The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons
Abstract
The heat shock transcription factor Hsf1p and the stress-responsive transcription factors Msn2p and Msn4p are activated by heat shock in the yeast Saccharomyces cerevisiae. Their respective contributions to heat shock protein induction have been analysed by comparison of mutants and wild-type strains using [35S]-methionine labelling and two-dimensional gel electrophoresis. Among 52 proteins induced by a shift from 25°C to 38°C, half of them were found to be dependent upon Msn2p and/or Msn4p (including mostly antioxidants and enzymes involved in carbon metabolism), while the other half (including mostly chaperones and associated proteins) were dependent upon Hsf1p. The two sets of proteins overlapped only slightly. Three proteins were induced independently of these transcription factors, suggesting the involvement of other transcription factor(s). The Ras/cAMP/PKA signalling pathway cAMP had a negative effect on the induction of the Msn2p/Msn4p regulon, but did not affect the Hsf1p regulon. Thus, the two types of transcription factor are regulated differently and control two sets of functionally distinct proteins, suggesting two different physiological roles in the heat shock cellular response.
Introduction
When shifted to high temperatures, yeast cells induce the synthesis of heat shock proteins, some of which are chaperones and associated proteins involved in the care of damaged proteins. In Saccharomyces cerevisiae, this response is controlled by the heat shock transcription factor (Hsf1p), which binds the heat shock-responsive element (HSE) (Wu, 1995). The heat shock response also involves the synthesis of metabolic enzymes and antioxidant defence proteins (Boucherie et al., 1996). Several of these proteins are also induced at the diauxic transition by Msn2p and/or Msn4p transcription factors (Msn2/4p) (Boy-Marcotte et al., 1998). The genes encoding these proteins generally contain stress response elements (STRE: CCCCT) in their regulatory region, a sequence that mediates the response to many environmental changes, including heat shock, osmotic stress and the diauxic transition (Marchler et al., 1993). In response to various stresses, the Msn2/4p transcriptional factors translocate to the nucleus and activate transcription of target genes by binding to STREs (Martinez-Pastor et al., 1996; Görner et al., 1998). The Msn2/4p-dependent gene induction through the STREs is sensitive to the cAMP-dependent protein kinase (PKA), which is under the control of the Ras/cAMP signalling pathway (Marchler et al., 1993; Martinez-Pastor et al., 1996; Boy-Marcotte et al., 1998). One role of this pathway is to control the nuclear localization of Msn2/4p negatively (Görner et al., 1998).
We have analysed the contribution of Hsf1p and Msn2/4p to the yeast heat shock responses, using a global approach for the analysis of gene expression based on two-dimensional gel electrophoresis. We compared the heat shock response between wild-type cells and cells lacking either a functional HSF1 gene or MSN2 and MSN4 genes. We observed that, whereas Msn2/4p controls the expression of most of the carbon metabolic enzymes and antioxidant defence proteins of the heat shock response, Hsf1p is very specific for the induction of the chaperones and chaperone-associated heat shock proteins.
Results
The heat shock response stimulon
The heat shock response was obtained by a temperature shift from 25°C to 38°C. The proteins were labelled with [35S]-methionine 15 min after the shift (cf. Experimental procedures ), as it has been reported previously that the induction of heat shock proteins is transient, with a maximum expression at 15–20 min (Smith and Yaffe, 1991a; Martinez-Pastor et al., 1996). This response was analysed in three different wild-type strains, MYY290, W303-1A and OL526, by comparative two-dimensional gel electrophoresis. This two-dimensional gel analysis allows the detection of about 1000 soluble proteins, of which more than 350 have been identified (Boucherie et al., 1996). We detected 52 proteins (Fig. 1) whose synthesis was induced by more than twofold in at least two of the three genetic backgrounds. The results obtained in the OL526 strain are presented in Fig. 2. Forty-one of these proteins are the products of known genes, of which 13 had not been identified previously as heat shock proteins: these are products of ALD2/5, ALD7, DAK1, DNM1, GAL1, TFS1, TKL2, YBR149w, YGL037c, YLR206w, YNL274c, YOR021c and WTM1. The 11 other proteins were not identified as gene products. Numerous proteins were also repressed in these conditions; they involved essentially amino acid biosynthetic enzymes, ribosomal proteins and translation factors (data not shown).
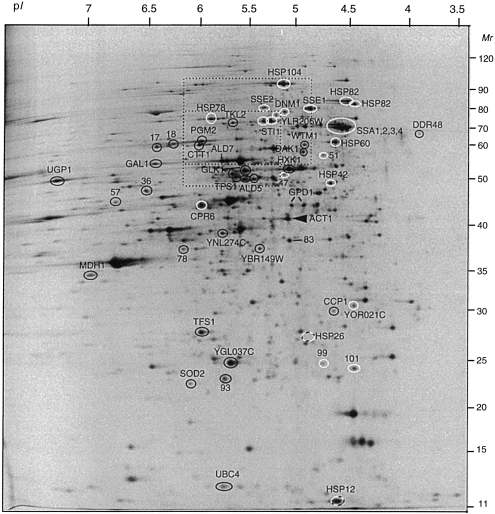
. Two-dimensional gel electrophoresis of total yeast proteins expressed under heat shock conditions. The wild-type strain W303-1A was labelled with [35S]-methionine after transfer from 25°C to 38°C, and two-dimensional gel electrophoresis was performed as described in Experimental procedures. All proteins induced by heat shock and Act1p (used as internal standard) are indicated on the map. The unidentified spots are defined by a number. The protein spots are designated with: a white circle when they are less induced in the MYY385 hsf1-m3 mutant (class 1); a black circle when they are less induced in the Wmsn2msn4 mutant (class 2); a white and black circle when they are less induced in hsf1-m3 mutant and in msn2msn4 mutant; a bar when they are normally induced in the two mutants (class 3). The position of the HSP26 gene product is also indicated, although not detectable as 35S-labelled protein in W303-1A (Fig. 2). The two framed regions are enlarged in Fig. 3.
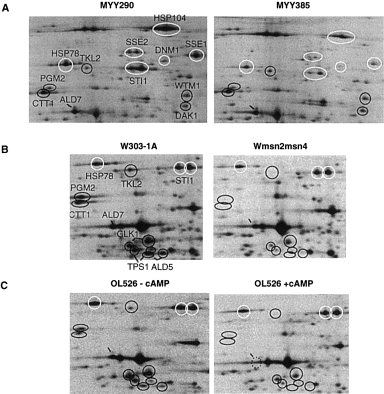
. Two-dimensional gel electrophoresis of wild-type and mutant extracts under heat shock conditions. Enlargement of the two regions framed in Fig. 1: Strains were labelled with [35S]-methionine after transfer from 25°C to 38°C. A. Strains MYY290 and MYY385 (hsf1-m3 ). B. Strains W3031A, Wmsn2msn4. C. OL526 grown without and with 3 mM cAMP. (B) and (C) are enlargements of the same region. Designations of induced proteins are the same as in Fig. 1. The expected position of the Ald7p, which is absent in the map OL526 + cAMP, is indicated with a dotted circle.
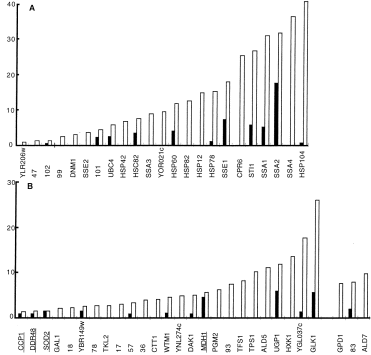
. Induction of heat shock proteins in OL526 strain. [35S]-methionine-labelled extracts of strain OL526 under standard and heat shock conditions were subjected to two-dimensional gel electrophoresis, and the 52 induced proteins were quantified as described in Experimental procedures. The values (in pixel units) were normalized to Act1p spot. Expression level is represented by a black bar under standard conditions and by a white bar under heat shock conditions. Experiments have been repeated twice, and the standard deviation ranged from 20% to 30%. Similar results were obtained in W303-1A and MYY290 strains (data not shown). Underlined gene names correspond to gene products that were not significantly induced in OL 526, but were induced more than twofold in the two other genetic backgrounds. In the case of Hsp26p, quantitative data with [35S]-methionine were obtained only in the MYY290 background. In the other backgrounds, Hsp26 does not contain methionine or cysteine residues, and the induction was qualitatively observed by Coomassie blue coloration. A. Class 1 gene products. B. Class 2 and 3 gene products, as referred to in Fig. 1 and Table 1.
Genes dependent upon Hsf1p
To identify among the 52 induced proteins those that are dependent upon Hsf1p, we used a mutant strain (MYY385) containing the thermosensitive allele hsf1-m3. The heat shock response was compared between the mutant strain MYY385 and the corresponding isogenic wild-type strain MYY290. An effect of the Hsf1p defect was considered as significant when the induction was reduced at least twofold in the mutant compared with the wild type. The heat shock induction of 24 proteins was undetectable or significantly reduced in the hsf1-m3 background (Fig. 1, Fig. 3A for details). Twenty of these proteins are known, most of them being chaperones and associated proteins (Table 1, class 1). In addition to some heat shock proteins described previously as regulated by Hsf1p (Ssa1p, 2, 3 and 4, Hsp104, Hsc82 and Hsp82, Hsp78, Hsp26, Hsp12, Sti1p; Nicolet and Craig, 1989; Smith and Yaffe, 1991a,b; Lindquist and Kim, 1996; Zarzov et al., 1997; Treger et al., 1998), we found new proteins belonging to this regulon: the Hsp70 chaperones Sse1p and Sse2p, the mitochondrial Hsp60 chaperonin (a homologue of Escherichia coli GroE), the ubiquitin-conjugating enzyme Ubc4p, the dynamin homologue Dnm1p and Hsp42. It is noteworthy that the proteins that were the most expressed at high temperature (i.e. Hsp104 and the Ssap family) belong to this class (Fig. 2). The effect of the hsf1-m3 thermosensitive mutation was variable depending on the gene; some proteins were not induced at a detectable level in the mutant, and others were induced but at a lower level than in the wild-type strain (Table 1, class 1).
Genes dependent upon Msn2/4p
Msn2/4p are redundant transcription factors (Martinez-Pastor et al., 1996). Therefore, we analysed the effect of the null deletion of both genes on the heat shock response in the W303-1A genetic context. Twenty-seven proteins of the 52 analysed were undetectable or have at least a twofold reduced expression at 38°C compared with the wild type (Fig. 1 and Fig. 3B for details). Among them, 21 are the products of known genes (Table 1, class 2). Most of them are involved in carbon metabolism (PGM2, UGP1, TPS1, HXK1, GLK1, GAL1, TKL2, YBR149w, DAK1, ALD2/5 and MDH1 ) or in response to oxidative stress (CTT1, SOD2 and CCP1 ). We also found some gene products with diverse functions (DDR48, TFS1 and WTM1/YOR230w ). Msn2/4p also control the induction of Hsp12 and Hsp26. These data are consistent with previous results showing that CTT1, HSP12, HSP26, PGM2 and TPS1 are regulated by Msn2/4p in response to heat shock (Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996; Winderickx et al., 1996; Treger et al., 1998).
The overlap observed between the Hsf1p and the Msn2/4p regulons is very small: only Hsp12 and Hsp26 are controlled by both factors. Most of the heat shock-induced proteins were dependent upon Hsf1p or Msn2/4p. However, the induction of three proteins (including Gpd1p and Ald7p) by heat shock was not affected by the hsf1-m3 or msn2msn4 mutations (Table 1, class 3). Interestingly, the set of repressed proteins was also independent of both factors (data not shown).
The cAMP effect on the heat shock response
We analysed the effect of cAMP on the heat shock response in strain OL526 grown in the presence or absence of cAMP. In this strain, the rca1 mutated allele of the PDE2 gene causes a defect in the high-affinity phosphodiesterase and allows the manipulation of the intracellular cAMP level by adding cAMP to the culture medium (Boy-Marcotte et al., 1996). All the Msn2/4p-dependent proteins had their induction reduced twofold or more by cAMP (Table 1, class 2 and Fig. 3C for details). There was a good correlation between the effect of the msn2msn4 deletion and the cAMP effect for an important fraction of the gene products analysed. This correlation has been described previously during the diauxic transition (Boy-Marcotte et al., 1998). Only a few class 1 proteins (Hsp104p, Ssa3p and Ubc4) and class 3 proteins (Ald7p and Gpd1p) had their induction significantly affected by cAMP (Table 1).
Distribution of the STRE and HSE sites in the promoter regions
HSE and STRE sequences were searched in the 700 bp upstream region of the Hsf1p- and Msn2/4p-dependent genes (Fig. 4). The distribution of the STRE sites in the Msn2/4p-dependent genes is very different from that in the Hsf1p-dependent genes: STRE elements are present at a frequency of 2.7 sites in the upstream region of the genes controlled by Mn2/4p and at a frequency of 1.3 in the Hsf1p-dependent genes. The fraction of genes with more than three STRE sites is 38% for the Msn2/4p-dependent and 10% for the Hsf1p-dependent genes. It is also remarkable that a large fraction of these sites are clustered in less than 60 bp. We also observed that the intensity of Msn2/4p-dependent expression measured by the differential expression at 38°C between the wild-type W303-1A and the mutant msn2msn4 is correlated with the number of STRE sites (Fig. 4B), suggesting an additive contribution of these sites to Msn2/4p-dependent expression. as in the HSP12 promoter (Varela et al., 1995). In the case of ALD2/5, DAK1, GAL1 and SSA3, no STRE motif was found in their promoter regions. However, the post-diauxic shift (PDS) sites present in the ALD2/5, DAK1 and SSA3 promoter regions could be responsive to Msn2/4p (Marchler et al., 1993).
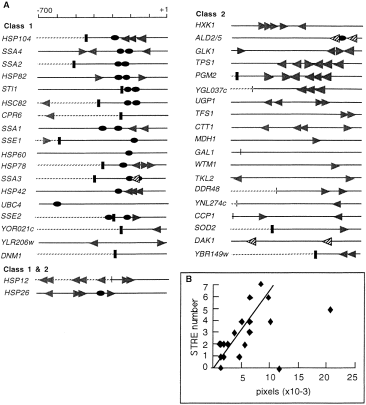
. STRE and HSE consensus sites found in the promoter regions of Msn2/4p- and Hsf1p-dependent genes. A. STRE sites (CCCCT, grey arrows) and HSE sites (NGAANNTTC repeated more than once, black circles) were searched in the promoter region (700 bp upstream of the ATG) of each heat shock-induced gene. For this search, yeast genomic DNA sequences were obtained from the Saccharomyces Genome Database (SGD). PDS sites (TCCCT) present in promoter regions of Msn2/4p-dependent genes with no STRE sites are indicated by striped arrows. Intergenic regions and ORFs are represented, respectively, by a plain line and a dotted line. Vertical bars indicate the border lines between intergenic regions and ORFs (thin bar, ATG; and large bar, terminator). B. The level of Msn2/4p-dependent activation is correlated with the number of STRE motifs in Msn2/4p target genes: the number of STREs in the promoter region of each Msn2/4p target gene was plotted against the difference in protein expression (in pixel units) between the strains W303-1A and Wmsn2msn4 under heat shock conditions.
Fifteen out of 20 of the Hsf1p-dependent genes contain HSE motifs in their promoter region, whereas one gene out of the 19 genes only controlled by Msn2/4p contains an HSE (Fig. 4A). In the case of HSP26, HSC82, SSA1, SSA3 and SSA4, the HSE motifs have been shown to be important for Hsf1p-dependent induction (Boorstein and Craig, 1990a,b; Stone and Craig, 1990; Chen and Pederson, 1993; Erkine et al., 1996).
The presence of HSE motifs in the promoter regions of the Hsf1p-dependent genes, but not in those dependent on Msn2/4p, and the significant excess of the STRE motifs in the Msn2/4p-dependent genes suggest that many of these genes are direct targets for Hsf1p or Msn2/4p. The absence of HSE and STRE motifs in some genes may indicate an indirect role for Hsf1p and Msn2/4p in the control of these genes. However, a direct effect via degenerated sites cannot be ruled out.
Acquired thermotolerance is not reduced in the msn2msn4 mutant
One property of cells treated with a mild heat shock is to acquire thermotolerance to an otherwise lethal heat stress. As Msn2/4p controls a large fraction of the proteins induced by mild heat shock, we analysed whether the thermotolerance is controlled by Msn2/4p. The msn2msn4 mutant was more sensitive to a shift to 50°C than the wild type, as described previously (Martinez-Pastor et al., 1996) (Fig. 5). A 30 min pretreatment at 38°C restored the resistance of both mutant and wild-type strains to a subsequent shift to 50°C. Thus, the Msn2/4p regulon is involved in the capacity of the cell to resist high temperatures but is not required for the thermotolerance acquired after a mild heat shock.
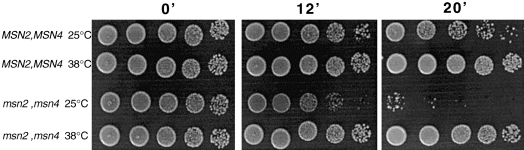
. The acquisition of thermotolerance is not impaired in the msn2msn4 mutant. W303-1A and Wmsn2msn4 strains were grown in YPD at 25°C. When the OD710 reached 1.3, two aliquots of each culture were either transferred to 38°C or maintained at 25°C. After 30 min, samples were transferred to 50°C. After 0, 12 and 20 min, aliquots were placed on ice and serially diluted (fivefold at each step). Five microlitres was spotted onto YPD plates and incubated at 25°C to measure colony-forming units.
Discussion
Although not exhaustive, this genome-wide approach based on two-dimensional gel electrophoresis allowed us to establish that heat shock-induced proteins are mainly controlled by the transcriptional activators Hsf1p and Msn2/4p. The Hsf1p regulon is mostly composed of chaperones, and the Msn2/4p regulon includes enzymes of carbon metabolism, indicating that the two regulons have distinct physiological functions in the heat shock response. Moreover, whereas the Msn2/4p regulon is repressed by the Ras/cAMP/PKA pathway, the Hsf1p regulon is not under this control.
The Msn2/4p factors mainly control a metabolic response of the cell during heat shock
The proteins of the Msn2/4p regulon consist mainly of carbon metabolism and antioxidant enzymes. It is remarkable that a similar (if not identical) set of proteins is also induced by Msn2/4p at the diauxic transition (Boy-Marcotte et al., 1998) and by oxidative stress (M. Toledano and J. Labarre, unpublished results). Their physiological role in the heat shock and other stress responses is unclear. Although speculative, considerations for some of these enzymes are discussed here.
Trehalose metabolism.
Some enzymes of the trehalose metabolism were found to be induced by Msn2/4p in this work (i.e. Pgm2p, Ugp1p and Tps1p). It has also been shown that the expression of TPS2 is Msn2p dependent (Schmitt and McEntee, 1996), as well as that of TPS3 (D. Tadi, unpublished data). Consistently, the trehalose accumulation in response to a mild heat shock is defective in the msn2msn4 mutant (Parrou et al., 1997). The trehalose could contribute to thermoresistance, as this compound increases the thermal stability of proteins in vitro (Hottiger et al., 1994) and strains unable to produce trehalose (tps1, tps2 and msn2msn4 null strains) are sensitive to heat shock (De Virgilio et al., 1994; this work). Trehalose is also important for thermotolerance when pretreatment is performed at temperatures higher than 40°C (Ribeiro et al., 1997). Accordingly, in our experimental conditions (pretreatment at 37°C), thermotolerance is not impaired in the mutant strain. However, it should be noted that the accumulation of trehalose as a result of heat shock is limited, as the neutral trehalose Nth1p is also induced under these conditions (Parrou, 1997), suggesting a recycling of trehalose in response to stress.
Hexokinase regulation.
One function of this futile cycling of trehalose may be to increase the intracellular level of trehalose-6-phosphate, which is a potent inhibitor of the main hexokinase Hxk2p (Blazquez et al., 1993; Teusink et al., 1998). This enzyme could be replaced by the Glk1p and Hxk1p hexokinases, which are induced under heat shock conditions (this work) and are, respectively, not and less inhibited by trehalose-6-phosphate (Blazquez et al., 1993). This replacement may have a role in the control of glycolytic flux and/or alternative pathways (trehalose cycle and pentose phosphate pathway).
Antioxidant defence and NADPH regeneration.
Msn2/4p induce Ctt1p, Sod2p and Ccp1p enzymes that have antioxidant activities. These activities could enhance the cellular defence towards the oxidative stress, which is thought to be associated with heat shock (Davidson et al., 1996). In addition, an increase in the regeneration of NADPH, an important cofactor in the oxidative stress response, can also be postulated from the induction of Tkl2p and could participate in the antioxidant defence, as suggested previously (Godon et al., 1998). The YBR149w gene product and Dak1p could also contribute to this NADPH regeneration (Godon et al., 1998), as well as the aldehyde dehydrogenases Ald2/5p and Ald7p.
All these metabolic effects should act in concert with the transient arrest of the cell cycle in G1 observed in response to various stresses (Rowley et al., 1993; Lee et al., 1996). This cell cycle arrest could be caused by Msn2/4p, which may antagonize the PKA-dependent growth by stimulating the expression of genes, such as YAK1, that inhibit growth (Smith et al., 1998). The role of the Msn2/4p regulon is not essential but is very general, as it is induced under a wide range of stress conditions and environmental changes (Martinez-Pastor et al., 1996). By a transient modification of the cellular metabolism, the Msn2/4p regulon could facilitate proper setting of a new protein machinery and the adaptation of the cell to the new conditions.
On the contrary, the Hsf1p regulon has an essential and specific role in the heat shock cellular response (Smith and Yaffe, 1991a; Lindquist and Kim, 1996). Indeed, it is mostly composed of chaperones and associated proteins that must be involved in the care and management of proteins denatured by the heat shock. The UBC4 gene product also belongs to this regulon. This ubiquitin-conjugating enzyme contributes to a major portion of the ubiquitin-dependent protein degradation in stressed cells (Seufert and Jentsch, 1990). Two gene products of unknown function (YOR021c and YLR206w ) were also found to be induced by Hsf1p and could also have chaperone-like functions.
The metabolic response to heat shock is under the control of the Ras/cAMP pathway
The Ras/cAMP pathway controls STRE motifs and acts on Msn2/4p, at least on their nuclear localization induced by heat shock (Marchler et al., 1993; Görner et al., 1998). Accordingly, we found that all the genes induced by Msn2/4p under heat shock conditions were repressed by an excess of cAMP. The same correlation was also observed during the diauxic transition (Boy-Marcotte et al., 1998). A control of the cAMP intracellular level must be part of the cellular response to heat shock and could be achieved through Ssa1p, which is complexed with the guanine nucleotide exchange factor Cdc25p, the main activator of cAMP synthesis (Geymonat et al., 1998). In addition to the Msn2/4p genes, a few others were significantly repressed by cAMP. It is possible that the cAMP pathway exerts its effect through other regulators in addition to Msn2/4p, as observed at the diauxic transition (Boy-Marcotte et al., 1998). Most of the Hsf1p-dependent genes are insensitive to cAMP, consistent with the absence of control by the Ras/cAMP pathway over HSE heat shock-induced transcription (Marchler et al., 1993; Engelberg et al., 1994).
Hsf1p and Msn2/4p are the two main transcriptional activators in heat shock response
Most of the heat shock proteins belong either to the Hsf1p regulon or to the Msn2/4p regulon, indicating the prominent role of these two factors in the heat shock response. The expression of only three proteins is not impaired in the absence of Hsf1p or Msn2/4p. It is possible that some other transcriptional activators are involved in the heat shock response (Kamada et al., 1995). However, a residual activity of Hsf1p (Lindquist and Kim, 1996) and a different sensitivity of the different Hsf1p-regulated genes to the particular defect of the hsf1-m3 thermosensitive mutation cannot be excluded. This hypothesis is supported by the phenotype of the hsf1-82 thermosensitive mutant, which specifically reduces the transcription of Hsp82 and Hsc82p (Zarzov et al., 1997).
The overlap between the two heat shock regulons is limited to Hsp12 and Hsp26. Ssa3p and Hsp104 could also be added to this group, as a small effect of the msn2msn4 mutation is correlated with a significant cAMP effect. It is also possible that other genes are redundantly induced by Hsf1p and Msn2/4p, but that compensatory effects lead to a normal heat shock induction in one or both mutants. Such an effect has been described for HSP104 and HSP78, whose induction is only impaired in the triple mutant hsf1-m3,msn2,msn4 (Treger et al., 1998). Therefore, the number of genes controlled by both factors is probably underestimated in our analysis. Nevertheless, most of the Hsf1p-independent genes described in this work do not contain an HSE site and, thus, it is very improbable that they are actually controlled by Hsf1p.
Two-dimensional gel and yeast genome microarray analysis
Using DNA microarrays to analyse the gene expression of 2479 open reading frames (ORFs) of S. cerevisiae, 17 genes were found to be induced by heat shock (Lashkari et al., 1997). Some of them belong to the Hsf1p regulon defined in this work. On the contrary, none of the Msn2/4p-dependent genes was found. This difference may result from the difference in kinetics of the two procedures: whereas the analysis of the mRNA by hybridization of the DNA microarrays was performed 1 h after the heat shock, we analysed the heat shock protein induction 15 min after the temperature shift. After 1 h, it is possible that a large part of the transient response is not detectable any more, as observed for the heat shock-induced genes SSA1 and CTT1 (Smith and Yaffe, 1991a; Martinez-Pastor et al., 1996). Nevertheless, a difference resulting from unknown post-transcriptional regulation cannot be excluded, as the DNA microarrays analyse mRNA levels, whereas two-dimensional gel analysis measures protein synthesis rate. This DNA microarray analysis allowed the detection of heat shock-responsive genes whose products are not detectable on two-dimensional gels as membrane proteins (for example Hsp30p), basic proteins (i.e. most of the ribosomal proteins) or proteins expressed at a low level. An exhaustive analysis with the yeast genome microarrays should complete the description of the heat shock regulons in yeast.
Experimental procedures
Yeast strains and growth conditions
OL526 (α rca1, leu2, ura3, his3, trp1, this work). W303-1A (a, ade2, his3, leu2, trp1, ura3 ) and Wmsn2-msn4 (a, ade2, his3, leu2, trp,1 ura,3 msn2-Δ3::HIS3, msn4–1::TRP1 ) are isogenic strains (Estruch and Carlson, 1993). MYY290 (a, leu2, his3, ura3, phoc, phoE ) and MYY385 (a leu2, his3, ura3, phoC, phoE, hsf1-m3 ) are isogenic strains (Smith and Yaffe, 1991a). Strains were grown in the glucose medium YNBS described previously (Boy-Marcotte et al., 1996), supplemented with the required amino acids. Heat shock experiments were performed as follows: cells growing exponentially at 25°C (OD 710 of 0.3 in a Jenway 6061 colorimeter) were shifted to 38°C in a prewarmed flask.
Protein synthesis analysis
For protein labelling, 5 ml of log-phase culture (OD710 of 0.3) was labelled for 15 min with 5.5 × 106 mBq of [35S]-methionine (> 3.7 × 1013 mBq mmol−1). In the case of heat shock, [35S]-methionine was added 15 min after the transfer to 38°C. The control labelling was performed at 25°C. The temperature of 38°C was chosen as it was reported that, above 40°C, the induction of some STRE-regulated genes was abolished (Parrou et al., 1997). Preparation of cell extracts and two-dimensional gel electrophoresis were performed as described previously (Maillet et al., 1996). Quantitative analysis of the synthesis of the polypeptides separated on the two-dimensional gel was performed as follows: after drying, gels were exposed to phosphor-screens scanned in a Molecular Dynamics PhosphorImager. Image files were then exported into the bioimage software for image analysis and spot quantification. The spot intensities were obtained in pixel units and normalized to the value of Act1p spot. For proteins that are present as several distinct polypeptides of different pI values, the spot intensities were added.
Acknowledgements
We thank Sylvie Kieffer and Jérôme Garin for mass spectrometry experiments, Michel Toledano and Carl Mann for helpful discussions and critical reading of the manuscript. This work was supported by grants from the Association pour la Recherche sur le Cancer (ARC), the Ligue Nationale Française contre le Cancer and the Groupement de Recherche et d'Etudes sur les Génomes (GREG).