Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein
Summary
The ability to gain access to iron is pivotal for bacterial pathogens during infection. Although much is known about iron acquisition systems in Gram-negative bacteria, comparatively little is known about how Gram-positive pathogens access iron from host iron sources. A previous study showed that, in the Gram-positive human pathogen Staphylococcus aureus, a cell surface-associated glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enzyme (Gap, or Tpn) is capable of binding human transferrin, representing a potential means by which this bacterium is able to access iron in vivo. We have investigated this property of S. aureus further and shown that, in S. aureus RN6390, GAPDH is expressed on the S. aureus cell surface independent of exogenous iron concentrations, and that overexpressed and purified Gap, although retaining GAPDH activity, has no affinity for human transferrin. Moreover, although a S. aureus gap mutant was devoid of surface-associated and cytoplasmic GAPDH activity, it retained the ability to bind human transferrin, equivalent to wild type. We concluded from these results that the Gap protein is not involved in S. aureus binding to human transferrin. We identified the transferrin-binding protein as a novel cell wall-anchored protein, designated StbA for staphylococcal transferrin-binding protein A, which shared no significant similarities with any other bacterial transferrin-binding proteins. StbA contained a C-terminal cell wall-anchoring motif (LPKTG), and expression of StbA in the cell wall was strictly controlled by exogenous iron concentrations. The stbA gene is found within a 7 kb region in the S. aureus chromosome that contains a total of six iron-regulated genes. Immediately downstream from stbA is an iron-regulated gene whose product was predicted to be another cell wall-anchored protein with no significant similarity to proteins with characterized functions. Transcribed in the opposite direction from stbA is a four-gene operon whose expression is also regulated by iron. While the deduced products of the first two genes lack similarity to known proteins, the last two genes encode, respectively, putative lipoprotein and permease components of an ABC transporter that shares significant similarities with several iron(III) ABC transporters in a variety of bacteria.
Introduction
Staphylococcus aureus causes of a wide variety of infections in humans, ranging from minor skin and wound infections to more serious and invasive infections such as septicaemia, endocarditis and osteomyelitis (Archer, 1998). The ability of this bacterium to cause such an extensive range of diseases can be attributed, at least in part, to its vast array of cell-surface and secreted virulence factors. S. aureus secretes numerous toxins and exoenzymes capable of damaging host proteins and tissues (Bohach et al., 1997; Arvidson, 2000). S. aureus also expresses numerous surface proteins that are involved in adherence to host proteins, including fibrinogen, fibronectin, collagen and the Fc domain of IgG molecules (Höök and Foster, 2000). One essential factor among the numerous and complex host–pathogen interactions is the ability of the invading pathogen to multiply successfully within the host. A wealth of information now exists showing that the availability of iron in the host environment is extremely limiting, and that this has dramatic effects on invading pathogens (Bullen and Griffiths, 1999).
Due to its role in many biochemical processes (Griffiths, 1999), iron is an essential nutrient for bacterial growth. The bacteriostatic nature of human serum is due to the presence of the c. 80 kDa, high-affinity iron-binding glycoprotein transferrin (hTf), which transports iron to tissues throughout the body. hTf is a monomeric protein that comprises an N-terminal and C-terminal lobe, each of which possesses a ferric ion and bicarbonate anion binding site (Baker and Lindley, 1992).
Williams and co-workers identified a 42 kDa cell-surface protein, expressed in Staphylococcus aureus and several coagulase-negative staphylococci, with the ability to bind hTf (Modun et al., 1994). More recently, they showed that S. aureus strain BB expresses a cell-surface localized protein (which they called Tpn) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity that is also capable of binding hTf and human plasmin (Modun and Williams, 1999). Although surface expression of GAPDH activity in strain BB was found to be higher on cells cultured in iron-limited than in cells cultured on iron-replete media, this activity was not tightly regulated by the iron content in the bacterial growth media (Modun et al., 1994; Modun and Williams, 1999), an unusual property for proteins intimately involved in high-affinity iron transport processes. Multifunctional, extracytoplasmic GAPDH enzymes are not unprecedented. An extracytoplasmic GAPDH in Streptococcus spp. can bind plasmin and possesses an ADP-ribosylating activity (Pancholi and Fischetti, 1992), while in Candida albicans the extracellularly localized of GAPDH has been shown to bind fibronectin and laminin (Gil-Navarro et al., 1997; Gozalbo et al., 1998).
In this study, we investigated transferrin binding in S. aureus in greater detail. We overexpressed and purified Gap (or Tpn; genome sequences of S. aureus Mu50 and N315 refer to this protein as Gap, which is how we will refer to this protein) from S. aureus RN6390 and showed that the purified Gap protein, although possessing GAPDH activity, was incapable of binding to hTf. Moreover, we showed that, in a gap knockout strain of S. aureus RN6390, hTf binding to cell wall fractions was unaltered. We identified the protein involved in binding hTf, and found it to be an iron-regulated cell wall-anchored protein. We designated this protein StbA, for staphylococcal transferrin-binding protein A. Adjacent to stbA on the staphylococcal chromosome lie several other iron-regulated genes. The products of two, in particular, share significant similarity with ABC transporter components involved in iron transport in several other bacteria. We also identified a second putative cell wall-anchored protein whose expression was regulated by exogenous iron concentrations.
Results
Construction and characterization of a gap mutant
The goal of this study was to characterize the ability of S. aureus to bind human transferrin (hTf) in S. aureus in greater detail. Although Modun and Williams (1999) have studied transferrin binding in S. aureus using strain BB, we have used strain RN6390 to study this property in S. aureus because this isolate is amenable to genetic manipulation. A previous report implicated Gap, a cell surface-associated protein with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity, as the transferrin-binding protein in S. aureus (Modun and Williams, 1999). Genomic sequences were searched for coding regions whose deduced product resembled the published N-terminal sequence of Gap. These searches identified the gap gene in several strains of S. aureus. The gap gene is the second coding region in a putative operon, comprising gapR (gap regulator), gap (glyceraldehyde-3-phosphate dehydrogenase), pgk (phosphoglycerate kinase), tpi (triosephosphate isomerase), pgm (phosphoglycerate mutase) and eno (enolase), whose functions are dedicated to glycolysis. Initial attempts to construct a S. aureus gap mutant by homologous recombination with a mutated gap gene were unsuccessful. We hypothesized that a gap mutation could be lethal to cells growing in rich (or glucose-containing) media as it would yield a strain deficient in glycolysis. To circumvent this problem, S. aureus RN6390 was grown in Tris-minimal succinate during the final stages of the mutagenesis procedure. Using this strategy, we were able to construct the RN6390 gap::Tet mutant strain H330. This growth media-specific phenotype has been reported in other GAPDH mutants, specifically E. coli (Hillman and Fraenkel, 1975; Irani and Maitra, 1974) and Salmonella typhimurium (Fraenkel and Horecker, 1964). Similarly, attempts to mutagenize gap in Streptococcus were unsuccessful using rich growth media (Winram and Lottenberg, 1998). We speculate that, by maintaining H330 on a glucose-free medium (succinate and casa-mino acids, in this case), catabolite repression of Krebs citric acid cycle enzymes was avoided. Consistent with this idea, strain H330 (RN6390 gap::Tet) was incapable of growth in the presence of 0.4% glucose, and showed decreased growth rates in the presence of 0.4% fructose or 0.4% maltose (data not shown).
Cell wall fractions from wild-type RN6390 possessed GAPDH activity (Fig. 1A), in agreement with previous results using S. aureus strain BB (Modun and Williams, 1999). In contrast, cell wall fractions from strain H330 were devoid of GAPDH activity (Fig. 1A). Cell surface-associated GAPDH activity, along with the ability to grow in glucose-containing media (not shown), was restored to H330 upon introduction of pJT23, which carried a wild-type copy of the gap gene (Fig. 1A). To characterize Gap in more detail, we cloned the gap gene into an expression vector and overexpressed Gap as a His6-tagged derivative. The Gap–His6 hybrid, purified to near homogeneity (Fig. 1B), was enzymatically active (Fig. 1A). Purified protein was used to raise rabbit anti-Gap antibodies and the localization of Gap in S. aureus RN6390 was investigated by Western blot analysis with polyclonal Gap antiserum. These experiments demonstrated that Gap was expressed in staphylococcal cell wall fractions and also in protoplasts, suggesting a cell-surface and cytoplasmic location for this enzyme (Fig. 1C and D). Moreover, Gap expression appeared to be independent of the iron status of the growth media, in contrast to previous observations in S. aureus strain BB (Modun and Williams, 1999). That Gap is not detected among proteins derived from H330, along with the observation that H330 is incapable of growth in the presence of glucose, suggests that the mutated gap gene is the only copy of a gene encoding GAPDH activity on the S. aureus chromosome, even though genome-sequencing projects indicate that Mu50 and N315 possess two gap homologues (gap and gapB) sharing approximately 50% total similarity. We therefore conclude that surface-associated and cytoplasmic forms of GAPDH in strain RN6390 are derived from the same gene.
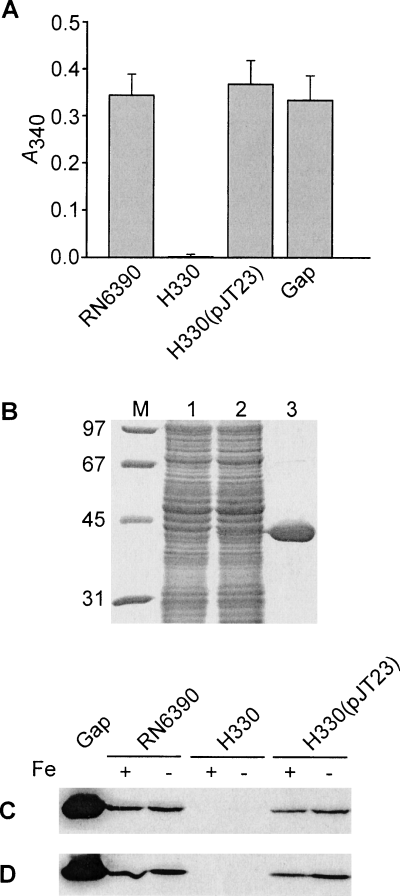
Characterization of the staphylococcal Gap protein.
A. GAPDH activity is observed in cell wall fractions of wild-type RN6390 and H330 (RN6390 gap::Tet) complemented with pJT23, but not in H330 alone (RN6390 gap::Tet). Activity for purified S. aureus Gap (5 μg) is also shown. For cell wall fractions, 50 μg of total protein was used in the assay.
B. The purified Gap protein, described in Experimental Procedures, was analysed by 12% SDS-PAGE, and the gel stained with Coomassie brilliant blue R250. Lane M, molecular weight markers in kDa. Cell lysates were prepared from E. coli DH5α and E. coli DH5α(pJT11), both grown in the presence of arabinose, and loaded in lanes 1 and 2 respectively. Lane 3 was loaded with 10 μg of purified His6-Gap.
C and D. Western blot analysis demonstrating that Gap is expressed in the cell wall (C) and protoplast (D) fractions of S. aureus, irrespective of the iron content in the growth media. Proteins were separated by 12% SDS-PAGE, blotted and probed with antisera raised against purified Gap (see Experimental Procedures). Approximately 10 μg of purified Gap was loaded into the first lane and approximately 15 μg total protein was loaded in the remainder of the lanes. Strain designations are indicated, as is whether bacterial cultures were grown in iron-replete (+) or iron-deficient (−) media.
The staphylococcal transferrin-binding protein is not Gap
We showed that S. aureus expressed a protein, with an approximate molecular mass of 38.5 kDa [based on mobility in sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)], that possessed an ability to bind hTf (Fig. 2A). Expression of this hTf-binding protein was strictly regulated by exogenous iron concentrations, and was associated only with the cell wall and was not found in protoplasts (Fig. 2A). The transferrin-binding protein was also expressed in a several other S. aureus type strains, including S. aureus ATCC 12900 and ATCC 6538, and, as in RN6390, only in cultures that were grown under iron starvation conditions (data not shown).
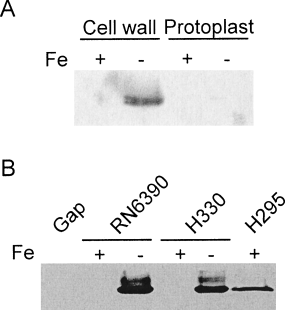
Characterization of hTf-binding by S. aureus.
A. Expression of a transferrin-binding protein in the cell wall of iron-limited S. aureus. Cells were grown under either iron-replete (+) or iron-limiting (−) conditions, and cell wall or protoplast fractions were electrophoresed on a SDS-polyacrylamide gel and blotted. Immobilized proteins were probed with HRP-labelled hTf, and detection was performed using the ECL Plus kit from Amersham Pharmacia Biotech.
B. Western immunoblot showing that expression of a transferrin-binding protein in the cell wall of S. aureus is independent of Gap expression. Proteins were separated by 12% SDS-PAGE, blotted and probed with HRP-labelled hTf. Approximately 10 μg of purified Gap was loaded into the first lane and approximately 15 μg total protein was loaded in the remainder of the lanes. Strain designations are indicated, as is whether bacterial cultures were grown in iron-replete (+) or iron-deficient (−) media.
It was previously reported that the staphylococcal GAPDH is a transferrin-binding protein (Modun and Williams, 1999). However, no genetic studies were performed to link this protein unequivocally to a role in hTf binding. We sought to characterize the transferrin-binding ability of this enzyme in greater detail. However, using purified, and enzymatically active, Gap (see Fig. 1A and B), we could not detect binding of hTf to Gap, even using 10 μg amounts of Gap (Fig. 2B). This surprising result raised the possibility that Gap expression in E. coli, and/or the purification steps, could have altered the transferrin-binding properties of this protein. However, further investigation revealed that strain H330 (RN6390 gap::Tet) still expressed a protein capable of binding hTf, and that expression of this hTf-binding protein was strictly regulated by exogenous iron concentrations, as it was in wild-type RN6390 (Fig. 2B). This contrasts with the iron-independent expression of Gap, both within the cell wall and in protoplasts (Fig. 1C and D). The iron regulation of the hTf-binding proteins was Fur-dependent in S. aureus, as the hTf-binding protein was expressed in H295 (RN6390 fur::Km) grown under iron-replete conditions (Fig. 2B). Based upon our biochemical and genetic information, we conclude that it is not Gap, but rather a protein whose expression is tightly regulated by exogenous iron concentrations, that is involved in the direct binding to hTf.
Identification of an iron-regulated cell wall transferrin-binding protein
Cation-exchange chromatography was utilized to fractionate cell wall proteins isolated from iron-starved S. aureus RN6390 (Fig. 3A). Probing protein fractions with HRP-hTf identified three fractions that were enriched for hTf bind-ing while containing the least amount of contaminating proteins (Fig. 3B). The transferrin-binding protein was isolated and the N-terminal sequence was obtained. The resulting sequence consisted of the following 14 amino acids: Ala-Thr-Glu-Ala-Thr-Asn-Ala-Thr-Asn-Asn-Gln-Ser-Thr-Gln. This sequence was then used to query the online S. aureus genome sequences, and was found to be identical to amino acids 47–60 of a predicted translation product identified in the genome sequences of S. aureus N315 and Mu50 (Kuroda et al., 2001) and to the deduced sequence of an unpublished gene called sai-1 and identified as a 29 kDa cell-surface protein from S. aureus strain Cowan I (accession no. AB042826). The open reading frame is 1062 nucleotides in length and encodes a predicted product of 354 amino acids that would yield an unmodified product of 39 kDa. We have labelled the gene stbA for staphylococcal transferrin-binding protein A. In agreement with our findings, the StbA protein possessed a classical cell wall-anchoring motif (317LPKTG321) in the C-terminal region, suggesting that it is covalently linked to the S. aureus cell wall. Following cleavage of the 46-amino acid-long N-terminal signal peptide and the C-terminus, the processed protein would consist of 274 amino acids with a predicted molecular mass of 30.5 kDa, which is smaller than the estimated 38.5 kDa mass based on mobility in SDS-polyacrylamide gels. Interestingly, StbA shares no similarity with known bacterial transferrin-binding proteins identified in the Pasteurellaceae and Neisseriaceae families (Gray-Owen and Schryvers, 1996), nor with other proteins in the databases.

Enrichment of the S. aureus transferrin-binding protein, StbA. Cell wall fractions (CW) and protoplasts (PP) were isolated from iron-starved S. aureus RN6390. Cell wall proteins were fractionated using cation-exchange chromatography and separated by SDS-PAGE. The polyacrylamide gel was either stained with Coomassie brilliant blue R250 (A) or blotted and probed with HRP-hTf (B). Shown are unfractionated cell wall and protoplast preparations, as well as three fractions of cell wall proteins that were enriched for the transferrin-binding protein.
To prove unequivocally its involvement in binding to hTf, the stbA gene was insertionally inactivated in RN6390 to create strain H518. Cell wall fractions of iron-starved S. aureus H518 showed no interaction with hTf (Fig. 4). Introduction of multicopy stbA (plasmid pJT35) into H518 restored the ability of S. aureus cell wall fractions to bind hTf (Fig. 4). A slight reactive protein band can be seen in cell wall fractions of iron-replete H518(pJT35) (Fig. 4), indicating expression of small amounts of StbA. We attribute this to titration of the Fur protein, as cloned DNA in pJT35 possesses the Fur box upstream of the stbA coding region.

Western immunoblot demonstrating that StbA is a cell wall-anchored, iron-regulated transferrin-binding protein. Proteins were separated by 12% SDS-PAGE, blotted and probed with HRP-hTf. Strain designations are indicated, as is whether bacterial cultures were grown in iron-replete (+) or iron-deficient (−) media.
StbA, expressed in Escherichia coli, retains its ability to bind hTf
Expression of gonococcal TbpA in E. coli allowed this bacterium to bind hTf with the same specificity shown in the gonococcus (Cornelissen et al., 1993). These data showed that this was the only protein that was required for transferrin binding, and that transferrin binding did not require the presence of TbpB or any other gonococcal protein. Likewise, we were interested in determining whether S. aureus StbA was able to function on its own in binding hTf. When StbA was expressed in E. coli DH5α, encoded from plasmid pJT35, a c. 42 kDa protein was shown to bind hTf (Fig. 5), whereas E. coli DH5α alone did not contain any proteins that bound hTf (Fig. 5). The StbA protein in E. coli runs slightly higher in the gel than the c. 38.5 kDa protein expressed in S. aureus (Fig. 5). We attri-buted this to C-terminal processing of the protein, which would probably occur in S. aureus (given the presence of the LPKTG motif that would be recognized by sortase) but which would not occur in E. coli. Therefore, we conclude that the ability of S. aureus to bind hTf is due solely to the expression of StbA and is independent of other staphylococcal proteins, including Gap.

Western immunoblot demonstrating StbA retains transferrin-binding activity, even when expressed in E. coli. Bacterial cell lysates were separated by 12% SDS-PAGE, blotted and probed with HRP-hTf. Strains used were as follows: S. aureus RN6390, E. coli DH5α and E. coli DH5α-containing plasmid pJT35.
stbA is situated amidst a number of other iron-regulated genes on the S. aureus chromosome
The stbA coding region and surrounding DNA sequences (Fig. 6A) are well conserved among different S. aureus strains, as they are present in all S. aureus genomes that have been completely sequenced (Kuroda et al., 2001), or that are currently being sequenced at the University of Oklahoma, The Institute for Genome Research (TIGR) and the Sanger Centre. Nucleotide sequences upstream of the stbA coding region contained a putative Fur box (Fig. 6B) that was highly conserved with the consensus S. aureus Fur box previously identified (Horsburgh et al., 2001). This is in full agreement with our data showing that expression of StbA was regulated by iron (2, 4), and that this regulation was Fur dependent (Fig. 2). Adjacent to stbA and transcribed in the opposite direction are four open reading frames (ORFs) (Fig. 6A) that are predicted to exist within an operon structure, as the start and stop codons for the individual ORFs either overlap or are separated by no more than 26 bp. The operon is preceded by sequences bearing striking similarities to known Fur boxes (see Fig. 6B), which would suggest that transcription of the operon is regulated by the Fur protein in response to exogenous iron concentrations. Given this, we have designated the ORFs sirDEFG, for staphylococcal iron-regulated genes D, E, F and G. This nomenclature follows from a previous report that identified sirABC as three genes (located elsewhere on the chromosome) whose expression was regulated by iron (Heinrichs et al., 1999). The functions of the sirABC genes remain undetermined. The deduced product of sirD shares limited similarity with an unknown protein in Bacillus halodurans and the p64 protein from Listeria monocytogenes. The deduced product of sirE shares low similarity with several transcription factors. Deduced products of sirF and sirG share significant similarity with a number of bacterial iron transport proteins. SirF is a predicted lipoprotein with similarity to several iron-regulated lipoproteins in Gram-positive bacteria and iron-regulated periplasmic binding proteins in Gram-negative bacteria, whose functions are involved in iron–siderophore transport. SirG is highly hydrophobic, with several predicted membrane-spanning domains, and shares significant similarity with permease proteins involved in iron–siderophore transport.
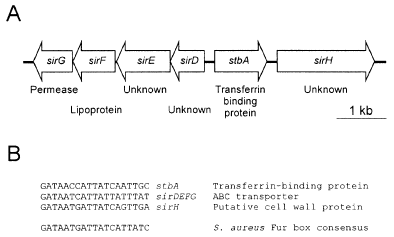
A. Physical map of the 7.0 kb stbA locus. The two monocistronic genes, stbA and sirH, encoding iron-regulated cell wall-anchored proteins are shown, as is the iron-regulated operon encoding sirD, sirE, sirF and sirG and sirE. SirF and SirG are predicted to function as part of an ABC transporter involved in iron uptake. Genes equivalent to sirD, sirE, sirF, sirG, stbA and sirH are designated SA0978, SA0979, SA0980, SA0981, SA0977 and SA0976, respectively, in the genome of S. aureus N315 (Kuroda et al., 2001).
B. Alignment of putative Fur box sequences identified upstream of stbA, the sirDEFG operon, and sirH. The S. aureus Fur box consensus sequence is taken from Horsburgh et al. (2001).
To investigate the regulation of these genes, a lacZ fusion to sirF was constructed in RN6390, creating strain H536, to monitor transcription from the promoter region upstream of sirD. Measurements of β-galactosidase activity in strain H536 grown under iron-replete and iron starvation conditions indicated that transcription of the operon was regulated in an iron-dependent fashion (Fig. 7), and was controlled by the Fur protein (data not shown). Although these data suggest a role for this operon in high-affinity iron transport, the functions of sirF and sirG, as well as those of sirD and sirE, remain unknown.
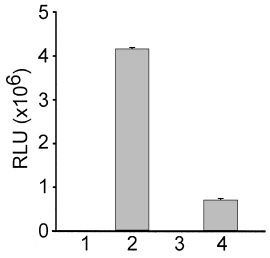
Influence of iron on sirF and sirH gene expression. β-galactosidase activity, measured in relative light units (RLU), was monitored as a function of sirF or sirH expression in strains bearing chromosomal sirF–lacZ and sirH–lacZ transcriptional fusions. β-galactosidase activity was measured from a lysate of 25 μl of cell culture, from cultures grown to an optical density of 1.0. 1, Iron-replete H535; 2, iron-starved H535; 3, iron-replete H536; iron-starved H536.
Immediately downstream of stbA, and transcribed in the same direction, is a larger ORF, designated sirH (Fig. 6A), whose predicted product appears to be a cell wall protein, as its amino acid sequence contains a C-terminal wall-anchoring motif (610LPQTG614) which would be recognized by the S. aureus sortase enzyme (Mazmanian et al., 2001). The unprocessed protein encoded by this orf is predicted to be 654 amino acids in length, encoding a protein with a molecular mass of 72 kDa. The putative cell wall protein encoded by sirH shares a low degree of simi-larity with another unknown protein from B. halodurans, as well as p64 from L. monocytogenes. An alignment between StbA and the C-terminal half of the protein encoded by sirH show a low but significant degree of homology, 20% identity and 26.9% total similarity. Nucleotide sequences upstream of sirH contain a consensus Fur box (Fig. 6B), indicating that transcription of sirH may also be regulated by iron. To investigate this further, a lacZ fusion was constructed to sirH in the chromosome of RN6390, creating strain H535. In agreement with the presence of a Fur box upstream of sirH, expression of the gene was regulated by exogenous iron concentrations (Fig. 7), and this regulation was Fur dependent (data not shown). The sirH–lacZ fusion interrupts the sirH coding region and therefore yields a S. aureus sirH mutant. Loss of sirH expression did not alter hTf binding to cell wall fractions in S. aureus H535 (data not shown).
Discussion
Iron uptake systems in Gram-positive bacteria, unlike their Gram-negative counterparts, largely remain a mystery, and only now are studies beginning to unravel their complexity. In the iron-limiting environment of the mammalian host, there are a number of host proteins which can serve as a source of iron, and it is clear that many pathogenic bacteria are able to utilize most, if not all, of these sources. In this paper, we describe the identification of a transferrin-binding protein in S. aureus, called StbA, which is localized to the cell wall and is regulated by exogenous iron levels via the Fur protein. Analysis of a strain containing an insertionally inactivated stbA gene demonstrated that the StbA protein binds hTf, and this is the first identification of a cell wall-anchored protein in S. aureus which plays a role in iron acquisition.
Mammalian iron-binding proteins, such as transferrin, lactoferrin and haemoglobin, are fairly large in size; transferrin is an 80 kDa monomeric bilobed protein (Evans et al., 1999). It is unlikely that proteins of this size would easily penetrate the thick peptidoglycan layer of S. aureus. Thus, a cell wall-anchored protein would serve as the initial contact for transferrin. Indeed, studies have shown that the size cut-off of globular proteins able to penetrate the peptidoglycan layer in both Gram-negative bacteria (E. coli) and Gram-positive bacteria (B. subtilis) is approximately 50 kDa (Demchick and Koch, 1996; Dijkstra and Keck, 1996).
S. aureus produces a number of cell wall-associated proteins which are covalently linked to the peptidoglycan layer through a conserved LPXTG motif located near the C-terminus (Schneewind et al., 1992). Not surprisingly, many of these surface-localized proteins contribute to staphylococcal pathogenesis, given that they would be in intimate contact with the host environment. Along with StbA, we have identified another iron-regulated gene, sirH, predicted to encode a second, and larger, cell wall-anchored protein. Although the function of sirH is, as yet, undetermined, it is possible that it serves a role in iron uptake in the host environment by binding to a host iron-binding protein or protein complex. As iron–siderophore complexes are of a sufficiently small size as to not be restricted by the cell wall layer, the involvement of a cell wall-anchored protein in their transport is unlikely. Indeed, iron–siderophore uptake systems in S. aureus and other Gram-positive bacteria probably only require membrane-associated ABC transporters for their internalization (Schneider and Hantke, 1993; Sebulsky et al., 2000; Sebulsky and Heinrichs, 2001).
A number of cell wall-anchored proteins in staphylococci possess repeated amino acid sequences at some distance from the C-terminus. For example, the two tandem fibronectin-binding proteins of S. aureus, FnBPA and FnBPB, both possess three consecutive sets of 37 or 38 repeated amino acids that play an important role in the high-affinity fibronectin-binding activity exhibited by the proteins (Sun et al., 1997). These repeats lie outside of the wall-spanning domains that traverse the peptidoglycan layer, and are therefore free to associate with fibronectin (Hartford et al., 1997). Similarly, we have identified nearly identical tandem repeats that lie N-terminal to what would be predicted to be the wall-spanning domain of StbA. We are currently investigating the importance of these repeats to transferrin binding.
The transferrin receptor in Gram-negative bacteria has been extensively characterized and shown to consist of a pair of outer membrane proteins, TbpA and TbpB, which, individually or together, can bind and remove iron from transferrin (West and Sparling, 1985). As N. gonorrhoeae is unable to produce siderophores, it relies on the transferrin receptor for growth within the host environment (West and Sparling, 1985; Cornelissen et al., 1998). Conversely, S. aureus isolates have been demonstrated to produce at least three different siderophores (Meiwes et al., 1990; Haag et al., 1994; Courcol et al., 1997), and possess a hydroxamate transport system (Sebulsky et al., 2000; Sebulsky and Heinrichs, 2001) and genes en-coding at least two other putative iron uptake systems (Heinrichs et al., 1999; Morrissey et al., 2000; Sebulsky et al., 2000; Sebulsky and Heinrichs, 2001). In light of observations that S. aureus has the potential to express several putative iron uptake systems, the exact role of re-ceptor-mediated iron transport (e.g. via direct transferrin binding) is not clear and awaits further analyses.
In Gram-negative bacteria, ferric ions released from the transferrin molecule at the outer membrane are transported across the periplasm and cytoplasmic membrane by binding protein-dependent transporters, typified by the FbpABC proteins in gonococci (Adhikari et al., 1996). It is thus tempting to speculate that, in S. aureus, a membrane-bound ABC transporter would likewise be in-volved in the internalization of ferric ions released from transferrin. Adjacent to stbA, and transcribed in the opposite direction, is an operon of four genes. Deduced products from the last two of these genes, sirF and sirG, show significant similarity to ABC transporters involved in iron uptake, and we are currently investigating the pos-sibility that these proteins function in ferric iron transport. We have shown that the sirF, sirG and sirH genes are strongly iron regulated. The observed differences in the level of β-galactosidase activity seen between the sirH and the sirF transcriptional fusions may be a result of the fusion being further away from the promoter region in the sirF fusion, or the fact that the sirH promoter is simply stronger than that of the sirDEFG operon. This would be resolved with the construction of a transcriptional fusion to the first gene of the sirDEFG operon, placing lacZ an equivalent distance from the promoter as that in the sirH–lacZ strain.
It was previously reported that the staphylococcal GAPDH was localized to the cell surface and involved in transferrin binding (Modun and Williams, 1999). However, no genetic studies were performed, until this study, to link this protein unequivocally to a role in transferrin binding. Our initial investigation involved the creation of a gap mutant. Not only was the gap mutant deficient in glycolysis, but it also maintained the ability to produce a cell wall protein capable of binding transferrin. Similarly, purified Gap, while maintaining enzymatic activity, failed to bind transferrin. Although scans of genome sequences re-vealed that strain N315 and Mu50 possessed two gap homologues (termed gap and gapB), our data support the conclusion that strain RN6390 contains only one functional GAPDH, as the gap-defective strain was incapable of growth in glucose containing media (indicating a defect in glycolysis) and lacked any detectable GAPDH enzymatic activity. Whether both gap homologues in strain N315 and Mu50 are functional as GAPDH enzymes is not known.
Although our data show unequivocally that Gap is not involved in hTf binding, a role for Gap in release of iron from transferrin cannot be ruled out. Indeed, in this study and another (Modun and Williams, 1999), GAPDH activity was localized on the bacterial cell surface, and whole cells were shown to convert diferric hTf to apotrans-ferrin (Modun et al., 1998). Moreover, iron removal from transferrin is accelerated by both acidification and the presence of phosphatic compounds, among other agents (Morgan, 1979). Liberation of 1,3-diphosphoglycerate through the activity of surface-associated GAPDH could, in theory, effect the release of ferric ions from the trans-ferrin molecule, and make it available to other proteins, such as membrane-localized ABC transporters, for transport across the cytoplasmic membrane.
In addition to its cytoplasmic location, staphylococcal Gap is present at the cell surface, yet its amino acid sequence does not contain a signal sequence or cell wall-anchoring motif. It is possible that staphylococcal GAPDH is maintained at the cell surface via protein–protein interactions and, in this regard, it was recently demonstrated that Streptococcus pyogenes mutants lacking M protein are also devoid of cell-surface GAPDH expression (D´Costa et al., 2000). We are currently investigating the possible role of staphylococcal surface proteins in maintaining Gap at the staphylococcal cell surface. Given its potential role in the release of transferrin-bound iron, this may aid us in identifying additional components of a putative transferrin–iron uptake system, and shed light on the role of transferrin as an iron source for staphylococci in the mammalian host.
Experimental procedures
Bacterial strains, plasmids, media and culture conditions
E. coli and S. aureus strains and plasmids used in this study are detailed in Table 1. Unless stated otherwise, E. coli strains were cultured in Luria–Bertani broth (LB), whereas S. aureus strains were cultured in tryptic soy broth (TSB). Tris-minimal succinate (TMS) served as the iron-poor growth media (Sebulsky et al., 2000). FeCl3 (50 μM) was added to TMS as required. Solid media were obtained by the addition of Bacto agar (Difco, 1.5% w/v). Unless otherwise stated, all bacterial growth was carried out at 37°C. Ampicillin (E. coli, 100 μg ml−1), tetracycline (S. aureus, 4 μg ml−1; E. coli, 10 μg ml−1), erythromycin (S. aureus, 3 μg ml−1; E. coli, 300 μg ml−1), chloramphenicol (S. aureus, 10 μg ml−1; E. coli, 30 μg ml−1), lincomycin (S. aureus, 20 μg ml−1) and X-gal (40 μg ml−1) were included in growth media where necessary. Iron-free water was used for all experiments and was obtained by passage through a Milli-Q water filtration unit.
| Name | Descriptiona | Reference/source |
|---|---|---|
| Strains | ||
| S. aureus | ||
| RN6390 | Prophage-cured wild-type strain | Novick et al. (1995) |
| 12900 | Wild-type strain | ATCCb |
| 6538 | Wild-type strain | ATCC |
| RN4220 | rK– mK+ | Kreiswirth et al. (1983) |
| H295 | RN6390 fur::Km; Kmr | Sebulsky et al. (2000) |
| H330 | RN6390 gap::Tet; Tcr | This study |
| H518 | RN6390 stbA::Tet; Tcr | This study |
| H535 | RN6390 sirH::pMUTIN4; Emr | This study |
| H536 | RN6390 sirF::pMUTIN4; Emr | This study |
| E. coli | ||
| DH5α | φ80 dLacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17(rk–mk+) supE44 relA1deoRΔ(lacZYA-argF)U169 | Promega |
| Plasmids | ||
| pAW11 | 3.8 kb E. coli–S. aureus shuttle vector; Emr | Wada and Watanabe (1998) |
| pBAD24 | Arabinose-regulatable expression vector; Apr | Guzman et al. (1995) |
| pBC SK(+) | Multicopy phagemid cloning vector derived from pUC19; Cmr | Stratagene |
| pAUL-A | 9.2 kb temperature-sensitive S. aureus suicide vector used for genereplacement experiments; Emr | Chakraborty et al. (1992) |
| pLI50 | 5.2 kb E. coli–S. aureus shuttle vector; Cmr Apr | Lee (1992) |
| pMUTIN4 | Promoterless transcriptional lacZ fusion vector; Apr (E. coli), Emr (S. aureus) | Vagner et al. (1998) |
| pDG1513 | Derivative of pMTL22 that carries a tetracycline resistance cassette; Apr, Tcr | Guérout-Fleury et al. (1995) |
| pDG1514 | Derivative of pMTL23 that carries a tetracycline resistance cassette; Apr, Tcr | Guérout-Fleury et al. (1995) |
| pJT6 | Derivative of pAUL-A carrying a 4.5 kb fragment containing the insertionallyinactivated gap gene; Tcr Emr | This study |
| pJT11 | Derivative of pBAD24 that carries the S. aureus gap gene; Apr | This study |
| pJT23 | pAW11 derivative carrying a 2.3 kb PstI/BamHI fragment that contains theS. aureus gap gene; Emr | This study |
| pJT34 | pAUL-A derivative that carries a 4.8 kb fragment containing theinsertionally inactivated stbA gene; Tcr Emr | This study |
| pJT35 | pLI50 derivative that carries the S. aureus stbA gene; Apr Cmr | This study |
| pJT36 | pMUTIN4 derivative that carries a 1079 bp fragment internalto the sirH coding region; Emr | This study |
| pJT37 | pMUTIN4 derivative that carries a 781 bp fragment internal tothe sirF coding region; Emr | This study |
| Oligonucleotidesc | ||
| gap1 | 5′-CTGCGTTCTGAAACAGATATGC -3′ | This study |
| gap2 | 5′-GGTCCATTCCATACAACAGTGT-3′ | This study |
| StbA1 | 5′-AACATTTCTCATTATTCCACATTGC -3′ | This study |
| StbA2 | 5′-GTCTTTTATTGCAGGGTTTT-3′ | This study |
| Gap6xHis 5′ | 5′-TTCCATGGTTCATCATCATCATCATCACATGGCAGTAAAAGTAGCAATTAATGG-3′ | This study |
| Gap6xHis 3′ | 5′-TGCTCCCCGCTTACTCATAA-3′ | This study |
| FeCW Sense | 5′-TTGCGGCCGCACTAACAATACATATCCTAT-3′ | This study |
| FeCW Antisense | 5′-TTGGATCCGAGTTATCTTGTTGTTCTTT-3′ | This study |
| FeLP Sense | 5′-TTGCGGCCGCTCAAGAATCAACTAAATCCG-3′ | This study |
| FeLP Antisense | 5′-TTGGATCCTGCCTTATCAGCATCAACAT-3′ | This study |
| pMUTIN4 Sense | 5′-CTGACTCCCCGTCGTGTAGA-3′ | This study |
| pMUTIN4 antisense | 5′-GTCAGACGCATGGCTTTCAA-3′ | This study |
- a. Ap, ampicillin; Cm, chloramphenicol; Em, erythromycin; Tc, Tetracycline.
- b. ATCC, American Type Culture Collection.
- c. Restriction enyzme sites are underlined.
DNA isolation and manipulation
Plasmid DNA was isolated from E. coli and S. aureus using Qiagen plasmid kits, with the modification that lysostaphin (50 μg ml−1) was incorporated into buffer P1 for lysis of S. aureus. Standard protocols were used for cloning, transformation, restriction digests and DNA ligations (Sambrook et al., 1989). Polymerase chain reaction (PCR) amplifications were all performed using the High-Fidelity PCR Amplification Kit (Roche Diagnostics). All oligonucleotides were obtained from Life Technologies.
Phage preparation and transduction
Phage 80α was used for all transduction experiments and was prepared as previously described (Sebulsky et al., 2000). Plasmids and chromosomal markers were transduced into various S. aureus backgrounds using published methodologies (Novick, 1991).
Mutagenesis of gap
The gap gene was amplified from the S. aureus RN6390 chromosome as a 2.3 kb PCR product, using primers gap1 and gap2, and cloned as a blunt PCR product into the Klenow-treated ClaI site in pBC-SK(+). A 398 bp ClaI fragment, internal to the gap coding region, was removed and replaced with a 2147 bp ClaI fragment from pDG1513 (Guérout-Fleury et al., 1995) which carried a tetracycline resistance cassette. The disrupted gap gene, present on a 4.5 kb BamHI fragment, was then introduced into pAUL-A (Chakraborty et al., 1992), to create plasmid pJT6. Plasmid pJT6 was passed through S. aureus RN4220 before being introduced into S. aureus RN6390. A transformant was cultured at 30°C in TMS medium containing tetracycline and grown in liquid culture to an OD600 of 0.2, before the temperature was shifted to 42°C for a further 4 h growth. The culture was then diluted and plated on tetracycline-containing plates and growth allowed to continue overnight. Clones were selected that showed resistance to tetracycline and sensitivity to erythromycin and the gap mutation was confirmed by PCR and Southern blot analysis (data not shown). For complementation of the gap mutation, the gap coding region, carried on the original 2.3 kb PCR product, was cloned into the PstI/BamHI site of pAW11, yielding plasmid pJT23.
Mutagenesis of stbA
The staphylococcal stbA gene sequence was amplified from the S. aureus RN6390 chromosome, using primers stbA1 and stbA2, as a 2.5 kb PCR fragment. The PCR product was digested with ClaI (ClaI cuts 332 bp from one end of the PCR product) and introduced into ClaI/EcoRV-digested pBC-SK(+). A 2172 bp Klenow-treated BamHI/HindIII fragment, derived from pDG1514 (Guérout-Fleury et al., 1995), was cloned into the unique HpaI site (blunted) present within the stbA coding region. The disrupted stbA gene, carried on a 4.8 kb BamHI/KpnI fragment, was introduced into pAUL-A, yielding plasmid pJT34. The stbA mutant derivative of RN6390 was derived in the same manner as was used to generate the gap mutation, except that TSB was used as the growth medium throughout. The stbA mutation was confirmed by PCR and Southern blot analysis (data not shown). For complementation of the stbA mutation, the stbA gene, carried on the original 2.5 kb PCR product, was cloned into the XbaI/HindIII site of pLI50, yielding plasmid pJT35.
Overexpression of Gap and generation of antisera
The gap gene was amplified from the chromosome of S. aureus RN6390 using oligonucleotide primers Gap6xHis 5′ and Gap6xHis 3′, cleaved with NcoI and HindIII and cloned into pBAD24 (Guzman et al., 1995), to create plasmid pJT11. For overexpression of His6-Gap, a 500 ml culture of E. coli DH5α containing pJT11 was grown to mid-log phase in LB plus 0.4% (w/v) glucose. Cells were washed in phosphate-buffered saline (PBS), resuspended in 500 ml of LB plus 0.02% (w/v) arabinose, and grown for 3 h. Cells were harvested, washed in PBS, resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 10 mg lysozyme), and kept on ice for 30 min The cells were then sonicated and cell debris was removed by centrifugation at 12 000 g for 20 min at 4°C. The supernatant was added to 3 ml Ni2+-NTA agarose (Qiagen), and incubated with shaking at 4°C for 2 h. The slurry was loaded into a column, washed with washing solution (50 mM NaH2PO4, 300 mM NaCl, 30 mM imidazole), and eluted with three column volumes of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 200 mM imidazole). The eluent was dialysed against PBS at 4°C, and the protein was stored at −20°C.
Polyclonal antisera to His6-Gap were generated in two New Zealand white rabbits (Charles River, Wilmington, MA, USA). In brief, 125 μg of protein mixed with Titre-Max Gold adjuvant (CytRx, Norcross, GA, USA) was injected subcutaneously into each rabbit. Rabbits were boosted with 60 μg of protein on days 16 and 36, and on day 42 the rabbits were sacrificed and antisera were recovered. Antisera were diluted 1:10 and adsorbed over induced cell lysates of E. coli DH5α(pBAD24) that were immobilized on nitrocellulose membranes. As a negative control, sera were taken from a preimmune bleed of each rabbit.
SDS-PAGE and immunoblot analysis
Protein samples were quantified by Lowry assay using reagents purchased from Bio-Rad. Proteins were separated by SDS-PAGE according to standard procedures (Laemmli, 1970). Proteins were visualized with Coomassie brilliant blue R250, or were transferred to nitrocellulose membranes (45 μm; Gelman), and washing and detection were per-formed using the ECL Plus chemiluminescent system and Hyperfilm (Amersham Pharmacia Biotech). Horseradish peroxidase (HRP)-labelled human transferrin (hTf) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) Western blots were performed as described previously (Modun et al., 1994; Modun and Williams, 1999). To eliminate reactivity with staphylococcal protein A, 5% horse serum was included in the blocking solution for all immunoblots. Adsorbed anti-Gap antisera were used at a dilution of 1:25 000. As a negative control for all blots, HRP-labelled antirabbit antiserum was used (Amersham Pharmacia Biotech).
Cell wall preparations and GAPDH assays
Staphylococcal cell wall proteins were prepared as described previously (Cheung and Fischetti, 1988), minus the protease inhibitor iodoacetamide, which inhibits GAPDH activity (Iglesias and Losada, 1988). Assays for GAPDH activity were performed as described previously (Winram and Lottenberg, 1996; Modun and Williams, 1999). Protein samples were quantified using a Lowry assay (Bio-Rad).
Ion-exchange purification of StbA and N-terminal sequencing
Staphylococcal cell wall proteins from a 500 ml culture of iron-starved S. aureus RN6390 were dialysed against two changes of 10 mM NaHPO4 buffer (pH 7.0) and applied to a 2.5 ml column of pre-equilibrated SP-Sepharose cation-exchange resin (Amersham). The column was washed (10 mM NaHPO4 buffer, pH 7.0, 50 mM NaCl) and proteins were eluted from the column with a 30 ml NaCl gradient (50 mM to 250 mM). Fractions were collected, run on polyacrylamide gels and blotted with HRP-transferrin as described above.
Protein fractions containing StbA were separated by SDS-PAGE and transferred to polyvinyl difluoridine (PVDF) membrane for N-terminal sequencing. For transfer, carbonate buffer (3 mM Na2CO3, 10 mM NaHCO3 pH 9.9, 10% methanol, 1% SDS) was used. The PVDF membrane was briefly stained with fresh and diluted Coomassie brilliant blue R250, and washed sequentially with 100% methanol, 50% methanol and water. N-terminal sequencing was performed at the Advanced Protein Technology Centre at the University of Toronto.
Construction of sirF–lacZ and sirH–lacZ fusions
To create a sirF–lacZ fusion, a 781 bp fragment internal to the sirF coding region was PCR amplified, using primers FeLP sense and FeLP antisense, digested with NotI and BamHI and cloned into pMUTIN4, creating plasmid pJT37. To create a sirH–lacZ fusion, a 1079 bp fragment internal to the sirH coding region was PCR amplified, using primers FeCW sense and FeCW antisense, digested with NotI and BamHI and cloned into pMUTIN4, creating plasmid pJT36. Plasmids pJT36 and pJT37 were introduced into RN4220 before being transduced into RN6390. A PCR product of 1640 bp, amplified from the chromosome of H535 and H536 using primers pMUTIN4 sense and pMUTIN4 antisense, verified the presence of the recombinant pMUTIN4 constructs in the chromosome.
β-Galactosidase assays
Staphylococcal strains bearing chromosomal pMUTIN4 insertions were assayed for β-galactosidase activity. Briefly, bacterial cells were harvested after overnight growth under iron-rich or iron-poor conditions, washed three times in PBS, and resuspended to an OD600 of 1.0. A 0.5 ml volume of cells was then centrifuged, resuspended in 200 μl of lysis buffer containing 15 μg of lysostaphin, and incubated for 10 min at 37°C. The bacterial lysate was centrifuged for 10 min at 14 000 g and the supernatants were measured for β-galactosidase activity using the Galacto-Light Plus kit (Tropix). Detection was performed in a Lumat LB 9507 luminometer (Berthold).
Computer analysis and nucleotide sequence accession numbers
DNA sequence analysis, oligonucleotide primer design and sequence alignments were performed using the Vector NTI Suite 6 software package (Informax, Inc., Bethesda, MD, USA). The nucleotide sequences described in this communication are registered in the GenBank database under accession number AY061874.
Acknowledgements
We are grateful to Miguel A. Valvano for helpful advice and critically reading the manuscript. This work was supported by operating grant MOP-38002, to D.E.H., from the Canadian Institutes of Health Research (CIHR). D.E.H. is the recipient of a CIHR New Investigator Award.