). DNA and amino acid sequence homology searches were performed at the National Center for Biotechnology Information with the BLAST network service. Carbohydrate was determined by the phenol/sulphuric acid method ( Dubois et al., 1956 ) and xyloglucan was quantified by the iodine–sodium sulphate method ( Kooiman, 1961). Protein was quantified by the method of Bradford (1976) with a protein assay kit (Pierce, Rockford, MD, USA) and bovine serum albumin as the standard.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (No. 05276103) from the Ministry of Education, Science, Sports and Culture, Japan.
References
DDBJ, EMBL and GenBank accession numbers D32166 (PopCel1), AB025796 (PopCel2) and AB025795 (poplar actin).