Gene transfer from mitochondrion to nucleus: novel mechanisms for gene activation from Cox2
Summary
The evolutionarily recent transfer of the gene for cytochrome c oxidase subunit 2 (cox2) from the mitochondrion to the nucleus in legumes is shown to have involved novel gene-activation steps. The acquired mitochondrial targeting presequence is bordered by two introns. Characterization of the import of soybean Cox2 indicates that the presequence is cleaved in a three-step process which is independent of assembly. The final processing step takes place only in the mitochondria of legume species, and not in several non-legume plants. The unusually long presequence of 136 amino acids consists of three regions: the first 20 amino acids are required for mitochondrial targeting and can be replaced by another presequence; the central portion of the presequence is required for efficient import of the Cox2 protein into mitochondria; and the last 12 amino acids, derived from the mitochondrially encoded protein, are required for correct maturation of the imported protein. The acquisition of a unique presequence, and the capacity for legume mitochondria to remove this presequence post-import, are considered to be essential adaptations for targeting of Cox2 to the mitochondrion and therefore activation of the transferred gene in the nucleus.
Introduction
Mitochondria are the direct descendants of a bacterium that was engulfed by a primitive eukaryotic host cell (reviewed by Gray et al., 1999). Over time the mitochondrion has lost or transferred the majority of its genetic information to the nucleus. Much of this transfer occurred soon after the establishment of the mitochondrial endosymbiosis (Gray, 1992). Gene transfer and functional activation appear to have ceased in animals, as evidenced by their nearly constant mitochondrial gene content (Boore, 1999). However, a number of evolutionarily recent gene transfer events from the mitochondrion to the nucleus have been identified in plants, indicating that gene transfer is still taking place (Adams et al., 2000; Adams et al., 2001; Covello and Gray, 1992; Figueroa et al., 1999a; Figueroa et al., 1999b; Grohmann et al., 1992; Kadowaki et al., 1996; Kobayashi et al., 1997; Kubo et al., 1999; Kubo et al., 2000; Nugent and Palmer, 1991; Sanchez et al., 1996; Wischmann and Schuster, 1995). These gene-transfer events provide a unique window of opportunity to study gene transfer, a process of fundamental importance to the establishment and evolution of organelles in eukaryotic cells.
Activation of a nuclear gene after transfer from an organelle requires the acquisition of a number of elements for regulation and targeting, including a promoter, polyadenylation signal, and a mitochondrial targeting presequence (Brennicke et al., 1993). On transfer to the nucleus, it is important that the gene acquires regulatory and trafficking sequences relatively quickly, before it becomes inactivated by random mutations (Thorsness and Weber, 1996). Several different possible mechanisms of acquisition of mitochondrial presequences have been observed (Adams et al., 2000; Adams et al., 2001; Figueroa et al., 1999a; Kadowaki et al., 1996; Kubo et al., 1999).
The transfer of the gene for cytochrome c oxidase subunit (cox2) in legumes (Adams et al., 1999; Covello and Gray, 1992; Nugent and Palmer, 1991) represents a gene that is almost exclusively located in the mitochondrial genome and whose product is a hydrophobic integral membrane protein (Lang et al., 1999). (The nomenclature followed for subunit 2 of cytochrome c oxidase differs between organisms; here we follow the guidelines for plant gene nomenclature (Price et al., 1996) – mitochondrially encoded subunit 2 of cytochrome c oxidase is designated cox2, nuclear-encoded subunit 2 of cytochrome c oxidase Cox2, and the protein is designated Cox2.)
In all legumes examined, the nuclear copy of Cox2 has acquired a homologous N-terminal extension proposed to be a mitochondrial targeting signal, which is separated from the region transferred from the mitochondrial genome by a homologous intron (Adams et al., 1999; Covello and Gray, 1992; Nugent and Palmer, 1991). The study of Cox2 in legumes provides an opportunity to gain insights into the transfer of genes for membrane proteins, which may present unique problems in targeting, sorting or assembly. One view regarding the retention of such genes in mitochondrial genomes relates to the hydrophobic nature of their gene products, which are proposed to cause difficulties in retargeting and/or intraorganelle sorting (Claros et al., 1995; Popot and de Vitry, 1990; von Heijne, 1986).
In this study we have characterized the import of soybean nuclear Cox2 (GmCox2) into the mitochondrion. Our results identify key steps in the evolutionary activation of the transferred Cox2 gene.
Results
GmCox2 is flanked by introns in the nuclear genome
An understanding of the genomic structure of genes transferred to the nucleus is important for understanding the origin of the nuclear signals that have been acquired and the mechanisms of activation of the gene. The published GmCox2 sequence (cDNA and partial genomic sequence; Covello and Gray, 1992) was shown to contain a single intron (intron 2, Figure 1a), separating the transferred region (exon 2, Figure 1a) from the acquired mitochondrial targeting presequence (exon 1, Figure 1a). To further investigate the activation of GmCox2 in soybean, we extended the previous sequence of the genomic gene for GmCox2 using inverse PCR. A product of ≈1400 bp was obtained, and sequencing of this product confirmed that it represented GmCox2. This inverse PCR product extended the 3′ region of GmCox2 (relative to the published cDNA sequence) by 337 bp. In the 5′ direction the sequence was not identical to the published cDNA sequence upstream of the ATG start codon. To characterize this difference, primers to the 5′ end of the published cDNA sequence (cx2 5′.fwd and cx2 5′.rev, Figure 1a) were used to amplify genomic DNA, and yielded a fragment of ≈650 bp, which was ≈500 bp larger than the size predicted from the published cDNA sequence. Sequencing indicated there was a 494 bp insertion in the genomic sequence (AF314468), which has several features that are characteristic of a nuclear intron: 5′ GT and 3′ AG borders (Liu and Filipowicz, 1996), T-richness, polythymidine tracts (Ko et al., 1998), and branch-point motifs (YUNAN) (Simpson et al., 1996). Intron 1 (Figure 1a) immediately borders the 5′ end of the acquired mitochondrial targeting sequence (ATG start codon), while the 3′ end of the acquired mitochondrial targeting presequence is bordered by intron 2 (Figure 1a).
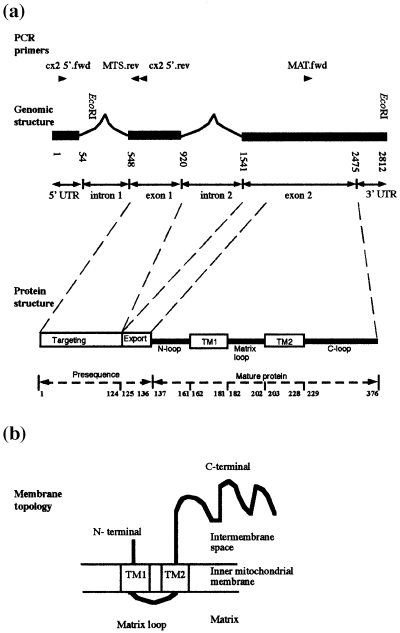
Characterization of the soybean genomic clone for cytochrome c oxidase subunit 2 (GmCox2).
(a) Genomic organization of the Cox2 gene in soybean, showing the relationship between genomic sequence and protein structure. Restriction sites and primers used in this study are indicated. Numbers refer to base pairs in the genomic sequence.
(b) Topology of Cox2 in the inner mitochondrial membrane (Bisson et al., 1982).
A similar gene structure is also present in Neonotonia, a close relative of soybean. PCR amplification of Neonotonia genomic DNA revealed a 443 bp insertion (AY009172) relative to the cDNA sequence (Adams et al., 1999) that is also characteristic of a nuclear intron. Intron 1 also appears to be present in the same position as Cox2 in the more distantly related legume Pachyrhizus, based on the upstream genomic sequence that was obtained by inverse PCR (data not shown). However, Pachyrhizus Cox2 is not expressed (Adams et al., 1999), and thus intron/exon boundaries cannot be defined.
To determine the origin of the acquired mitochondrial targeting presequence of GmCox2 (exon 1, Figure 1a), database searches of GenBank (release 8) for homology to other genes (or their mitochondrial targeting presequences) were undertaken, but no sequences with significant similarity were detected. Southern blot analysis of nuclear DNA with a probe specific for the acquired mitochondrial targeting sequence of GmCox2 produced a single band on hybridization (data not shown). Additionally, characterization of several independent phage clones from a genomic library indicated a single copy for the mitochondrial targeting presequence of GmCox2.
GmCox2 is cleaved in a three-step process on import into legume mitochondria
The N-terminal extension of the nuclear-encoded GmCox2 is 136 amino acids long, 124 acquired upon transfer to the nucleus and 12 present as an N-terminal extension from the mitochondrial-encoded copy (Figure 2a). In vitro translation of the soybean GmCox2 cDNA in the presence of [35S]-labelled methionine yielded a protein with an apparent molecular mass of 42 kDa (Figure 2b, lane 1, top band). Additional lower molecular mass products were also present in the translation lysate, due to initiation of translation from internal methionine residues encoded in the cDNA sequence, resulting in N-terminally truncated proteins. Changing the internal methionine residues to another residue can eliminate these proteins, but this process can change the nature of the protein, and is done only if one of the proteins interferes with the in vitro import reaction. Import of radiolabelled GmCox2 into mitochondria was assessed by (i) the generation of additional bands in the presence of mitochondria under conditions that support import (Figure 2b, lane 2); and (ii) the protection of these additional bands from externally added protease (Figure 2b, lane 3). Incubation of GmCox2 with isolated soybean cotyledon mitochondria followed by treatment of the mitochondria with proteinase K yielded a major band with an apparent molecular mass of 31 kDa, designated the mature protein (Figure 2b, lane 3, denoted M). A less intense band with an apparent molecular mass of 33 kDa, designated intermediate I2, was also generated and protected from proteinase K digestion (Figure 2b, lane 3). In addition to these bands, a protease-sensitive intermediate band (I1) was generated, with an apparent molecular mass of 36.5 kDa (Figure 2b, lane 2). The mobility of the mature GmCox2 generated on import was compared with (i) expressed mature GmCox2 (Figure 2b, lane 4, top band); and (ii) native GmCox2 detected by Western blotting with an antibody raised against soybean Cox2 (Figure 2b, lane 6). The N-terminal sequence for the native GmCox2 was determined by Edman degradation, and exactly matched that determined for an organelle encoded Cox2 (Figure 2a). The mature product generated upon in vitro import was therefore concluded to be the correct mature form for GmCox2.
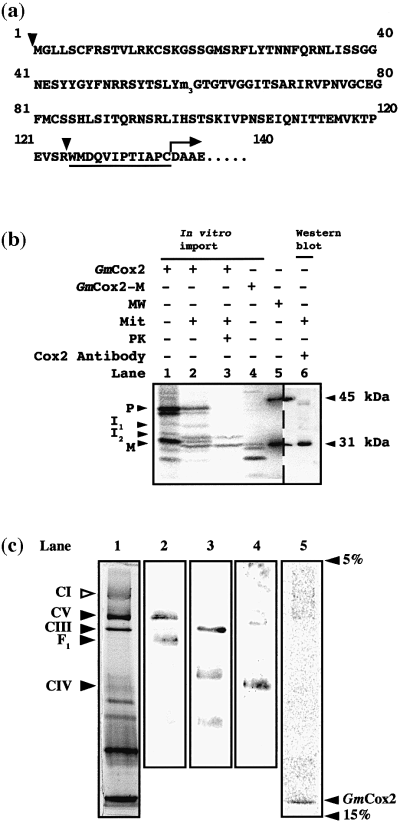
Import of GmCox2 into soybean mitochondria.
(a) Amino acid sequence of the presequence of GmCox2. Arrow indicates the start of the mature protein; triangles represent the location of introns in the genomic sequence. Underlined residues correspond to the 12 amino acid export sequence present from the mitochondrial encoded GmCox2. m3 corresponds to the methionine residue that was converted to isoleucine.
(b) Import of GmCox2 into soybean mitochondria. Lane 1, translation lysate containing precursor protein of GmCox2 (denoted P). Lane 2, precursor protein incubated with isolated soybean mitochondria. Additional products generated are labelled intermediate 1 (I1), intermediate 2 (I2) and mature (M). Lane 3, as lane 2 with proteinase K added; I2 and M are evident. Lane 4, translation lysate containing mature-sized GmCox2 (denoted M). Lane 5, molecular weight marker. Lane 6, isolated soybean mitochondria probed with an antisera to GmCox2. Molecular weight standards are indicated to the right of the panel.
(c) Characterization of soybean mitochondrial respiratory complex proteins by blue native (BN)–PAGE and localization of in vitro imported GmCox2. Lane 1, 1 mg of soybean mitochondrial membrane proteins separated by BN–PAGE and stained with Coomassie. Lane 2, immunodetection of F1 component of ATP synthase (CV) with F1 antibody. Lane 3, immunodetection of cytochrome bc1 complex (CIII) with bc1 complex antibody. Lane 4, immunodetection of cytochrome c oxidase (CIV) with Cox2 antisera. Lane 5, in vitro import of GmCox2 into soybean mitochondria and separation by BN–PAGE. Solid triangles indicate those complexes identified by immunodetection. Open arrow indicates the NADH dehydrogenase (CI), identified by comparison to map of potato BN–PAGE (Jänsch et al., 1996).
Three-step processing of GmCox2 is not linked to assembly
Blue native (BN)–PAGE was undertaken to isolate mitochondrial membrane protein complexes after the in vitro import reaction (Figure 2c, lane 1). Using antibodies for the F1 portion of the ATP synthase (Figure 2c, lane 2), the cytochrome bc1 complex (Figure 2c, lane 3), and Cox2 (Figure 2c, lane 4), it was possible to identify various respiratory complexes in the soybean mitochondrial membrane fraction. The banding pattern obtained for soybean mitochondrial membrane protein complexes was comparable to the published map from potato mitochondria (Jänsch et al., 1996). It was apparent that cytochrome c oxidase separates as a relatively weak and diffuse band (Figure 2c, lane 1, denoted CIV). Analysis of the in vitro import of GmCox2 by BN–PAGE (Figure 2c, lane 5) indicated that the radiolabelled GmCox2 mature protein did not localize with the cytochrome c oxidase holoenzyme (Figure 2c, lane 4), but rather ran as a lower molecular mass, unassembled polypeptide. As a control, a portion of the import reaction was separated by standard 12% SDS–PAGE, to confirm that import and processing had occurred as previously documented (data not shown). Because the imported GmCox2 was processed to its mature size and was isolated as a membrane protein, we conclude that GmCox2 was most probably located in the inner mitochondrial membrane. We conclude that processing of GmCox2 to the mature form is not dependent on assembly into a larger complex.
Three-step processing of the GmCox2 presequence is legume-specific
To investigate the generation of the intermediate products (I1 and I2), a time-course analysis of import into isolated soybean mitochondria was undertaken (Figure 3a,b). To clarify the appearance of I1, a protein of similar size present in the translation lysate, probably due to initiation from an internal methionine (data not shown), was removed by site-directed mutagenesis to change the methionine residue (m3, Figure 2a) to isoleucine. The resultant protein translation did not contain a radiolabelled protein band of similar molecular mass to I1 (Figure 2b, lane 1 versus Figure 3a, lane 1). Import of GmCox2 to its mature form in soybean mitochondria was observed after 60 min (Figure 3a, lane 7). Import and processing of GmCox2 to its mature form (M) was accompanied by the appearance of the protease-sensitive intermediate band (I1) (Figure 3a, lane 6) and the protease-insensitive intermediate (I2) (Figure 3a, lane 7). I1 and I2 were faintly evident at 30 min (Figure 3a, lanes 4 and 5), but were not evident at 10 min (Figure 3a, lanes 2 and 3).
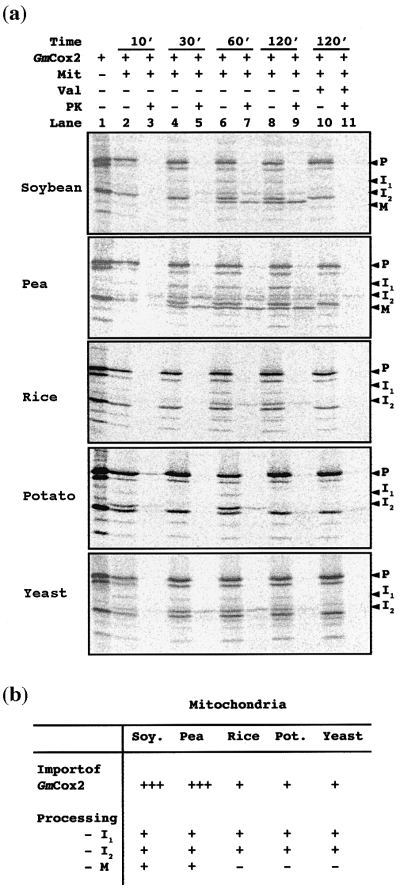
Import of GmCox2 into mitochondria from various species.
(a) Kinetic import of GmCox2 into isolated soybean pea, rice, potato and yeast mitochondria. Lane 1, translation lysate containing GmCox2 precursor protein. Lane 2, translation lysate incubated with mitochondria for 10 min. Lane 3, as for lane 2 with proteinase K added. Lanes 4–9 represent import assays allowed to proceed for increasing periods of time, as indicated. Lanes 10 and 11 were in the presence of valinomycin to dissipate the membrane potential.
(b) Summary of import and processing results obtained in (a).
Pea mitochondria were also able to import and process GmCox2 in a three-step process to its mature form (M) (Figure 3a,b), despite the fact that they have no functional need to do so in vivo as they maintain a mitochondrial-encoded Cox2 (Adams et al., 1999). The slightly faster import into pea as compared to soybean may simply reflect differences in tissue or developmental stage (pea leaf versus soybean cotyledon). In contrast, rice, potato and yeast mitochondria (which also maintain a mitochondrial-encoded Cox2 and are not known to contain Cox2 in the nucleus) could not process GmCox2 to its mature form post-import. In these species only the I1 and I2 intermediates were generated (Figure 3a,b), despite the fact that these mitochondria were able to import and process other precursor proteins correctly (data not shown). In addition, the imported product (I2) seen in rice, potato and yeast mitochondria after 60 min (Figure 3a, lane 7) was reduced or had disappeared by 120 min, indicating breakdown of the imported protein (Figure 3a, lane 9). We conclude that, although mitochondria from several diverse species can import the GmCox2 protein, only mitochondria from legumes can process GmCox2 to its mature form.
GmCox2 has a unique tripartite mitochondrial targeting presequence
Based on the three products of GmCox2 generated during the import process, we investigated the role for each of these regions using deletion constructs that lacked amino acids 1–21 (Δ1–21GmCox2), 72–124 (Δ72–124GmCox2), and 124–135 (Δ124–135GmCox2). Translation of Δ1–21GmCox2 yielded a lower molecular mass protein of 41 kDa (Figure 4a, lane 1) that was not imported into mitochondria (Figure 4a, lane 3). The Δ72–124GmCox2 construct was imported weakly into soybean mitochondria (Figure 4a, lane 6) compared to the wild type GmCox2 (Figure 4a, lane 12). Δ72–124GmCox2 was processed to mature size for GmCox2 (Figure 4a, lanes 6 versus 12); however, the deletion of these residues seemed to affect the efficiency with which it was imported. Wild-type GmCox2 was imported with an efficiency at least twice that of Δ72–124GmCox2 (based on several individual experiments). This was estimated by determining the pixel density of the PK protected products (I2 and M), compared to the amount of precursor added to the import assay. The Δ124–135GmCox2 construct was imported to a protease-insensitive location in isolated soybean mitochondria, but was not processed to mature size for GmCox2 (Figure 4a, lane 9 versus lane 12). This suggests roles in targeting, translocation and sorting for the three regions of the GmCox2 presequence, respectively.
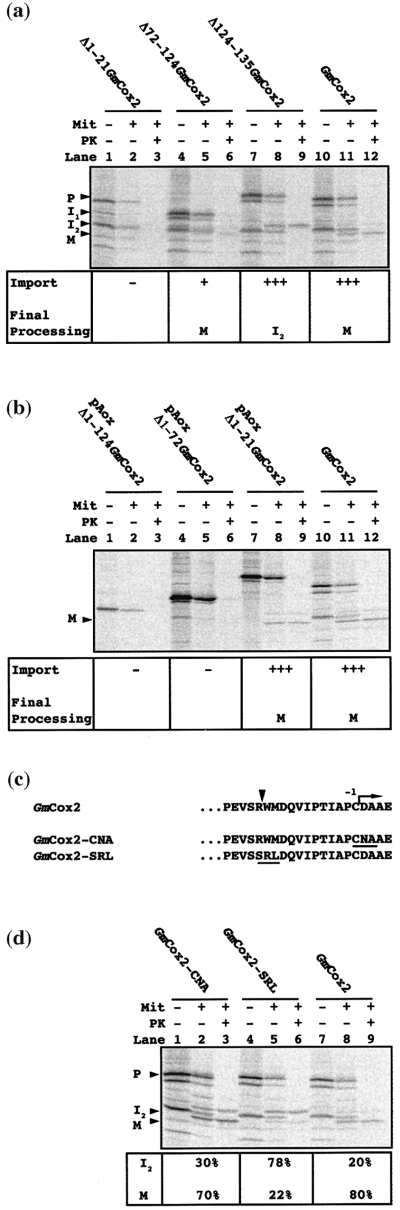
Import of GmCox2 deletion and chimeric precursor proteins into soybean mitochondria.
(a) Import of the deletion constructs Δ1–21GmCox2, Δ72–124GmCox2 and Δ124–136GmCox2 into soybean mitochondria. Lane 1, translation lysate containing the Δ1–21GmCox2 precursor protein. Lane 2, Δ1–21GmCox2 incubated with isolated soybean mitochondria. Lane 3, as lane 2 with proteinase K added. Lanes 4–6, as for lanes 1–3 except with Δ72–124GmCox2. Lanes 6–9, as for lanes 1–3 except with the Δ124–135GmCox2 precursor protein. Lanes 9–12, as for lanes 1–3 except with the GmCox2 precursor protein. Annotation as for Figure 2(b). Table below summarizes import results. (–) no import; (+) weak import; (+++) normal import.
(b) Import of the chimeric proteins pAoxΔ1–124GmCox2, pAoxΔ1–72GmCox2 and pAoxΔ1–21GmCox2 into soybean mitochondria. Lanes 1–3, as for lanes 1–3 of (a) except with the pAoxΔ1–124GmCox2 precursor protein. Lanes 4–6, as for lanes 1–3 except with the pAoxΔ1–72GmCox2 precursor protein. Lanes 7–9, as for lanes 1–3 except with the pAoxΔ1–21GmCox2 construct. Lanes 10–12, as for lanes 1–3 except with the GmCox2 protein. Annotation as for Figure 2(b). Table below summarizes import results. (–) no import; (+) weak import; (+++) normal import.
(c) Amino acid alignment of GmCox2 and mutants used for import. Arrow at −1 position indicates the start of the mature protein. Inverted triangle represents the position of intron 2 in the corresponding genomic sequence. Underlined residues indicate those changed from the wild-type sequence.
(d) Import of GmCox2 mutants into soybean mitochondria. Lane 1, translation lysate containing the GmCox2-CNA precursor protein. Lane 2, GmCox2-CNA incubated with soybean mitochondria. Lane 3, as lane 2 with proteinase K added. Lanes 4–6, as lanes 1–3 except with GmCox2-SRL. Lanes 7–9, as lanes 1–3 except with GmCox2. The amount of each precursor imported was calculated and set to 100%. The relative contribution of I2 and M forms are indicated for each precursor. Abbreviations as for Figure 2.
To determine if this presequence could be replaced by another mitochondrial targeting presequence, various chimeric proteins were constructed that contained the presequence of the alternative oxidase protein (Aox). The 41 amino acid presequence of Aox1 is a well characterized mitochondrial targeting presequence (Tanudji et al., 1999) which can target other passenger proteins (FAd subunit of F1-F0-ATP synthetase, glutathione reductase, ferrochelatase; J.W., unpublished results) to the mitochondrion. However the Aox presequence could not replace the first 124 or 72 amino acids of the GmCox2 presequence, as the chimeric constructs pAox Δ1–124GmCox2 and pAox Δ1–72GmCox2 were not imported to a protease-insensitive location within the mitochondrion (Figure 4b, lanes 3 and 6). In contrast, the pAox Δ1–21GmCox2 construct was imported into the mitochondrion and cleaved to the mature size for GmCox2 (Figure 4b, lane 9 versus lane 12). These data indicate that the N-terminal part of the GmCox2 presequence fulfils the mitochondrial targeting function and can be replaced by a standard targeting signal, but that the remainder of the presequence fulfils additional roles.
In an effort to trap I2, the processing site corresponding to the mature-sized GmCox2 was changed to inhibit the final processing step of GmCox2. The maturation of a mitochondrially encoded Cox2, by removal of its export sequence, is a well characterized process in Saccharo myces cerevisiae, requiring the Imp peptidase (Nunnari et al., 1993; Pratje and Guiard, 1986; Pratje et al., 1983; Schneider et al., 1991). The processing site for Imp has been characterized in S. cerevisiae for both the mitochondrially encoded Cox2 and the nuclear-encoded cytochrome b2 (Chen et al., 1999; Pratje and Guiard, 1986). The processing site can remain functional with a number of residues at the −1 position (Chen et al., 1999), but appears to require a negatively charged residue at the +1 position. To abolish the proposed processing of GmCox2 by this peptidase, site-directed mutagenesis of the negatively charged aspartic acid residue at the +1 site of GmCox2 was performed (Figure 4c, GmCox2-CNA). The accumulation of I2 was determined as its relative proportion of the total imported product. As a control, when GmCox2 was imported into soybean mitochondria 20% of the imported product was in the I2 form and 80% had been processed to the M form after 60 min incubation (Figure 4d, lane 9). Import of GmCox2-CNA into isolated soybean mitochondria (Figure 4d, lane 3) indicated that 70% of the imported product had been converted to the mature form and 30% was present as I2. Another chimeric construct, GmCox2-SRL (Figure 4c), was able more efficiently to trap 78% of the imported GmCox2 as the I2 form (Figure 4d, lane 6). It is believed that this mutant did not disrupt a processing site, but affected the proposed sorting/export function of this region and therefore accessibility of the presequence to the processing peptidase.
Discussion
Successful gene transfer from the mitochondrion to the nucleus requires that the nuclear gene should acquire a number of signals necessary for expression and regulation. In addition, the gene product must be retargeted to the mitochondrion, sorted to its correct intramitochondrial location, and assembled into the appropriate holoenzyme. The successful gene transfer of cox2 in legumes displays novel features for import to the mitochondrion and maturation of the precursor protein that enabled the activation of Cox2 following gene transfer (Figure 5).
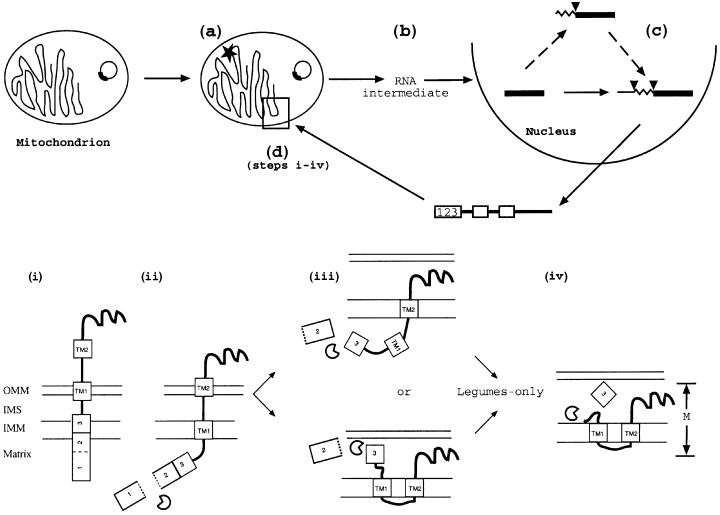
A proposed model for the transfer of cox2 to the nucleus in legumes and subsequent activation.
(a) Prior to the divergence of legumes and before the transfer of cox2 to the nucleus, an event occurred that altered the mitochondrial sorting/processing machinery in legumes.
(b,c) cox2 was transferred to the nucleus via an edited RNA intermediate (b), and on integration into the nucleus it acquired a mitochondrial targeting presequence in a process involving two introns (c). Alternatively, the mitochondrial targeting presequence may have been acquired by a process involving one intron, and the 5′cis-regulatory elements acquired by similar mechanism involving the other intron.
(d) Cox2 is imported into legume mitochondria involving three-step removal of the presequence. (i) The N-terminal region of the acquired mitochondrial targeting presequence directs GmCox2 to the mitochondrion, and (ii) is then cleaved to give the intermediate product I1, which is still susceptible to protease digestion. (iii) Once the first cleavage has been performed, the mitochondrial targeting presequence functions to further translocate the remainder of Cox2 through the outer mitochondrial membrane (OMM), but it is not known where the second processing event takes place. The protein is protected from externally added protease at this stage. (iv) The sorting sequence (Exp) is cleaved (only in legume mitochondria) to produce the mature protein with an Nout–Cout topology. Abbreviations: IMM, inner mitochondrial membrane; IMS, intermembrane space.
Nuclear-encoded GmCox2 has acquired a mitochondrial targeting presequence of 124 amino acids which is flanked by introns in the soybean nuclear genome. An intron is present between the acquired mitochondrial targeting sequence and the transferred region (Covello and Gray, 1992) and an intron upstream of the start ATG in soybean and Neonotonia. The location of intron 1, only 1 bp upstream of the start codon in soybean Cox2 and 34 bp upstream of the start codon in Neonotonia, suggests that this intron could have been involved in an exon shuffling-like process during presequence acquisition (Figure 5c). Intron 1 may also have been involved in the acquisition of 5′cis-regulatory elements by an exon shuffling-like process, either during the same event as presequence acquisition, or during a separate, but almost concurrent, process. Some other cases of mitochondrial gene transfer have involved the gain of introns in the nucleus that may have played a role in functional activation. An intron located between the mitochondrial targeting sequence and the mature coding region is found in Arabidopsis Rps10 and Marchantia nad7 (Kobayashi et al., 1997; Wischmann and Schuster, 1995); an intron is present in the 5′-UTR of Rps10 and Sdh3 in some grass species (Adams et al., 2000; Adams et al., 2001; Kubo et al., 2000); and an intron is located in the 3′-UTR of Arabidopsis rps14 (Figueroa et al., 1999b).
The mitochondrial targeting sequence of GmCox2 appears to be single copy in the soybean genome, and database searches identify no sequences with significant similarity. Thus the presequence probably was not derived from another gene, as has occurred with several other cases of gene transfer (Adams et al., 2000; Adams et al., 2001; Figueroa et al., 1999a; Kadowaki et al., 1996; Kubo et al., 1999), but instead the sequence recruited became a functional presequence only when it became associated with Cox2. It has been proposed that the nuclear genome contains a number of ‘dormant’ mitochondrial targeting presequences (Kobayashi et al., 1997), and ≈2.5% of random Escherichia coli clones generated in a shotgun screen exhibited mitochondrial targeting activity (Baker and Schatz, 1987). However, the Cox2 targeting sequence is not a typical mitochondrial presequence, as it cannot be replaced by another mitochondrial targeting presequence (Sjöling and Gloser, 1998).
Novel import machinery in legume mitochondria has enabled the activation of GmCox2
GmCox2 was imported into soybean mitochondria in a process that produced two intermediate products (I1 and I2), and the mature protein (M) (Figure 5d, i–iv). To our knowledge there are no reports of three-step processing of mitochondrial precursor proteins on import. In contrast to the first two processing steps, which were evident in the mitochondria of all species examined (Figure 5d, ii–iii), the third processing step was only evident in mitochondria from legumes (Figure 5d, iv). In all other species, breakdown of the imported but not fully cleaved product was observed. As the processing of GmCox2 to its mature form in legumes was not linked to assembly into the cytochrome c oxidase holoenzyme, it was concluded that the maturation and stability of GmCox2 are independent of assembly.
These observations suggest that legume mitochondria have either acquired a new activity that is necessary for the correct processing of GmCox2, or else have lost an inhibitory activity that prevents correct processing of GmCox2 in non-legumes. Pea mitochondria could also process the imported GmCox2 to the correct-sized mature form, despite the fact that pea is unrelated to the clade of legumes with Cox2 in the nucleus (Adams et al., 1999). We therefore conclude that the evolution of the proper machinery for this final processing step probably took place independently of, and prior to, the cox2 gene transfer event (Figure 5a). This may have involved the peptidase required to remove the export signal, or perhaps a component of the export machinery required to redirect the N-terminal region of imported proteins back across the inner membrane. Nevertheless, the activity that enables the processing of GmCox2 to its mature form in legumes has been instrumental in activating the nuclear GmCox2 gene.
Deletion constructs of the tripartite presequence were constructed to determine the function of each region. We conclude that the N-terminal region of the mitochondrial targeting presequence is required for targeting to the mitochondria; the middle region is required for efficient translocation of the protein; and the C-terminal region is required for sorting the protein to its correct topology in the inner mitochondrial membrane. The unique nature of the GmCox2 presequence was evident in the observation that a standard, well characterized mitochondrial targeting presequence (for Aox) could not support the import of the mature GmCox2. Although the Aox presequence could restore the N-terminal targeting function of the GmCox2 presequence, it could not replace the middle and C-terminal regions of the latter presequence.
Gene transfer and evolution of sorting pathways
The evolution of a new sorting pathway has been suggested previously in a case of organellar gene transfer. The CFo-II subunit of the chloroplast F1F0 ATP synthetase has arisen through gene duplication of CFo-I and is therefore structurally very similar to it. CFo-II has been transferred to the nucleus and is now imported into chloroplasts. The mechanism of integration of CFo-II into the thylakoid membrane differs from the plastid encoded CFo-I (Michl et al., 1999), despite the fact that both proteins require the same membrane topology.
In Chlamydomonad algae the gene for Cox2 has also been lost from the mitochondrial genome; however, in this situation Cox2 is now encoded by two genes –Cox2a and Cox2b– that are the product of gene fission (Pérez-Martinez et al., 2001). These two genes are independently transcribed and their products are probably imported as separate polypeptides. Mitochondrial sequencing projects indicate that there are other species that lack a mitochondrially encoded cox2 gene; Pedimonas minor and Plasmodium falciparum (reviewed by Gray et al., 1998). These projects further indicate that only two proteins, apocytochrome b and CoxI, are encoded in the mitochondrial genomes of all organisms examined to date (Gray et al., 1998). Therefore it is likely that other organisms have novel targeting or assembly pathways for ‘recalcitrant’ (membrane-spanning) proteins. Such proteins may have more demanding import pathways, and therefore limit the chances of a successful gene transfer to the nucleus compared to proteins (e.g. ribosomal proteins) that can be imported into mitochondria under the direction of a standard mitochondrial targeting sequence or, occasionally, without acquiring a presequence at all.
Experimental procedures
Nucleic acid extraction and manipulation
Soybean genomic DNA was prepared from 7-day-old soybean cotyledons (Glycine max L. Merr, cv. Stevens) according to Jofuku and Goldberg (1988). Neonotonia and Pachyrhizus genomic DNA were prepared as described by Adams et al. (1999). Inverse PCR was carried out with 100 ng EcoRI-digested genomic DNA. Linearized DNA was self-ligated at 14°C overnight using T4 DNA ligase in a final volume of 10 µl, and amplification was performed using the MTS.rev and MAT.fwd primer set for soybean and the primer set Pac.fwd and Pac.rev for Pachyrhizus. The amplification profile consisted of 94°C for 2 min, followed by 35 cycles of 94°C for 1 min, 50°C for 2 min, and 72°C for 2 min. Direct amplification from soybean genomic DNA was performed in a 20 µl reaction volume containing 200 ng genomic DNA, and 30 pmol each of primers cx2 5′.fwd and MTS.rev. The amplification profile consisted of 94°C for 3 min, followed by 30 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min. A final extension cycle of 94°C for 2 min and 72°C for 5 min was applied. Amplification from Neonotonia genomic DNA was performed as just described, using the primer set Neon.fwd and Neon.rev.
Primers
MTS.rev 5′-CACATCCTACATTTGGCACCC-3′
MAT.fwd 5′-CTAGAAGTGGACAATAGAGTGG-3′
cx2 5′.fwd 5′-ATCCTCTGTCCTCTCGAAGC-3′
cx2 5′.rev 5′-CGTATACAGGAATCTTGACATGCC-3′
Neon.fwd 5′-AAGAGTGCAGCCCTCACCG-3′
Neon.rev 5′-WAAGTGAAGWGTATGATCTCT-3′
Pac.fwd 5′-AATTGCGGTTTTTGTAGTAT-3′
Pac.rev 5′-GAGCTATCGTAGGCATCAAT-3′
Mitochondrial isolation and in vitro import assays
Mitochondria were prepared from 7-day-old soybean cotyledons, 10-day-old pea leaves (Pisum sativum L. Greenfeast), and 10-day-old etiolated rice (Oryza sativa L. cv. Amaroo) as described by Day et al. (1985). Potato (Solanum tuberosum L.) tuber mitochondria were prepared according to Neuberger et al. (1982). Saccharo myces cerevisiae mitochondria were prepared as described by Pratje and Michaelis (1977) using Zymolyase to lyse the cells. [35S]-labelled precursor proteins were synthesized from cDNA clones as described by Whelan et al. (1995). In vitro import assays were carried out as described by Whelan et al. (1996) unless otherwise stated. In vitro import assays into S. cerevisiae were varied from Whelan et al. (1996) to contain 2 mm ATP, 2 mm NADH and 2 mm GTP. Processing assays using lysed soybean mitochondria and a purified mitochondrial processing peptidase (MPP) from spinach were performed according to Tanudji et al. (1999). Proteins were separated by 12% SDS–PAGE, and gels were dried and exposed to a BAS TR2040S plate for 24 h. Detection was carried out on a BAS 2500 according to the manufacturer's instructions (Fuji, Tokyo, Japan).
Plasmid constructions
The nuclear-encoded Cox2 gene from soybean was amplified from genomic DNA using primers to the published sequence (Covello and Gray, 1992) and cloned into the pCR2.1 vector (Invitrogen, Adelaide, Australia). The genomic intron situated between the putative presequence and the mature region was removed by inserting flanking SalI restrictions sites using the Quick-Change Site-Directed Mutagenesis Kit (Stratagene, La Jolla, California, USA), followed by SalI digestion and plasmid religation. The gene sequence was corrected to its wild-type cDNA form by site-directed mutagenesis, and upstream ATG codons were changed from the multiple cloning sequence of the pCR2.1 vector in the same manner. This construct (GmCox2) was used as the template for synthesis of radiolabelled GmCox2 that was used for in vitro import assays. All other constructs were derived from this clone: Δ1–21GmCox2, Δ72–124GmCox2, Δ124–135Cox2, GmCox2-M. The Aox1 gene encoding the mitochondrial alternative oxidase from soybean was used to make the chimeric constructs (Tanudji et al., 1999) pAoxΔ1–21GmCox2, pAoxΔ1–72GmCox2 and pAoxΔ1–124GmCox2. All constructs were verified by DNA sequencing using an ABI 310 genetic analyser (Perkin Elmer, Melbourne, Australia).
Blue native–PAGE
Blue native (BN)–PAGE and preparation of mitochondrial membrane samples were carried out as described by Jänsch et al. (1996). For import and subsequent BN–PAGE, the standard import reaction was scaled up to contain 1 mg mitochondrial protein. Western blots of blue native gels were modified from Murcha et al. (1999). Because Coomassie blue strongly binds to blotting membranes (Jänsch et al., 1996), blue native gels were soaked overnight in 2% (w/v) SDS, 0.37 m Tris–HCl pH 6.8 to remove the Coomassie blue from the gel.
Cox2 antibody production and Western blots
A 700 bp fragment encoding a partial fragment of the mature region of the nuclear-encoded copy of GmCox2 was cloned into the pQE31 expression vector downstream of a 6x His tag (Qiagen, Clifton Hills, Australia). The recombinant His-tagged construct was overexpressed in the bacterial strain JM109 and purified using Ni-NTA resin (Qiagen). The overexpressed Cox2 protein (150 µg) was inoculated into rabbits, and the polyclonal antibody to Cox2 was isolated as sera (Coligan et al., 1996). Bands that reacted with this antibody on Western blots were detected using chemiluminescence (Roche, Sydney, Australia) and visualised using a LAS1000 (Fuji).
Acknowledgements
We thank Farag Ahmed for technical assistance inoculating rabbits, and the UWA Graduates' Association and Professor A. Robson for travel bursaries to D.O.D. for work performed in the laboratory of E.P. We are grateful to Dr T. Lithgow for useful comments. This work was supported by the ARC (J.W.) and by the NIH (J.D.P.). A.H.M. is supported by an ARC Australian Postdoctoral Fellowship. K.L.A. was supported by a USDA graduate fellowship.