Enhanced bacterial virulence through exploitation of host glycosaminoglycans
Summary
Present in the extracellular matrix and membranes of virtually all animal cells, proteoglycans (PGs) are among the first host macromolecules encountered by infectious agents. Because of their wide distribution and direct accessibility, it is not surprising that pathogenic bacteria have evolved mechanisms to exploit PGs for their own purposes, including mediating attachment to target cells. This is achieved through the expression of adhesins that recognize glycosa-minoglycans (GAGs) linked to the core protein of PGs. Some pathogens, such as Bordetella pertussis and Chlamydia trachomatis, may express more than one GAG-binding adhesin. Bacterial interactions with PGs may also facilitate cell invasion or systemic dissemination, as observed for Neisseria gonorrhoeae and Mycobacterium tuberculosis respectively. Moreover, pathogenic bacteria can use PGs to enhance their virulence via a shedding of PGs that leads to the release of effectors that weaken the host defences. The exploitation of PGs by pathogenic bacteria is thus a multifaceted mechanistic process directly related to the potential virulence of a number of microorganisms.
Bacteria and host proteoglycans: a multifaceted interaction
Cell-to-cell interactions are essential for many physiologi-cal processes such as embryogenesis, development or tissue repair, as well as in infectious diseases, as infection involves a continuous interplay between a microbial pathogen and host cells, including those of the immune system. Following entry into a susceptible host, a pathogen adheres to target tissues to escape mechanical clearance and to establish an infectious focus from which dissemination may occur. This adherence step is mediated by specialized microbial surface-exposed molecules, termed adhesins, that recognize complementary receptors on host cells. The molecular characterization of these adhesin–receptor interactions may lead to the development of new therapeutic and/or prophylactic strategies against infectious diseases (Karlsson, 1998).
For more than a decade, it has been demonstrated that an increasing number of infectious microorganisms express adhesins that bind sulphated glycosaminoglycans (GAGs) borne by proteoglycans (PGs) (for comprehensive reviews, see Sawitzky, 1996; Rostand and Esko, 1997; Wadström and Ljungh, 1999). Present in the extracellular matrix (ECM) or anchored in the membrane of virtually all eukaryotic cells, GAGs constitute a homogeneous family of polysaccharides made of alternating residues of amino sugars (N-acetyl-D-glucosamine or N-acetyl-D-galactosamine) and uronic acids (D-glucuronic acid or L-iduronic acid). Despite such an apparent homogeneity, GAGs nevertheless exhibit some polymorphism as a result of enzymatic modifications including N- and O-sulfation, epimerization and deacetylation (Salmivirta et al., 1996). Their wide distribution and accessibility on the outermost surface of host tissues make GAGs ideal targets for the attachment of pathogens. It is therefore not surprising that virulent bacteria have evolved mechanisms to interact specifically with these compounds. Several in vitro studies have demonstrated that heparin used as a prototype of GAG can inhibit binding of bacteria to eukaryotic cells. It can also displace bound bacteria. The aim of this review is to describe some specific examples of the complex inter-actions between GAGs and bacteria. These multifaceted interactions can initiate a cascade of events leading to tissue colonization, cell invasion and systemic dissemination and can potentially affect functions of the host cell itself.
GAG-binding adhesins of Bordetella
The filamentous haemagglutinin (FHA) of Bordetella is a remarkable protein and in some ways similar to large eukaryotic attachment factors such as fibronectin or laminin, which are secreted into the extracellular matrix and found on the cell surface. Many studies have demonstrated that FHA is required for colonization by Bordetella pertussis, the aetiological agent of whooping cough (Locht et al., 1993). It was shown a decade ago that B. pertussis can attach to sulphated glycosphingolipids, and that attachment of the pathogen to tracheal cells can be inhibited by sulphated sugars such as heparin (Brennan et al., 1991). This suggested that B. pertussis may attach to GAGs, which are found in abundance in the mucosa of the bronchial airways. Menozzi et al. (1991) demonstrated that FHA binds to heparin, a property that can be utilized to purify the protein, and that the heparin-inhibitable attachment of B. pertussis to epithelial cells is mediated largely by FHA (Menozzi et al., 1994). FHA binds to the same sulphated glycolipids as virulent B. pertussis, and heparan sulphate proteoglycans have been identified as FHA receptors on epithelial cells (Hannah et al., 1994). Heparin can also inhibit FHA-induced haemagglutination.
Through the use of recombinant fragments of FHA the heparin-binding domain has been identified in its N-terminal region (Menozzi et al., 1994). This domain is also one of the two immunodominant domains of FHA (Leininger et al., 1997). Both Bordetella bronchiseptica and Bordetella parapertussis express FHA-like molecules (Cotter et al., 1998). The FHA of B. parapertussis mediates attachment to lung epithelial cells, but adherence is not inhibited by heparin (van den Akker, 1998), suggesting that the B. parapertussis FHA lacks the heparin-binding domain.
In addition to its heparin-binding domain, FHA contains an RGD motif, which mediates B. pertussis attachment to macrophages via integrin receptors (Relman et al., 1990), and a lectin-like domain, which mediates interactions of FHA with cilia (Liu et al., 1997). Therefore, FHA appears similar to fibronectin in that it contains multiple binding sites including a heparin-binding domain and an RGD motif (Fig. 1).
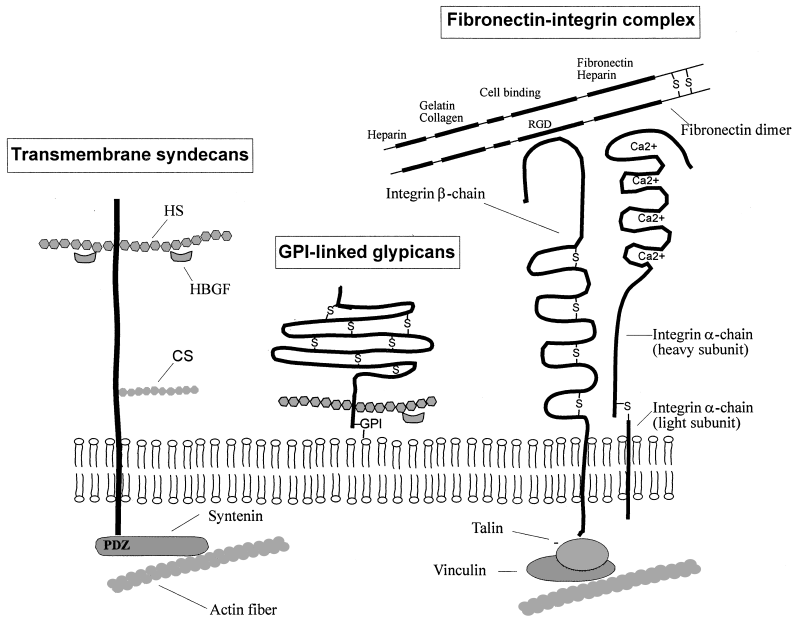
Schematic depiction of major components of the eukaryotic extracellular matrix. Among the cell-surface heparan sulphate (HS) proteoglycans, the syndecans and glypicans are present on virtually all adherent cells. Syndecans are transmembrane proteins that bear HS chains in their ectodomain and may also contain chondroitin sulphate (CS) chains near the plasma membrane (Bernfield et al., 1999). Glypicans are covalently linked to a glycosyl phosphatidyl inositol (GPI), which anchors them in the outer leaflet of the plasma membrane. Glypicans contain multiple disulphide bonds (–S–) and bear HS chains near the plasma membrane. The HS chains of syndecans and glypicans may sequester heparin-binding growth factors (HBGF) such as FGF, TGFβ or VEGF. The integrins constitute a family of cell-surface heterodimers (α and β chains), which act as receptors for various ligands present on adjacent cells or in the extracellular matrix, such as collagen, laminin or fibronectin (Albelda and Buck, 1990). The fibronectin dimer , which on each chain carries five distinct binding domains (heparin-, gelatin/collagen-, fibrin-, cell- and heparin/fibrin-binding domains), interacts with the β-integrin chain through an arginine-glycine-aspartic acid (RGD) motif present in its cell-binding domain. The syndecans and integrins are linked to the cytoplasmic F-actin network through the syntenin and the talin–vinculin protein complex respectively. See text for details.
FHA-related proteins have been identified in many other bacterial species, including Haemophilus ducreyi (Ward et al., 1998), Haemophilus influenzae (Barenkamp and Leininger, 1992), Pasteurella multocida, Yersinia pestis, Xylella fastidiosa, Pseudomonas aeruginosa, Neisseria meningitidis and many others (Locht et al., 2001). Whether any of them also bind sulphated carbohydrates remains to be established.
In addition to FHA, B. pertussis also produces fimbriae that can bind both sulphated sugars and integrins found on monocytes (Geuijen et al., 1996). The genes required for the expression of B. pertussis fimbriae are clustered and closely associated with the genes responsible for FHA expression (Willems et al., 1994). By using specific fim-brial mutants, both the major fimbrial subunit and the minor adhesin subunit FimD have been found to bind sulphated sugars (Geuijen et al., 1996; 1997). The sulphated sugar specificity for fimbriae is not yet known, but the receptors for the B. pertussis fimbriae may be more restricted than those for FHA, as fimbriae appear to mediate attachment to laryngeal epithelial cells but not to bronchial cells, as observed for FHA (van den Berg et al., 1999).
Role of the mycobacterial heparin-binding haemagglutinin in extrapulmonary dissemination
Mycobacterium tuberculosis synthesizes on its surface a heparin-binding haemagglutinin adhesin (HBHA), which is involved in the mycobacterial interaction with epithelial cells, but not with professional phagocytes (Menozzi et al., 1996; Pethe et al., 2001). The HBHA-mediated mycobacterial adherence relies on the interaction of the C-terminal lysine-rich domain of HBHA with heparan sulphate-containing proteoglycans (HSPGs) (Menozzi et al., 1998; Delogu and Brennan, 1999; Pethe et al., 2000). This domain is composed of several repeats, and the affinity of HBHA for heparan sulphate is directly depend-ent on the number of these repeats. A M. tuberculosis mutant strain deficient for HBHA production colonizes the lungs of mice as efficiently as does the wild-type parent strain (Pethe et al., 2001). However, colonization of the spleen upon intranasal infection was severely affected by deletion of the hbhA gene. The mutant strain nevertheless colonized the spleen as effeciently as the wild-type strain when the mice were infected by the intravenous route, indicating that HBHA is required for the extrapulmonary dissemination of M. tuberculosis. These findings suggest that a mycobacterial GAG-binding adhesin that targets epithelial cells may trigger systemic dissemination, which is an important step in the pathogenesis of tuberculosis. Although the precise mechanisms leading to dissemination of M. tuberculosis from the lungs to other tissues are still unknown, in vitro experiments using confluent cell monolayers suggest that HBHA may induce transient opening of cell junctions (K. Pethe, F. Soncin and F.D. Menozzi, unpublished observations). Inhibition of extrapulmonary mycobacterial dissemination was also observed when the wild-type mycobacteria were incubated with monoclonal antibodies that recognize the HBHA heparin-binding domain prior to intranasal administration. These antibodies also block HBHA binding to GAGs, suggesting that the mycobacterial interactions with GAGs are important steps in dissemination (Pethe et al., 2001). Altogether, these observations suggest that phagocytic cells are not the major vehicle used by mycobacteria to disseminate, and that a mucosal immune response towards HBHA could potentially protect against extrapulmonary tuberculosis.
Syndecans as gonococcal receptors
Neisseria gonorrhoeae, the aetiological agent of gonorrhoea, is strictly adapted to its human host. The initial attachment of N. gonorrhoeae to the urogenital mucosa is mediated by pili, whereas subsequent adherence steps leading to tighter binding are mediated by the outer membrane opacity-associated (Opa) proteins (van Putten and Duensing, 1997). N. gonorrhoeae may express up to 11 genes encoding Opa proteins, which exhibit high amino acid sequence similarities except for variable domains present in three of the four surface-exposed loops. Opa proteins interact through protein–protein interactions with various members of the carcinoembryonic antigen-related cellular adhesion molecule (CEACAM; previously CD66), which are present on epithelial cells and neutrophils, two cell types targeted by N. gonorrhoeae in the course of natural infection (Bos et al., 1998; Virji et al., 1999). Besides this interaction, mediated by virtually all Opa proteins, it has been shown that syndecan receptors are required for N. gonorrhoeae entry into human mucosal cells (van Putten and Paul, 1995). Syndecans constitute a family of HSPGs, which, along with glypicans (Fig. 1), are the major carriers of cell-surface heparan sulphate (Bernfield et al., 1999). So far, four mammalian syndecans have been identified and termed syndecans 1 to 4. Each of them is encoded by a distinct gene. Syndecans 1 and 4 have been shown to be implicated in the attachment and uptake of N. gonorrhoeae by epithelial cells (Freissler et al., 2000). This adherence process is mediated by the interaction of the heparan sulphate (HS) chains of syndecans with the hypervariable region 1 of the N. gonorrhoeae OpaA protein (Grant et al., 1999). Syndecans have an intracellular EFYA amino acid motif located at their C-terminus, which interacts with the PDZ domain of syntenin (Fig. 1). PDZ motifs have been shown to be involved in the transmission of extracellular signals to the cytoskeleton and in the activation of signal pathways (Grootjans et al., 1997). Thus, OpaA+N. gonorrhoeae may perhaps modulate the host cytoskeleton–membrane dynamics and the redistribution of adherens junctions by means of syndecans–syntenin interactions (Zimmermann et al., 2001), leading to enhanced bacterial invasion. However, the entry of OpaA+ gonococci into specific cell types appears to be a multifactorial phenomenon as it requires the concerted action of fibronectin and integrin receptors (van Putten et al., 1998) in addition to PGs. Besides its involvement in cytoadherence, the binding of heparin by strains producing Opa proteins has been demonstrated to induce in vitro protection from human serum killing, suggesting that N. gonorrhoeae also uses GAGs to persist within the human urethra, where sulphate-containing PGs are abundant and where antibodies and serum components may transude from the blood (Chen et al., 1995).
GAG mimicry by Chlamydia
The adherence of Chlamydia trachomatis, an obligate intracellular Gram-negative pathogen, to epithelial cells can be inhibited by heparin or heparan sulphate, but not by chondroitin sulphate ABC or keratan sulphate, and the attachment and infectivity can be neutralized by treatment of C. trachomatis elementary bodies with heparitinase, suggesting that C. trachomatis synthesizes on its surface a heparan sulphate-like ligand able to bind to a complementary GAG-binding receptor of the host cell (Zhang and Stephens, 1992). Attachment can be inhibited by an anti-heparan sulphate antibody that binds to the surface of C. trachomatis (Rasmussen-Lathrop, et al. 2000), and both attachment and infectivity of heparitinase-treated bacteria can be restored by the addition of exogenous heparin or heparan sulphate (Zhang and Stephens, 1992).
The chlamydial heparan sulphate-like ligand has been isolated from infected CHO cells that are intrinsically deficient in GAG synthesis, suggesting that it is produced by the bacteria and not appropriated from host cell. Although the precise molecular structure of the chlamydial GAGs has yet to be determined, the size of a decasaccharide derived from heparin corresponds to the minimal chain length requirement to rescue infectivity of heparitinase-treated C. trachomatis (Chen et al., 1996). These observations suggest that some intracellular bacterial pathogens can infect host cells through mimicry of the eukaryotic cell-surface component GAG. The GAG-dependent adherence mechanism is common to C. trachomatis trachoma and lymphogranuloma venereum biovars (Chen and Stephens, 1994).
In spite of these observations, GAG synthesis by Chlamydia remains controversial, as Taraktchoglou et al. (2001) failed to detect chlamydial heparan sulphate-like compounds on some serovars. Moreover, analysis of the completed genomes of C. trachomatis and Chlamydia pneumoniae provided no evidence for chlamydial GAG biosynthesis-related genes (Hackstadt, 1999). Other investigations have shown that the C. trachomatis major outer membrane protein (MOMP) is a cytoadhesin with lectin activity for heparan sulphate-containing receptors on the surface of epithelial cells, suggesting that chlamydiae can use GAGs present on host cells to mediate their adherence (Su et al., 1996). Recently, it has been demonstrated that the 60 kDa cysteine-rich outer membrane complex protein, OmcB, which is present on the surface of chlamydial elementary bodies also binds GAGs (Stephens et al., 2001). Although heparin and synthetic sulphated polysaccharides are potent inhibitors of C. trachomatis infectivity in vitro (Zaretzky et al., 1995), these compounds lack protective efficacy when tested in vivo in the murine model of chlamydial genital tract infection (Su and Caldwell, 1998).
Syndecan shedding
Host HSPGs are used by pathogenic bacteria not only to mediate their attachment but also to enhance their virulence by a mechanism termed shedding, as recently described for Pseudomonas aeruginosa and Staphylococcus aureus (Park et al., 2000). The shedding mechanism involves cleavage of cell-surface proteins and release of their ectodomains from the surface as soluble effectors (Bernfield et al., 1999). In P. aeruginosa, the LasA protein, a secreted 20 kDa virulence factor, has been shown to specifically enhance shedding of syndecan-1. This process does not involve a direct interaction of LasA with the syndecan-1 ectodomain but relies on the activation by LasA of the host cell’s own shedding mechanism. Syndecan-1 shedding is an important trait of P. aeruginosa pathogenesis as mice deficient in syndecan-1 resist lung infection but become susceptible when given purified syndecan-1 ectodomains or heparin, indicating that the HS chains are the effectors (Park et al., 2001). The precise mechanism by which shedding enhances P. aeruginosa virulence is still unknown, but shed syndecan-1 ectodomains bind tightly to cationic antimicrobial peptides in the lung and may therefore interfere with innate host defences. A similar inhibition of host antimicrobial peptides through dermatan sulphate release from PGs has been described in P. aeruginosa, Enterococcus faecalis and Streptococcus pyogenes (Schmidtchen et al., 2001). Host proteoglycan shedding in response to inflammation during natural infection may also represent a defence mechanism to inhibit the adherence of pathogens that require tight GAG-mediated interactions with host cells to establish infection.
GAG-mediated recruitment of host proteins
It has been shown that N. gonorrhoeae binds vitronectin without the involvement of a specific gonococcal vitronectin receptor (Duensing and van Putten, 1998; Duensing et al., 1999). In this interaction, vitronectin, which is also a heparin-binding protein, and the gonococcal OpaA adhesin are bridged by heparin-related sulphated polysaccharides, leading to the possibility that heparin-like polysaccharides may be used by pathogenic bacteria to recruit host proteins. This strategy bypasses the need to synthesize specific receptors for each of these proteins, which may include growth factors, adhesive proteins, complement components, plasma lipoproteins, cytokines and regulators of haemostasis (Jackson et al., 1991). Such a broad recruitment mechanism has been recently demonstrated in several bacterial pathogens, including Helicobacter pylori, Streptococcus pyogenes and Yersinia, Staphylococcus and pathogenic Neisseria species (Duensing et al., 1999). Among the mammalian proteins recruited through GAG bridges are the monocyte chemotactic protein 3 (MCP-3), γ-interferon and the macrophage inflammatory protein 1α (MIP-1α). These observations suggest that pathogenic bacteria may hijack cytokines and chemokines to manipulate the host immune response by inhibiting chemokine-directed immune re-actions, such as leucocyte infiltration, a primary defence mechanism against microbial pathogens during the inflammatory response (Standiford et al., 1997). Obviously, GAG-mediated recruitment of host proteins could also be facilitated by syndecan shedding.
Opening the Pandora’s box
Although the binding of bacteria to GAGs borne by PGs is well documented, it is not known whether this leads to metabolic changes within the host cells. As PGs are involved in many physiological processes, such as cellular adhesion, spreading and migration, and may serve as co-receptors for various growth factors (Steinfeld et al., 1996), it is possible that binding to GAGs may affect certain metabolic pathways, which could be exploited by bacterial pathogens. It is certainly conceivable that adherence of pathogens to cell-surface HSPGs prevents them from participating in the formation of growth factor–receptor complexes, such as fibroblast growth factor 2 (FGF-2)–FGFR1 (Rapraeger et al., 1991; Kan et al., 1993) or transforming growth factor β (TGFβ)–βglycan (Lopez-Casillas et al., 1993), which may disrupt the proliferative signals and lead to cell death and tissue destruction. HSPGs have also been shown to protect growth factors from degradation and to modulate their bioavailability by serving as a reservoir for these factors (Andres et al., 1992; Iozzo, 1998). By competing for the binding of FGF, TGFβ or vascular endothelial growth factor (VEGF) (Cheng et al., 1997) to HSPGs, GAG-binding prokaryotic adhesins could potentially induce the release of growth factors trapped by the cell-surface HSPGs, thereby increasing their bioavailability.
Interference of bacterial adhesins with cell-to-cell contacts mediated by PGs could also potentially result in the disruption of tissue integrity and lead to the dissemination of the bacteria by crossing barriers composed of cells with tight junctions in epithelial or endothelial layers. Through their ability to rapidly (within minutes) open interendothelial cell junctions (Senger et al., 1983), growth factors such as the GAG-binding VEGF can transiently and non-selectively increase the permeability of the endothelium. Although the VEGF receptors responsible for this transient permeability have not yet been identified, a pathogen expressing a specific GAG-binding adhesin could have a similar effect by binding to VEGF receptors. This may help the bacteria to cross endothelial cell layers and to migrate into host tissues. Another example of possible disruption of tissue integrity could be related to the HGF-induced epithelial cell scattering, which may be affected by the interaction of bacterial GAG-binding adhesins with HSPGs and dermatan sulphate PGs, both known to be required for the biological activity of HGF (Lyon et al., 2001).
In addition to interacting with growth factor pathways, HSPGs are known to affect directly cell adhesion and interaction with the ECM. Cell interactions with ECM molecules are mediated mainly by integrins (Fig. 1), which are found on all types of adherent cells. In addition to integrin binding sites, most ECM proteins, such as collagens, fibronectin (Walker and Gallagher, 1996), laminin (Parthasarathy et al., 1998) or vitronectin, have heparin/ HSPG-binding domains which, in some cases, are involved in cell adhesion and intracellular signalling (Woods and Couchman, 2000). Disruption of integrin– matrix interaction and cellular detachment is known to induce cell growth arrest and to lead to apoptosis (Chen et al., 1997; Ruoslahti, 1997). This phenomenon, potentially triggered by bacterial GAG-binding adhesins, could also promote the metastatic dissemination of the pathogen. Another putative target of GAG-binding adhesins could be the blood coagulation system. By interfering with thrombin or antithrombin III, both of which bind dermatan sulphate (Tollefsen et al., 1983), these adhesins could dramatically affect blood clotting.
These functions of PGs represent possible targets for bacteria to interfere with normal host cell activities. However, they have not so far been investigated thoroughly. It is not yet known whether PGs are mainly used as initial binding sites by pathogenic bacteria or whether they also facilitate subsequent steps of the infectious cycle by affecting cell adhesion or spreading. This represents undoubtedly an exciting emerging research field for a better understanding of bacterial pathogenesis.
Conclusions
GAGs borne by PGs are used by many bacterial pathogens to adhere to target tissues. Over the years this trait has emerged as a common theme in microbial pathogenesis. Recent observations have also shown that the interaction of bacterial adhesins with GAGs may not be limited to the initial adherence step, as exemplified by the fact that GAGs are necessary for extrapulmonary dissemination of M. tuberculosis (Pethe et al., 2001). This finding suggests that GAG-binding adhesins may have various effects within the infected host. To understand these effects and potentially develop new strategies for the treatment or prevention of bacterial diseases, it appears important to characterize not only the adhesins but also their receptors during the infectious cycle. Moreover, the bacterial GAG-binding adhesins could also represent new molecular tools to investigate physiological processes such as tissue remodelling or mitogenic reponses.
Acknowledgements
We thank all the workers in the field for the many exciting experiments and the excellent work that has been done over the years. The work in our laboratory was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Institut Pasteur de Lille, the Région Nord – Pas de Calais, and the Ministère de la Recherche. K. P. holds a fellowship of the Ministère de la Recherche and P. B. holds a fellowship of the Centre National de la Recherche Scientifique (CNRS).