Truncation of merozoite surface protein 3 disrupts its trafficking and that of acidic–basic repeat protein to the surface of Plasmodium falciparum merozoites
Summary
Merozoite surface protein 3 (MSP3), an important vaccine candidate, is a soluble polymorphic antigen associated with the surface of Plasmodium falciparum merozoites. The MSP3 sequence contains three blocks of heptad repeats that are consistent with the formation of an intramolecular coiled-coil. MSP3 also contains a glutamic acid-rich region and a putative leucine zipper sequence at the C-terminus. We have disrupted the msp3 gene by homologous recombination, resulting in the expression of a truncated form of MSP3 that lacks the putative leucine zipper sequence but retains the glutamic acid-rich region and the heptad repeats. Here, we show that truncated MSP3, lacking the putative leucine zipper region, does not localize to the parasitophorous vacuole or interact with the merozoite surface. Furthermore, the acidic–basic repeat antigen (ABRA), which is present on the merozoite surface, also was not localized to the merozoite surface in parasites expressing the truncated form of MSP3. The P. falciparum merozoites lacking MSP3 and ABRA on the surface show reduced invasion into erythrocytes. These results suggest that MSP3 is not absolutely essential for blood stage growth and that the putative leucine zipper region is required for the trafficking of both MSP3 and ABRA to the parasitophorous vacuole.
Introduction
The protozoan parasite Plasmodium falciparum causes the most severe form of malaria in humans. The majority of victims are young children, with adult populations nevertheless suffering significant morbidity. Drug resist-ance continues to increase and as yet there is no effective vaccine against malaria. A large number of potential vaccine candidates have been identified; however, little is known of their functions (Barnwell and Galinski, 1998). Many of the putative vaccine antigens are located on the merozoite surface. As a major interface between host and pathogen, the merozoite surface is an obvious target for the development of a vaccine. Proteins comprising the merozoite surface are exposed to the host immune system before and during invasion into the erythrocyte, albeit for a short time.
Thus far, several merozoite surface proteins have been identified, and included in this group are the mero-zoite surface proteins 1–6 and 8 (Smythe et al., 1988; Blackman et al., 1990; McColl et al., 1994; Oeuvray et al., 1994a; Marshall et al., 1997; Marshall et al., 1998; Black et al., 2001; Trucco et al., 2001). MSP1 is an important vaccine candidate, and there is evidence to suggest that it can bind to the surface of the eryth-rocyte (Kaslow et al., 1994), whereas MSP2–6 and 8 are less characterized. Indeed, there is very little data defining the roles of any of these antigens in parasite invasion.
Merozoite surface protein 3 (MSP3) is a polymorphic antigen, which has a number of structural domains (Fig. 1A). These domains include three blocks of four heptad repeats of the type AXXAXXX, a hydrophilic region and a putative leucine zipper sequence at the C-terminus (Fig. 1A) (McColl et al., 1994). The solution structure of the heptad repeats has been solved, and each block appears to form an α-helix, which may interact to form a somewhat unstable coiled-coil (Mulhern et al., 1995). Despite some sequence diversity of the msp3 gene between P. falciparum isolates, there is significant conservation (McColl et al., 1994; Escalante et al., 1998). Indeed, the alanine residues within the heptad repeat regions and the C-terminal half of the protein, which includes the putative leucine zipper region, are strongly conserved (McColl and Anders, 1997).
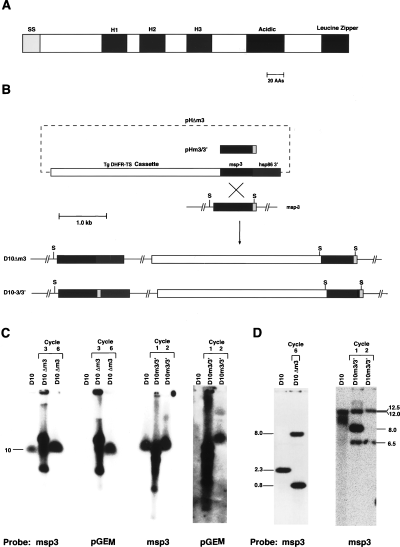
Structure of MSP3 and disruption of the msp3 gene.
A. Structure of MSP3 showing the putative signal sequence (SS), the three blocks of four heptad repeats (H1, H2, H3), the acidic region and the putative leucine zipper domain.
B. Disruption of the msp3 gene in D10 parasites. The plasmids pHΔm3 and pHm-3/3′ are shown and were transfected into D10 and transfected parasites selected with pyrimethamine. Cycling of transfected parasites on and off drug allowed selection of those with integrated copies of the plasmid into the msp3 gene as shown. The msp3 gene is divided into three shaded regions to show the regions used in pHΔm3 and pHm3/3′. The plasmid pHΔm3 integrated via a single recombination event to produce the parasite line D10Δm3 that contained a truncated msp3 gene. The plasmid pHm-3/3′ integrated via a single recombination event to produce the parasite line D10-3/3′, in which the msp3 gene had been reconstituted. S represents SwaI.
C. Pulsed-field gel electrophoresis analysis of chromosomes from D10 and transfected parasites to determine whether integration of the transfection plasmids had occurred. The first panel shows chromosomes from parasites after transfection with pHΔm3 and three and six cycles on and off drug probed with the msp3 gene (first panel) and the plasmid backbone pGEM (second panel). The third blot shows chromosomes from parasites after transfection with pHm-3/3′ and one and two cycles on and off drug probed with the msp3 gene (third panel) and the plasmid backbone pGEM (fourth panel). Chromosome 10 is indicated, and a single band migrating at the position of chromosome 10 suggests integration of the transfection plasmid.
D. Southern hybridization of genomic DNA from D10, D10Δm3 after cycle six (first panel) as well as D10, D10m3/3′ after one and two cycles on and off drug (second panel), indicating that the transfection plasmids had integrated into the msp3 gene in both D10Δm3 and D10m3/3′. Sizes of the hybridizing bands are shown in kb at the side.
Current evidence supports MSP3 as a vaccine candidate. MSP3 was first detected using human hyperimmune serum from Papua New Guinea (McColl et al., 1994) and also with antibodies that inhibit P. falciparum in vitro growth by co-operation with blood monocytes in an antibody-dependent cellular inhibition assay (ADCI) (Oeuvray et al., 1994a). The ADCI assay selects for molecules capable of producing a protective immune response. This strengthens the case for MSP3 as a vaccine candidate (Oeuvray et al., 1994a). Recently, it has been shown in an immunocompromised mouse model infected with P. falciparum that human antibodies purified on peptides from MSP3 are able to suppress P. falciparum growth in the presence of human monocytes (Badell et al., 2000).
Several MSP3 homologues have been found in other Plasmodium species. Plasmodium vivax express a protein similar in structure to PfMSP3, which contains blocks of heptad repeats predicted to form a coiled-coil and is also polymorphic (Galinski et al., 1999). Also, the Plasmodium knowlesi 140 kDa protein has homology with PfMSP3 (Hudson et al., 1983). Interestingly, MSP6 shows some structural similarity and homology with MSP3. MSP6 has been shown to form part of the MSP1 complex on the surface of the merozoite of P. falciparum (Trucco et al., 2001).
Given that MSP3 is conserved over diverse Plasmodium species, is recognized by hyperimmune sera from a malaria-endemic region and antibodies to MSP3 appear to be capable of inhibiting in vitro growth by ADCI assays, we sought to elucidate further the role of MSP3 in the P. falciparum merozoite. In this report, we describe the first targeted disruption of a gene expressing a protein located on the merozoite surface of P. falciparum. Targeting of msp3 resulted in truncation of 37 amino acids from the C-terminus of the MSP3 protein and removal of the putative leucine zipper sequence. This truncation disrupted the trafficking of this protein to the parasitophorous vacuole and interaction with the merozoite surface. Furthermore, the acidic–basic repeat antigen (ABRA), another merozoite surface protein, was also found to be absent from the merozoite surface. Interestingly, the loss of these proteins from the merozoite led to a reduction in invasion efficiency. These results have important implications for the function of MSP3 in merozoite invasion and its potential as a vaccine candidate.
Results
Targeted disruption of the msp3 gene
In order to study the role of MSP3 in merozoite invasion into human erythrocytes, we constructed two P. falciparum transgenic lines. In the first parasite line, the single wild-type copy of msp3 was disrupted, whereas in the second, the msp3 gene was replaced intact. This was achieved by introducing either the transfection vector pHΔm3 or pH-m3/3′, respectively, into D10 parasites (Fig. 1B). Transfected parasites were selected on pyrimethamine, cultured on drug alternating with cycles on and off drug to facilitate loss of episomal plasmid and selection for parasites with integrated copies (Crabb and Cowman, 1996; Wu et al., 1996; Crabb et al., 1997).
To determine whether the transfection plasmids had integrated into the parasite genome, chromosomes of the D10 parental line and the transfected lines were analysed by pulsed-field gel electrophoresis (PFGE) (Fig. 1C). When probed with the msp3 gene, D10 chromosomes exhibit a single band at a position corresponding to chromosome 10. However, after three rounds of cycling, chromosomes from the transfected parasites D10Δm3 show not only the endogenous gene, but also further bands consistent with migration of unintegrated plasmids under these electrophoresis conditions (Crabb and Cowman, 1996). In contrast, after six rounds of cycling, chromosomes from D10Δm3-transfected parasites show only a single band corresponding to chromosome 10, indicating complete integration of pHΔm3 into chromosome 10 (Fig. 1B). To prove that this single band did not arise as a result of complete loss of plasmid, the same chromosomes were subjected to hybridization with the plasmid backbone, pGEM (Fig. 1C). As expected, no hybridization occurred with D10 chromosomes; however, a pattern consistent with unintegrated plasmid occurred with chromosomes from D10Δm3 parasites after three rounds of cycling. A single band corresponding to chromosome 10 was seen in chromosomes from transfected parasites after six rounds of cycling. These results indicate that, after six rounds of cycling, complete integration of pHΔm3 into chromosome 10 had occurred in the D10Δm3-transfected parasites.
The plasmid pH-m3/3′ was also transfected into D10, and parasites were cycled through two rounds of off drug selection to obtain parasites with integrated copies of the plasmid. Chromosomes from D10 and the transfected line D10-M3/3′ were probed with the msp3 gene. After two cycles of selection, all the pH-m3/3′ plasmid was apparently integrated into chromosome 10. This was confirmed by hybridization of a pGEM probe (Fig. 1C).
In order to determine whether the pHΔm3 and pH-m3/3′ plasmids had integrated into the msp3 gene on chromosome 10 of the D10 transfectants, Southern blot analyses of SwaI restriction-digested genomic DNA were performed (Fig. 1D). Hybridization with an msp3 probe identified a single band of ≈ 2.3 kb in D10 corresponding to the endogenous gene. However, in D10Δm3-transfected parasites, two hybridizing bands of ≈ 0.8 kb and 8 kb were obtained, indicating that the plasmid had integrated into the msp3 gene. Further restriction mapping of the integration event confirmed that the pHΔm3 plasmid had integrated via a single homologous cross-over to generate the structure shown in Fig. 1B. This resulted in truncation of the msp3 gene in D10Δm3 by 111 bp. Expression of the ΔMSP3 protein from these parasites would lack 37 amino acids, which encompasses the putative leucine zipper sequence (Fig. 1A). Analysis of two independent clonal isolates derived from D10Δm3 parasites (designated D10Δm3/1 and D10Δm3/2) showed an identical restriction pattern. Similar analysis of the D10-m3/3′ parasites showed that the pH-m3/3′ plasmid had also integrated by a single homologous cross-over to replace the intact msp3 gene (Fig. 1C). This recombination event places the 3′ termination sequence hsp86 3′ at the 3′ end of the msp3 gene, resulting in reconstitution of the full gene including the region encoding the putative leucine zipper (Fig. 1B). This parasite line was designed to express the full-length MSP3 and thus served as a control for subsequent experiments.
Disruption of msp3 leads to the expression of truncated MSP3
In order to analyse expression of MSP3 in D10Δm3 compared with the parental line D10, we harvested late-stage schizonts and subjected them to SDS–PAGE and Western analysis using anti-MSP3 antibodies (Fig. 2A). When D10 proteins were probed with anti-MSP3 antibodies, the expected 62 kDa and 48 kDa protein bands were detected (McColl et al., 1994). In contrast, Western analysis of the D10Δm3 parasites containing the disrupted msp3 gene revealed two MSP3 bands of ≈ 58 kDa and 43 kDa. The degree of reduction in the size of both these bands compared with those seen in the D10 parental line is consistent with the expected size of proteins resulting from the truncation of the msp3 gene. Interestingly, there appears to be an increase in the expression of the truncated MSP3 in D10ΔM3 parasites, when compared with that observed for Hsp70 (Fig. 2A).

MSP3 is truncated in D10Δm3 parasites.
A. Protein from synchronized schizonts was probed with anti-MSP3 antibodies (first panel) and anti-HSP70 antibodies to show that MSP3 had been truncated in D10Δm3. The level of apparent expression of truncated MSP3 appears to be increased in D10Δm3 compared with the anti-HSP70 control (second panel).
B. Immunoblots on purified merozoites from D10 and D10Δm3 showing truncated MSP3 and an apparent increase in expression compared with a second parasitophorous vacuole-located protein, S-antigen (S-Ag). The first panel was probed with anti-MSP3 antibodies and the second panel with anti-S-Ag antibodies.
C. Immunoblots of purified merozoites from D10 and D10Δm3 probed with anti-ABRA (first panel) and anti-S-Ag antibodies (second panel). This suggests that the apparent expression level of ABRA has increased in D10Δm3 merozoites.
In order to determine whether truncated MSP3 in D10Δm3 parasites is associated with free merozoites, further Western analysis was performed. Merozoites purified from the D10 parental and D10Δm3 parasites were subjected to SDS–PAGE and Western blot analysis using anti-MSP3 antibodies. The 62 kDa and 48 kDa bands were obtained in the D10 merozoites; however, a more intensely truncated 58 kDa was seen in D10Δm3 (Fig. 2B). This is in contrast to the results observed with S-antigen, a protein that is also secreted into the parasitophorous vacuole of the schizont stage (Anders et al., 1983). The S-antigen protein was detected in both D10 and D10Δm3 merozoites at approximately equal levels (Fig. 2B). We also analysed the expression of the acidic–basic repeat antigen (ABRA), which is also expressed in the parasitophorous vacuole, and surprisingly found differences in D10Δm3 compared with D10. ABRA is a protease that is expressed in the parasitophorous vacuole and has also been shown to be associated with the merozoite surface (Nwagwu et al., 1992; Kushwaha et al., 2000). Purified merozoites were analysed by SDS–PAGE and Western blots using antibodies to the ABRA protein (Kushwaha et al., 2000). Interestingly, ABRA also displayed an apparent overexpression in D10Δm3 compared with the parental D10 parasites (Fig. 2C). ABRA appears to be present in D10Δm3 merozoites at levels at least three or four times that observed with D10 relative to the S-antigen loading control. These results suggest that truncation of the MSP3 protein is linked to an accumulation of ABRA within D10Δm3 merozoites.
MSP3 and ABRA in D10Δm3 parasites do not localize to the merozoite surface
Immunofluorescence microscopy was performed on D10 and D10Δm3 schizont and merozoite stages in order to determine the subcellular localization of the truncated MSP3 protein and ABRA in D10Δm3 compared with the D10 parental parasites. Late schizonts and free merozoites were incubated with anti-MSP1, -MSP3 and -ABRA antibodies in immunofluorescence experiments (Fig. 3). In late D10 schizonts, MSP3 and ABRA had a localization consistent with the parasitophorous vacuole and the merozoite surface, as described previously (Oeuvray et al., 1994a; Kushwaha et al., 2000). This was similar to the subcellular localization of MSP1 (Holder and Freeman, 1984), which is present on the surface of the developing merozoite as a glycosylphosphatidyl inositol-anchored protein (Holder et al., 1985). This subcellular localization was confirmed in free merozoites, in which both MSP3 and ABRA co-localized with MSP1 (Fig. 3, top). Localization of MSP3 and ABRA in D10Δm3 schizonts showed a very different pattern of localization compared with that observed in D10. Neither truncated MSP3 nor ABRA fully co-localized with MSP1, suggesting that these proteins were not able to traffic to the parasitophorous vacuole in D10Δm3 (Fig. 3, lower). In order to determine whether truncated MSP3 and ABRA were present within free merozoites, co-localization studies were carried out with MSP1. A significant concentration of both MSP3 and ABRA proteins was observed in the form of small dots within the merozoites. These results suggest that MSP3 and ABRA accumulate within the merozoite of D10Δm3 during development and that they are unable to be trafficked to the parasitophorous vacuole.
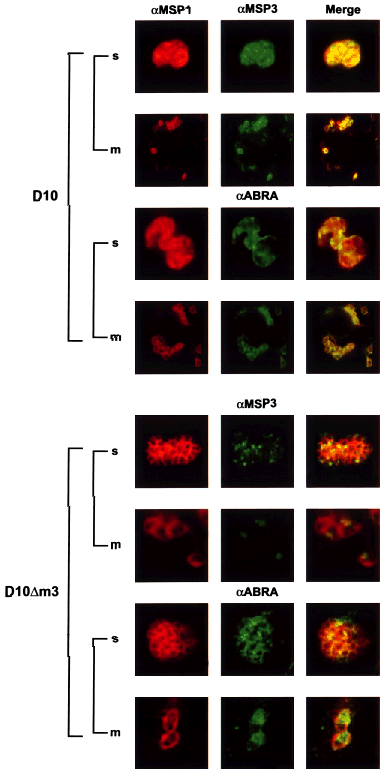
Subcellular localization of MSP3 and ABRA shows that deletion of the putative leucine zipper region is linked to incorrect localization of ΔMSP3 and ABRA in D10Δm3. Smears of late schizonts of D10 and D10Δm3 were incubated with anti-MSP1 (located on the surface of the merozoite) and anti-MSP3 or anti-ABRA antibodies. Anti-MSP1 mouse antibodies were labelled with rhodamine-conjugated anti-mouse antibodies (red). Anti-MSP3 and anti-ABRA rabbit antibodies were labelled with anti-rabbit antibodies conjugated with FITC (green). Co-localization is shown in the row and indicated by yellow. The first panel is D10 smears labelled with anti-MSP1 (first column) and anti-MSP3 or anti-ABRA (second column). The second panel is D10Δm3 labelled with anti-MSP1 (first column, red) and anti-MSP3 or anti-ABRA antibodies (green). D10Δm3 merozoites in the second and fourth panels have been magnified by a factor of two compared with the other blots to enable increased detail to be visualized.
MSP3 localization in D10Δm3 free merozoites is not associated with the endoplasmic reticulum
It is possible that the aberrant localization of MSP3 in D10Δm3 merozoites resulted from retention in the endoplasmic reticulum (ER) as has been described for RAP2 in parasites that express truncated RAP1 (Baldi et al., 2000). To determine whether this was the case in D10ΔM3 merozoites, antisera were raised to the P. falciparum ERC protein (La Greca et al., 1997). No co-localization of MSP3 and ERC was observed with either D10 (data not shown) or D10ΔM3 parasites (Fig. 4). MSP3 appears to be contained in vesicular-like structures or large aggregates at the apical end of the merozoite, and this was distinct from the ER and nucleus as shown by anti-ERC antibodies and DAPI staining.
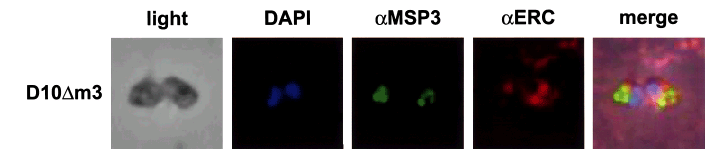
ΔMSP3 does not co-localize with the ER marker PfERC in merozoites. Merozoite smears incubated with anti-MSP3 and anti-ERC antibodies. The first panel shows light transmission of the merozoites. The second panel shows staining with the nuclear stain DAPI. The third panel shows localization of ΔMSP3. The fourth panel shows localization of PfERC, and the fifth blot shows merging of blots two, three and four.
Lack of MSP3 and ABRA on the merozoite surface has an effect on merozoite invasion in vitro
To determine whether truncation of MSP3 had any effect on the ability of merozoites to invade human erythrocytes, D10, D10-m3/3′ and the two D10Δm3 clones, D10ΔM3/1 and D10ΔM3/2, were compared in merozoite invasion assays. Percoll-purified trophozoites were incubated overnight with fresh human erythrocytes, and the number of ring-stage parasites was counted. The invasion efficiency was calculated and expressed as percentage parasitaemia (Fig. 5). The parental D10 parasites invaded at a higher rate than the transfection control D10-m3/3′, suggesting that insertion of the plasmid at the 3′ end of the msp3 gene may have affected its expression, although the difference in merozoite invasion was small but significant. Importantly, the difference in merozoite invasion between D10-m3/3′ and D10Δm3 expressing the truncated MSP3 protein was significantly decreased (P < 0.05). This suggests that truncation of MSP3 and/or altered localization of ABRA is not necessary for merozoite invasion per se.
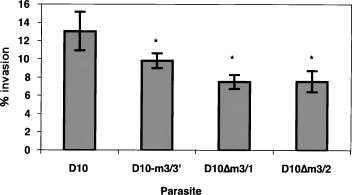
Comparison of merozoite invasion rates for D10, D10-3/3′ and the two cloned lines, D10Δm3/1 and D10Δm3/2. Purified schizonts were incubated with uninfected erythrocytes and allowed to reinvade, and this allowed us to measure differences in merozoite invasion rates. Parasitaemias were counted and ranged from 6% to 15%. D10Δm3/1 and D10Δm3/2 were significantly lower than D10-3/3′ and D10.
Discussion
The merozoite interacts initially with the erythrocyte surface randomly before invasion and then undergoes a process of reorientation. Proteins on the surface of the merozoite are likely to play a role in this initial interaction and reorientation and are furthermore exposed to the host immune system during this process. MSP3 is located on the surface of the invasive merozoite and is an important vaccine candidate. We have used transfection in P. falciparum to disrupt the msp3 gene in order to understand the role of this protein in merozoite invasion. Disruption of the msp3 gene has resulted in the expression of truncated MSP3 lacking the putative leucine zipper domain at the C-terminus (Fig. 1A). This has resulted in decreased ability of the merozoite to invade erythrocytes. Interestingly, truncation of MSP3 was linked to altered trafficking of ABRA, a second protein normally localized to the merozoite surface. Our results suggest that the putative leucine zipper region is required for normal trafficking of both MSP3 and ABRA and that these proteins play a role in the merozoite invasion process.
The absence of truncated MSP3 on the surface of merozoites would suggest that either the protein was unable to interact with the surface or, alternatively, there was a defect in its normal trafficking to the parasitophorous vacuole. The putative leucine zipper region in MSP3 is likely to be an important domain for protein–protein interactions (Anantharamaiah et al., 1991; Legrain and Chapon, 1993; Hohjoh and Singer, 1996; Lupas, 1996). The data suggest that removal of the putative leucine zipper region has caused a defect in trafficking of this protein. This is further supported by the accumulation of truncated MSP3 within structures in the merozoite.
Truncated MSP3 protein is still released during schizont rupture; however, it is unlikely that this is caused by the presence of protein within the parasitophorous vacuole. The ER-localized protein PfERC is also released into the supernatant, with some of the protein retained in the remnant ER within the merozoite (Baldi et al., 2000). Similarly, RAP2 is retained in the ER of mutant parasites that express a truncated RAP1 protein, and this protein is also released at schizont rupture. Despite the release of truncated MSP3 during schizont rupture, the protein is unable to interact with the merozite surface. This suggests that either the putative leucine zipper region is essential for this interaction or, alternatively, localization within the parasitophorous vacuole is a prerequisite for allowing the interaction to take place.
Recently, a protein termed MSP6 has been identified that has some homology and structural similarity to MSP3, including a putative leucine zipper region at the C-terminus. MSP6 has been shown to be part of the MSP1 complex on the merozoite surface. It is possible that MSP3 interacts with a complex in a manner similar to MSP1–MSP6 and that interaction of MSP3 with other proteins may be required for correct targeting to the parasitophorous vacuole. It has already been shown that truncation of RAP1 disrupts formation of the RAP1–RAP2 complex, resulting in retention of RAP2 in the ER rather than correct trafficking to the rhoptries (Baldi et al., 2000). Similarly, in Toxoplasma gondii, a transmembrane micronemal protein MIC6 has been shown to function as an escort for the accurate targeting of two soluble proteins, MIC1 and MIC4, to the rhoptries (Reiss et al., 2001). It is possible that MSP3 and ABRA interact with each other and that deletion of the putative leucine zipper region of MSP3 disrupts interaction with an escort protein, resulting in mislocalization of both MSP3 and ABRA. A number of genes have recently been identified in the P. falciparum genome that have structural similarities to MSP1, and the proteins encoded by these are located on the merozoite surface (Black et al., 2001). These proteins are candidates to act as binding partners for MSP3 and ABRA in a complex on the merozoite surface.
The truncation of MSP3 and/or alterations in ABRA localization result in reduced invasion of merozoites in D10Δm3. The D10-3/3′ control parasites also showed a small decrease in merozoite invasion compared with the parental D10 line. One possibility to explain this result is that there is a slight decrease in the level of expression of MSP3 due to insertion of an altered 3′ untranslated region. However, the important point is that the D10Δm3 is significantly decreased in merozoite invasion compared with both the parental line D10 and D10-3/3′. This is the first example to be described in which alteration of a protein such as MSP3 results in reduction in invasion efficiency. For instance, truncation of the RAP-1 protein, which resulted in the loss of RAP2 from merozoites, had no effect on invasion efficiency (Baldi et al., 2000), and truncation of EBA-175, despite resulting in a switch in invasion phenotype (Reed et al., 2000), similarly had no significant effect on invasion (Kaneko et al., 2000; Reed et al., 2000). Taken together, these results give weight to the argument that MSP3 or an MSP3–ABRA complex plays a role in invasion. Furthermore, these results are consistent with the demonstration that antibodies to MSP3 peptides in co-operation with monocytes can inhibit P. falciparum growth in an immunocompromised mouse model (Badell et al., 2000) and the ability of anti-MSP3 antibodies to inhibit merozoite invasion in an ADCI assay (Oeuvray et al., 1994a,b).
The requirement for MSP3 and/or ABRA for efficient merozoite invasion of human erythrocytes supports these proteins as vaccine candidates. However, lack of expression of these on the surface of the merozoite does not ablate invasion. This suggests that they are not absolutely essential and that there is some redundancy in the invasion process. Indeed, several different pathways of invasion have been identified in P. falciparum (Orlandi et al., 1992; Dolan et al., 1994; Okoyeh et al., 1999). Furthermore, the same parasite strain is able to ‘switch’ invasion pathways when a particular ligand is unavailable (Dolan et al., 1990; Reed et al., 2000). Given this redundancy, it is important that this be considered in evaluating any P. falciparum protein as a vaccine candidate.
These results suggest that MSP3 may be part of a complex on the surface of the merozoite and that interactions with other proteins are important for correct targeting of both MSP3 and ABRA to the parasitophorous vacuole. Interestingly, MSP3 does not appear to be absolutely essential for merozoite invasion, and it is very likely that it will be possible to construct P. falciparum mutant parasites that do not express MSP3. Construction of MSP3 null P. falciparum parasites would be useful to evaluate the role of antibodies to MSP3 in ADCI assays (Oeuvray et al., 1994a,b) and in P. falciparum infections of immunocompromised mice (Badell et al., 2000).
Experimental procedures
Parasites and transfection
Plasmodium falciparum was cultivated (Trager and Jensen, 1976) and synchronized as published previously (Lambros and Vanderberg, 1979). Parasites of the cloned line D10 were transfected as described previously (Crabb and Cowman, 1996; Wu et al., 1996; Crabb et al., 1997) with 80 μg of plasmid purified using Qiagen maxiprep columns. Transfected parasites were selected on 0.1 μM pyrimethamine and subjected to successive cycles of culture on and off drug for periods of 4 weeks. Parasites containing integrated plasmids were cloned by single-cell cloning.
pHΔm3 and pH-m3/3′ were created for transfection by the cloning of an 800 bp msp3 insert into the pHC1 transfection vector (Crabb et al., 1997). This fragment was amplified from D10 genomic DNA with the following oligonucleotides: pHΔm3 forward (5′-GGTAACTCGAGGGAATTTGGAGAAGGTTTTA GTGCAG-3′); pHΔm3 reverse (5′-TTTCCTCGAGTTCTTTT TTACGTTTTTCTCATTATTATTC-3′); pH-m3/3′ forward (5′-GCTGCTCGAGCTGCTTTAAAGGC-3′); pH-m3/3′ reverse (5′-TTAACTCGAGTTAATGATTTTTAAAATATTTGGATA-3′). This amplification resulted in the introduction of XhoI restriction sites on both ends of each fragment (bold). These sites were used for cloning into the pHC1 vector.
Nucleic acids and pulsed-field gel electrophoresis
Genomic DNA was extracted from late-stage parasites as described previously (Corcoran et al., 1986). Analysis of genomic DNA was carried out using standard Southern blot hybridization techniques. Pulsed-field gel electrophoresis (Chu et al., 1986) was used for chromosome separation as described previously (Corcoran et al., 1986).
Antisera and immunoblot assays
MSP3 antisera were raised in the following manner: full-length msp3 (excluding putative signal sequence) was amplified from D10 genomic DNA using the oligonucleotides 5′-GCGGATCCAAAGAAATTGTAAAAAAATATAATC-3′ and 5′-CGAATTCAATGATTTTTAAAATATTTGGATAATTC-3′, introducing BamHI and EcoRI restriction sites respectively. The resulting fragment was cloned into pPro-EX-HTb (Life Technologies). Expression of this plasmid in the Escherichia coli strain BL21 produced a protein in fusion with hexa-histidine. MSP3-hexa-his was purified on nickel–NTA beads and by reversed-phase chromatography. Purified protein was inoculated into NZ White rabbits. Serum generated was used throughout this study.
ERC antisera were raised in the following manner: ERC protein (La Greca et al., 1997) was obtained from L. Tilley (La Trobe University, Melbourne, Australia) and inoculated into eight mice. Serum from each mouse was tested for correct localization by immunofluorescence microscopy before being used throughout this study.
Proteins from late-stage parasites were separated on SDS–12% PAGE and transferred to nitrocellulose membranes as described previously (Cowman et al., 1991). MSP3, ABRA, S-antigen and HSP70 antisera were diluted 1:500, 1:1000, 1:1000 and 1:5000, respectively, in 5% (w/v) skimmed milk in phosphate-buffered saline (PBS). Secondary antibody was sheep anti-rabbit Ig horseradish peroxidase (HRP)-conjugated antibody (Silenus). Antigen– antibody complexes were detected by ECL (Amersham) and autoradiography.
Immunofluorescence
Thin blood smears fixed at room temperature in methanol were used for immunofluorescence. MSP1 was detected using the monoclonal anti-MSP1-19 antibody (Cooper et al., 1992). MSP3, ABRA and ERC were detected using the antibodies described above. Antibodies to MSP1, MSP3, ABRA and ERC were diluted 1:2000, 1:100, 1:10 and 1:75, respectively, in 5% (w/v) skimmed milk in PBS. Anti-MSP1 and -ERC antibodies were detected using goat anti-mouse rhodamine-labelled antibodies (Chemicon). Anti-MSP3 and -ABRA antibodies were detected using sheep anti-rabbit fluorescein isothiocyanate (FITC)-labelled antibodies (Silenus). All parasites were also labelled with 4′,6-diamidino-2-phenylindole (DAPI) DNA stain (Molecular Probes).
Invasion assays
Merozoite invasion assays were conducted essentially as described previously (Dolan et al., 1994). Briefly, 8 × 105 synchronized, Percoll-purified trophozoites were mixed with 4 × 107 erythrocytes in a total volume of 200 μl with 50/50 culture medium in a 96-well, flat-bottomed microtitre plate. Assays were performed in triplicate. After 28 h incubation, wells were resuspended, and thin blood smears were made and stained with Giemsa. Approximately 1000 cells were counted per slide, and infected cells were expressed as a percentage of total cells. This allowed quantification of merozoite invasion of human erythrocytes.
Acknowledgements
We would like to thank Sonia Caruana for technical assistance, Leanne Tilley for the provision of ERC protein, Virander Chauhan for provision of the ABRA antibody, and Ross Coppel for the S-antigen antibody. We would like to acknowledge the Australian Red Cross Blood Bank (Melbourne, Australia) for supply of human erythrocytes and serum. K.E.M. is the recipient of a Melbourne Research Scholarship. A.F.C. and B.S.C. are International Research Fellows from the Howard Hughes Medical Institute. This work was funded by the National Health and Medical Research Council of Australia.