DB or not DB in translation?
Sir,
In a recent report (Tedin et al., 1999, Mol Microbiol31: 67–77), it has been stated that there are no cis-acting elements downstream of the initiation codon involved in the translation of leaderless mRNAs. In addition, these authors stated that Resch and his collaborators (Resch et al., 1996, EMBO J15: 4740–4748) have found no evidence for the involvement of the proposed downstream box/anti-downstream box interaction (Sprengart et al., 1996, EMBO J15: 665–674) in translation initiation of leaderless mRNAs. In this regard, it is interesting to notice that the conclusions of Resch et al. (1996) could be explained by the newly created DB sequence in their deletion constructs (see Fig. 1). As initially speculated by Sprengart and Porter (1997, Mol Microbiol24: 19–28), in all the DB deletion constructs of the λcI, tetR and P2V genes, a putative DB sequence was recreated, as shown in Fig. 1. This may account for their efficient formation of translation initiation complex observed by Resch et al. (1996). The newly created DB in the λcIΔ2/3 construct is composed of nine matches with one GC basepair (see Fig. 1A). Even though this new RNA duplex may be less stable than that of the DB–anti-DB interaction proposed by Resch et al. (1996), the new RNA duplex may be just enough for efficient translation initiation of the λcIΔ2/3 construct. Alternatively, 1Fig. 1A shows a second DB sequence in both wild-type λcI and λcIΔ2/3 consisting of 10 matches, in which three of them are GC basepairs. 1Figure 1B shows the DB–anti-DB interaction for the wild-type tetR (proposed by Resch et al., 1996) and the newly created DB in the tetRΔ2/3 construct (proposed here). The new DB–anti-DB interaction in the tetRΔ2/3 has nine matches with three GC basepairs. Alternatively, a second DB sequence consisting of nine matches with three GC basepairs is found in both wild-type tetR and tetRΔ2/3. The complementarity of the P2V mRNA with the 16S rRNA (proposed by Resch et al., 1996) has two GC basepairs compared with three GC basepairs between the newly created DB of the P2VΔ4/5 mRNA and 16S rRNA (Fig. 1C). A second DB sequence can also be found in both wild-type P2V and P2VΔ4/5, consisting of eight matches with four GC basepairs. Previously, Resch et al. (1995, FEMS Microbiol Rev 17: 151–157) also constructed base substitution mutations in the λcI and P2V phage mRNAs. However, the second DB sequences were not disturbed by their mutations (see Fig. 1A and C). Therefore, the conclusions of Resch et al. (1996) are not justified unless they remove the newly created DB sequences. In addition, it has been demonstrated that translation of the λcI mRNA requires DB and, furthermore, that the expression of the DB containing the λcI mRNA is enhanced in S2-depleted ribosomes (Shean and Gottesman, 1992, Cell70: 513–522). It has been proposed that, in the absence of ribosomal protein S2, the anti-DB sequence in the 16S rRNA would be more exposed and therefore available to interact with a DB-containing mRNA (Shean and Gottesman, 1992, Cell70: 513–522; Powers et al., 1998, J Mol Biol201: 697–716). Alternatively, Shean and Gottesman (1992) have speculated that the S2-depleted 30S ribosomal subunit may have an altered shape that could favour the DB–anti-DB interaction. This is supported by the altered mobility of the S2-depleted 30S subunit in sucrose gradients (Shean and Gottesman, 1992, Cell70: 513–522).
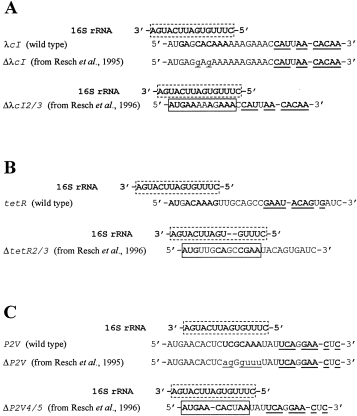
. DB–anti-DB complementarity in the ΔDB-leaderless mRNAs constructed by Resch et al. (1995, 1996). A. Putative DB sequences in the λcIΔ2/3 constructs. B. Putative DB sequences in the tetRΔ2/3 constructs. C. Putative DB sequences in the P2VΔ4/5 constructs. The anti-DB sequence in the 16S rRNA is boxed with a dotted line. The DB–anti-DB base pairings in the wild-type leaderless mRNAs proposed by Resch et al. (1996) are shown in bold. The second DB sequences proposed here are bold and underlined. The base substitution deletions in the λcI and P2V mRNAs performed by Resch et al. (1995) are shown in lowercase and underlined. The newly created DB sequences in the ΔDB-leaderless mRNAs are boxed.
The role of the DB in translation has also been observed in the expression of the lysyl-tRNA synthetase gene lysU (Ito et al., 1993, Proc Natl Acad Sci USA90: 302–306). A series of translational lysU–lacZ fusions shows a direct correlation between the complementarity of the DB–anti-DB and β-galactosidase activity. It is also interesting to note that the rpoH-DB sequence is hidden by a stable mRNA secondary structure that becomes disrupted after a temperature upshift (Nagai et al., 1991, Proc Natl Acad Sci USA88: 10515–10519; Morita et al., 1999, J Bacteriol181: 401–410).
We have shown that DB plays a crucial role in the expression of the major cold shock proteins, CspA (Mitta et al., 1997, Mol Microbiol26: 321–335) and CspB (Etchegaray and Inouye, unpublished) at low temperature. Moreover, we have recently provided compelling evidence for the role of the DB as a translational enhancer by inserting an artificial perfectly matching DB sequence (15 matches) after the fifth codon of the lacZ gene (Etchegaray and Inouye, 1999, J Biol Chem274: 10079–10085). Upon induction of the lacZ gene at 37°C, the production of β-galactosidase as well as polysome formation efficiency were highly enhanced. In addition, the β-galactosidase production from the DB-containing lacZ mRNA was further enhanced in S2-depleted ribosomes. These results are in agreement with the conclusions obtained by Shean and Gottesman (1992). Interestingly, our results indicate that the role of the DB in translation is dependent on the presence of the Shine–Dalgarno (SD) sequence, unlike leaderless mRNAs. It has been shown that, in leaderless mRNAs, an extra 5′-UTR sequence without SD has a negative effect on translation (Winzeler and Shapiro, 1997, J Bacteriol179: 3981–3988). This is also consistent with the findings of Shean and Gottesman (1992), in which primer extension inhibition (toeprinting) showed that the formation of preinitiation complex is less efficient in a leader-containing λc1 mRNA than in a leaderless λc1 mRNA.
Earlier (Etchegaray et al., 1998, Mol Microbiol27: 871–874), we noted the DB enrichment in the SELEX system performed by Ringquist et al. (1995, Biochemistry34: 3640–3648). When S1-depleted 30S ribosomes were used as a ligand, DB sequences ranging from 8 to 11 matches were located 1 and 5 nucleotides downstream of the SD sequence, indicating that DB sequences interact directly with 30S ribosomes or more likely with 16S rRNA. These results are quite compelling in support of the role of the DB in translation. Nevertheless, a more direct proof of the DB–anti-DB interaction can be achieved by cross-linking DB-containing mRNAs with purified active 30S ribosomal subunits and by point mutations within the 16S rRNA that alter the complementarity with DB.




