A novel mutation in the KH domain of polynucleotide phosphorylase affects autoregulation and mRNA decay in Escherichia coli
Abstract
Polynucleotide phosphorylase (PNPase) is a key 3′–5′ exonuclease for mRNA decay in bacteria. Here, we report the isolation of a novel mutant of Escherichia coli PNPase that affects autogenous control and mRNA decay. We show that the inactivation of PNPase by a transposon insertion increases the half-life of galactokinase mRNA encoded by a plasmid. When the bacteriophage lambda int gene retroregulator (sib/tI ) is placed between pgal and galK, it severely diminishes galactokinase expression because of transcription termination. The expression of galK from this construct is increased by a single base mutation, sib1, which causes a partial readthrough of transcription at tI. We have used this plasmid system with sib1 to select E. coli mutants that depress galK expression. Genetic and molecular analysis of one such mutant revealed that it contains a mutation in the pnp gene, which encodes the PNPase catalytic subunit α. The mutation responsible (pnp-71 ) has substituted a highly conserved glycine residue in the KH domain of PNPase with aspartate. We show that this G-570D substitution causes a higher accumulation of the α-subunit as a result of defective autoregulation, thereby increasing the PNPase activity in the cell. The purified mutant α-subunit shows the same electrophoretic mobility in denaturing gels as the wild-type subunit, as expected. However, the mutant protein present in crude extracts displays an altered electrophoretic mobility in non-denaturing gels that is indicative of a novel enzyme complex. We present a model for how the pnp-71 mutation might affect autoregulation and mRNA decay based on the postulated role of the KH domain in RNA–protein and protein–protein interactions.
Introduction
The processing and degradation of mRNAs by the concerted action of endoribonucleases and exoribonucleases are important events in the control of gene expression in all living cells (Belasco and Higgins, 1988). In Escherichia coli, the components of this mRNA processing/degradation machinery include the endoribonucleases ribonuclease III and ribonuclease E, and the exoribonucleases polynucleotide phosphorylase (PNPase) and ribonuclease II (Donovan and Kushner, 1986; Guarneros and Portier, 1990; Court, 1993; Cohen and McDowall, 1997). Stable secondary structures in many mRNAs block the processive action of exonucleases, thereby protecting the upstream sequences from degradation and increasing their stability in vivo (Higgins et al., 1993; Cisneros et al., 1996). A cellular factor called the exoribonuclease-impeding factor (EIF) has been proposed to bind the 3′ hairpin of some mRNAs and increase the time that exoribonucleases stall at the stem–loop structures (Causton et al., 1994). The degradation of such messages is initiated by a rate-determining endonucleolytic cleavage, either within the 3′ secondary structure or upstream from it, consequently generating products that become susceptible to rapid degradation by exonucleases.
The retroregulation of the bacteriophage lambda int gene is a remarkable example of a post-transcriptional processing–degradation mechanism promoted by a 3′ hairpin (Montañez et al., 1986; Guarneros, 1988). The int gene can be transcribed from either of two promoters, pI and pL. However, the pL transcript, which is subject to transcription antitermination control by the phage N protein, does not express the Int protein during infection of E. coli by phage lambda. This retroregulation involves the RNase III-mediated cleavage of the pL-int mRNA at the sib region just downstream from the int coding region and subsequent exonucleolytic degradation of the int message (Schmeissner et al., 1984a; Montañez et al., 1986). In contrast, the pI transcript activated by the lysogenic activator protein CII terminates at the tI terminator (a stem–loop structure that overlaps with sib ). This pI-int mRNA is not processed by RNase III and, hence, remains stable and expresses the Int protein efficiently (Miller et al., 1981; Schmeissner et al., 1984b). The two hairpins formed at sib/tI have profoundly different effects on the expression of genes placed upstream from them. When cloned as a module, the sib/tI sequence can protect the upstream RNA from exonucleolytic attack. The introduction of destabilizing point mutations, such as sib1, makes the terminated RNA susceptible to degradation by PNPase (Cisneros et al., 1996), while allowing the expression of a downstream gene resulting from defective transcription termination (Montañez et al., 1986).
A conserved enzyme in the bacterial kingdom, PNPase plays a critical role in RNA metabolism (Littauer and Soreq, 1982). In vitro, purified PNPase catalyses the processive 3′ to 5′ phosphorolytic degradation of RNAs, releasing 5′ nucleoside diphosphates. A unique property of the enzyme is its capacity to polymerize ribonucleoside diphosphates into long random polymers without a template requirement. PNPase also catalyses a phosphate-exchange reaction between inorganic phosphate and the β-phosphate of nucleoside diphosphates (Grunberg-Manago, 1963). The catalytic component of PNPase is a homotrimer of the α-subunit made up of 711 amino acids. It forms a multiprotein complex called the ‘degradosome’ that contains RNase E, EIF, polyphosphate kinase, DnaK, enolase and the RhlB helicase (Carpousis et al., 1994; Py et al., 1994; 1996; Miczak et al., 1996; Blum et al., 1997). This degradosome complex possesses an ATP-dependent activity that helps PNPase to degrade structured RNAs. PNPase can also associate with RNase III (Swatantra et al., 1982). Although mRNA degradation in E. coli is performed predominantly by RNase II (Deutscher and Racher-Reuven, 1991), PNPase does participate in the degradation of some specific mRNAs (Higgins et al., 1993), including the rpsO mRNA (Braun et al., 1995) and the antisense RNA that represses the replication of ColE1-type plasmids (Xu and Cohen, 1995). PNPase has also been implicated in competence development in Bacillus subtilis (Luttinger et al., 1996).
The expression of the PNPase catalytic subunit gene (pnp ) in E. coli is autoregulated post-transcriptionally (Robert-Le Meur and Portier, 1992). This autoregulation requires an initial cleavage of the pnp messenger by RNase III (Portier et al., 1987), and it may involve the direct recognition by PNPase of an exposed determinant at the 5′ end of the cleaved message (see Robert-Le Meur and Portier, 1994). The structure of PNPase has not been solved yet. However, the PNPase catalytic subunit contains an S1 RNA-binding domain made of a five-stranded anti-parallel β-barrel (Bycroft et al., 1997). The region immediately upstream of the S1 domain exhibits a high homology to the KH domain that has been implicated in RNA binding (Siomi et al., 1994) as well as protein–protein interaction (Zhang et al., 1995). The three-dimensional structure of the KH domain present in the human protein vigilin has been solved by nuclear magnetic resonance (NMR). It is composed of a stable βααββα fold, in which the two adjacent α helices are connected by a flexible loop that contains a highly conserved Gly-Lys-X-Gly motif (Musco et al., 1996). The carboxy-terminus of PNPase, which includes both the KH and the S1 domains, is obviously essential for catalytic activity, as shown by the isolation of a truncated mutant resulting from the insertion of the transposon Tn5 just upstream from the KH domain. This pnp ::Tn5 allele encodes an unstable polypeptide that possesses no enzymatic activity (Portier, 1980) and, remarkably, no apparent autoregulation capacity (Robert-Le Meur and Portier, 1992).
To explore the role of PNPase in post-transcriptional gene control in E. coli and to investigate its structure–function, we have devised a genetic selection scheme for the isolation of PNPase mutants. In this paper, we describe a novel PNPase mutant that has resulted from a substitution of the most highly conserved glycine residue within the KH domain's Gly-Lys-X-Gly motif. The resultant PNPase mutant increases the decay of galK mRNA dependent on lambda sib1. The mutant is also defective in autoregulation and, hence, it accumulates the catalytic subunit at a greater level in the cell. We present evidence that the mutant enzyme has a distinctive physical property indicative of altered protein–protein interaction that may explain the various properties of the mutant.
Results
The galactokinase mRNA is stabilized by inactivation of PNPase
To explore the role of PNPase in mRNA decay, we have initially investigated how the inactivation of PNPase affects the stability of a reporter gene, galK, encoded in a plasmid vector pKG1800 (Fig. 1A). In this vector, the galactokinase gene is placed under the transcriptional control of its cognate promoter, pgal, which causes a high level of expression of galactokinase. Total RNA isolated from wild-type cells and its pnp ::Tn5 mutant derivative, each carrying pKG1800, was analysed by Northern blot using an anti-galK 5′ RNA probe. 2Figure 2A shows that pKG1800 produces abundant transcripts of varying size, all carrying the galK coding region. The longest transcript of ≈1.8 kb represents the full-length RNA from the pgal promoter to the end of galK. The smaller transcripts most probably represent degradation products resulting from 3′ exonucleolytic degradation, endonucleolytic processing or a combination of both. The inactivation of PNPase by the Tn5 insertion increased the half-life of both the full-length message and the degradation products from less than 1 min to ≈2.2 min (Fig. 2A, compare lanes pnp and pnp ::Tn5 ). Thus, PNPase can influence galK mRNA degradation, at least when expressed from the plasmid vector.

. A. The galactokinase expression vectors. The pKG1800 vector expresses the galactokinase gene (galK ) from the pgal promoter (long arrow). In the pUS6 vector, the galK expression is avoided efficiently by transcription termination (short arrow) at the tI terminator located in the 0.23 kb sib region of lambda phage (co-ordinates 27724–27479). In the pMS1 vector, the sib1 mutation of tI causes a transcriptional readthrough, permitting low levels of galK expression (dashed arrow). Hairpins represent the sib(tI ) or the sib1(tI ) region, and open bars the galK cistron. Indented bars represent riboprobes used in the Northern blot analysis. pgal represents the galactokinase operon promoter. B. Galactokinase activity in different pnp alleles. Assays were performed as described in Experimental procedures. The results are the average of four independent determinations, except for Zgi:Tn10 pnp-71 with only two assays. PNPase alleles were in S165 backgrounds transformed with pKG1800 (Δsib ); pUS6 tI(sib ) and pMS1 tI(sib1 ) plasmids (see Experimental procedures ).
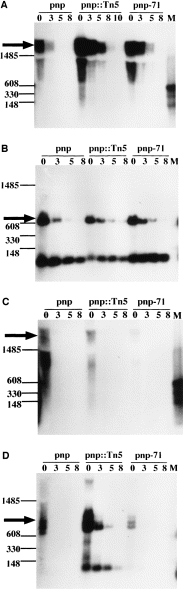
. pKG1800, pUS6 and pMS1 Northern blots. Total RNA was isolated from N99 wild-type, pnp ::Tn5 and pnp-71 bacteria carrying the indicated plasmids. The RNA was blotted and hybridized against the indicated riboprobe as described in Experimental procedures (Fig. 1A). The numbers at the top of the lanes correspond to time in min after inhibition of transcription with rifampicin. Markers are RNA molecules of indicated sizes. A. Northern blot of pKG1800 with anti-galK 5′ riboprobe. Solid arrow shows pgal-galK transcripts of ≈1800 bases long. B. Northern blot of pUS6 with anti-sib riboprobe. Solid arrow shows pgal-tI(sib ) transcription termination transcripts of ≈630 bases long. RNA transcripts of less than 150 bases are most likely products of processing at sib. C. Northern blot of pMS1 with anti-galK 5′ riboprobe. Solid arrow shows pgal-sib1-galK transcripts of ≈2000 bases long. RNA detected below these transcripts are products of degradation. D. Northern blot of pMS1 with anti-sib riboprobe. Solid arrow shows pgal-sib1 transcripts larger or equal to 630 bases long. High-molecular-weight RNAs detected above these are pgal-sib1-galK readthroughs. Low-molecular-weight RNAs detected below the pgal-sib1 transcripts are most likely products of processing degradation at sib1, more clearly visible in a pnp ::Tn5 background.
When the λsib region is cloned between pgal and galK (the pUS6 construct), the tI terminator acts efficiently to abolish galK transcription almost completely (Fig. 2B, pnp lanes), in agreement with previous reports (Schmeissner et al., 1984b; Montañez et al., 1986; Cisneros et al., 1996). The inactivation of PNPase increased the stability of the pgal-tI terminated transcripts, as shown by the Northern blot using an anti-sib probe (Fig. 2B); the half-life is increased from 2.4 min in the pnp background to 3.7 min in the pnp ::Tn5 background.
The sib1 mutation reduces transcription termination and galK mRNA stability
The sib1 mutation was originally isolated based on its resistance to processing by RNase III. This single base mutation disrupts the secondary structure of tI, thus decreasing transcription termination and allowing a moderate expression of galK in the pMS1 vector (see 1Fig. 1B; Montañez et al., 1986; Cisneros et al., 1996). The anti-galK blot of total RNA showed that sib1 decreases galK mRNA half-life in both wild-type and pnp ::Tn5 backgrounds (Fig. 2C). The longest transcript, ≈2.0 kb in size, represents the full-length pgal-sib1-galK RNA. Owing to the extreme lability of the pgal-sib1-galK message, however, we could not measure the half-life accurately using the anti-galK 5′ probe, even in the pnp ::Tn5 background.
Nevertheless, the steady-state level of the galK message from pMS1 was about 1.7-fold more in the pnp ::Tn5 mutant than in the wild-type strain (Fig. 3B). Moreover, when total RNA from the same strains was probed with anti-sib, both the pgal-sib1-galK and the pgal-sib1 transcripts were found to be more stable in the pnp ::Tn5 mutant (Fig. 2D). The steady-state level of the pgal-sib1 transcripts was about fivefold more in the pnp ::Tn5 strain than in the pnp parent (Fig. 3A). Together, these results indicate (i) that the sib1-tI structure decreases the stability of the downstream galK sequence; and (b) that PNPase promotes galK mRNA decay in this plasmid system.
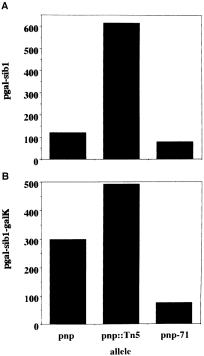
. RNA detection from pMS1. OD data were computed from autoradiographic films and membranes used in the actual Northern blots. The amounts of RNA detected by riboprobes were normalized to the amount of total RNA present in the nitrocellulose membranes at time zero of transcription inhibition by rifampicin. The galK mRNA was measured as indicated in Experimental procedures. A. pgal-sib RNA detected using anti-sib riboprobe. B. pgal-sib1-galK mRNA detected using anti-galK 5′ riboprobe.
Isolation of a polynucleotide phosphorylase mutant that decreases galactokinase expression
We decided next to use the pMS1 plasmid system to select E. coli mutants that depress galK expression. Potentially, some of these mutants could have acquired the ability to degrade galK mRNA rapidly, thus allowing the identification of components that might normally make up the mRNA degradation machinery in E. coli. The forward selection of E. coli mutants defective in galK expression is possible, as the expression of galK alone in the absence of the other gal enzymes is known to cause galactose toxicity as a result of the accumulation of galactose-1-phosphate (Shapiro and Adhya, 1969). As expected, the E. coli strain S165, which contains a deletion of the gal operon, becomes galactose sensitive when transformed by pMS1. However, the moderate expression of galactokinase from pMS1 does not inhibit E. coli growth completely; instead, it causes the production of flat rough colonies on LB-galactose plates. In contrast, a complete inhibition of growth is produced by pKG1800 containing no terminator, while round smooth colonies are obtained with pUS6 containing the wild-type sib/tI sequence between pgal and galK. Thus, there is a direct relationship between the level of galactokinase expression and the colony growth and morphology.
To select E. coli mutants that are depressed in galK expression, we subjected the strain S165 to mutagenesis by nitrosoguanidine. We then transformed the mutant bank with pMS1 and selected ampicillin-resistant transformants on LB-Gal-Amp plates. Upon sufficient growth of colonies, we looked for rare galactose-tolerant mutants, which formed round smooth colonies in the background of flat rough colonies. One such mutant is SGM71. In subsequent experiments, we have shown that this mutant expresses only 6 units of galactokinase from pMS1 compared with 29 units of galactokinase expressed by the same plasmid in S165.
The reduced expression of galK in the SGM71 mutant could result from a variety of reasons, including a defect in transcription initiation, an enhanced transcription termination, a decreased translation or hyperdegradation of galK mRNA. Indicative of the last possibility was the result of a Northern blot analysis, which suggested that the decay of mRNAs containing sib1 is in fact altered in the SGM71 mutant. This preliminary finding prompted us to analyse the PNPase activity in SGM71 mutant. For this purpose, crude extracts prepared from wild-type and mutant cells were resolved by electrophoresis in non-denaturing polyacrylamide gels, and the ADP polymerization activity was assayed according to standard procedures described previously (see Fig. 4A). Indeed, there was both a qualitative and a quantitative change in PNPase activity in the mutant. First, the PNPase band in the mutant migrated more slowly than the wild type. Secondly, the amount of poly(A) polymerization product that stains and identifies the PNPase band in these gels was considerably greater in the mutant than in the wild type. These results demonstrated that a mutation (or a combination of mutations) in the SGM71 mutant has two striking effects on PNPase: (i) an increase in PNPase activity, which could result from the elevated expression of PNPase α-subunit, the activation of its intrinsic catalytic activity or the inactivation of an inhibitor; (ii) an abnormal electrophoretic mobility of PNPase, which could result from either self-aggregation or interaction with another protein component.
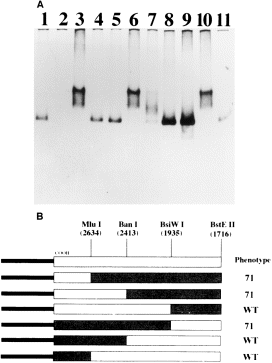
. A. PNPase polymerization activity of E. coli crude extracts. The ADP polymerization activity in N99 crude extracts, with different combinations of pnp alleles in chromosome and vectors, was analysed as explained in Experimental procedures. Lane 1, pnp ; lane 2, pnp ::Tn5 ; lane 3, pnp-71 ; lane 4, pnp ::Tn5/pCAS11; lane 5, pnp/pCAS11; lane 6, pnp ::Tn5/pCAS21; lane 7, pnp/pCAS21; lane 8, pnp ::Tn5/pCJ11; lane 9, pnp/pCJ11; lane 10, pnp ::Tn5/pnp:pnp-71 fusion in pCAS11; lane 11, pnp ::Tn5/pnp-71 :pnp fusion in pCAS11(see text). B. Localization of the pnp-71 mutation by gene fusion. Rectangles represent pnp gene (white) and pnp-71 gene (grey). The COOH indicates the carboxy-end, and the black solid bars represent the pCAS11 vector DNA. Numbers in parentheses are the co-ordinates in the pnp sequence. The phenotype of the different clones is indicated to the right. The 71 phenotype indicates retardation of the ADP polymerization complexes in native gels (see Experimental procedures ).
The pleiotropic biochemical phenotype of the SGM71 mutant is caused by a mutation in the PNPase α-subunit
Next, to determine whether one or more of the biochemical attributes of PNPase observed in the SGM71 mutant is caused by a mutation in the PNPase α-subunit itself, we performed phage P1 transduction experiments. A Tn10 (TetR) transposon linked to the pnp-nusA region (donor strain: Zgi:Tn10-pnp-nusA1) was used to transduce the SGM71 strain first, producing TetRnusA+ transductants that retained all of the PNPase71 phenotype (Fig. 4A, lane 3). Subsequently, one of these SGM71 Zgi::Tn10 transductants was used as the donor to transduce the pnp gene into the S165 parent as well as a set of other strains. In fact, a significant fraction of the TetR transductants in each case now displayed the SGM71 biochemical phenotype, establishing firmly that the responsible mutation is linked to the pnp locus. The newly constructed S165 pnp-71 strain expressed 11 units of galatokinase, compared with 29 units produced by the pnp parent (Fig. 1B). This showed clearly that the altered biochemical property of PNPase and the mRNA decay phenotype are both caused by the same pnp-linked mutation.
To determine whether the responsible mutation in the SGM71 mutation lies in the pnp gene or not, we next isolated the pnp gene from the SGM71 mutant chromosome by polymerase chain reaction (PCR) amplification and cloned it into a low-copy vector. For comparison, we have also PCR amplified and cloned the wild-type pnp gene from the plasmid pYN115 (see Experimental procedures ). The primers for the PCR were chosen to generate a DNA fragment that includes the region from the t1 terminator of the upstream rpsO gene to the t2 terminator downstream from the pnp gene (Regnier et al., 1987). The PCR products were cloned in the plasmid vector pGB2, yielding pCAS11 (pnp) and pCAS21 (pnp-71 ). Subsequent native gel electrophoresis of extracts made from transformants established that the responsible mutation in SGM71 maps within the cloned DNA, as each of the two distinct biochemical properties of the PNPase71 mutant (the higher level of PNPase activity and the altered mobility) was retained by the plasmid-borne copy of the pnp-71 gene. N99 pnp ::Tn5, a strain with no PNPase activity (Fig. 4A, lane 2), was transformed with pCAS11 and pCAS21 plasmids, and crude extracts were prepared and analysed for ADP polymerization activity. The pCAS11 plasmid complemented these cells to the PNPase wild-type phenotype as expected (Fig. 4A, lane 4), while the pCAS21 plasmid complemented the same strain to the SGM71 phenotype (Fig. 4A, lane 6). Curiously, transforming the pnp+ strain with pCAS21 showed that the co-expression of the wild-type and the mutant PNPase subunits in the same cell can form PNPase enzymes with an intermediate mobility in the non-denaturing gels (Fig. 4A, lane 7).
The pnp-71 mutation substitutes a conserved glycine in the KH domain of PNPaseα-subunit
An initial sequencing of the 5′ end of the cloned inserts in pCAS11 and pCAS21 showed that the sites critical for pnp transcription initiation and the processing of PNPase message were not affected in the pnp-71 mutant gene. We next decided to shuffle the various parts of the pnp-71 gene with wild type to localize the responsible mutation within the large pnp coding region conveniently. Digestion of the pCAS11 and pCAS21 vectors by the BstEII restriction enzyme produced two fragments. The larger of the two contained the 1312 bp of the 5′ end, while the smaller fragment contained the 1150 bp of the 3′ end. Upon interchange, the 5′-pnp-71::pnp-3′ fusion complemented the pnp ::Tn5 strain to PNPase wild type (Fig. 4A, lane 11), while the 5′-pnp ::pnp-71-3′ fusion complemented it to the PNPase71 phenotype (Fig. 4A, lane 10). Thus, the pnp-71 mutation is located between the BstEII sites within the pnp open reading frame (ORF) and the 3′ end. The same approach was continued to narrow the gene fragment in which the mutation was located, using the BsiWI, BanI and MluI sites. The mutation was finally mapped between the BanI and BsiWI sites within a 478 bp fragment (Fig. 4B). This fragment was then purified from both wild-type and mutant plasmids and cloned into pUC19. DNA sequencing showed that the only difference between the wild-type and the mutant sequences was a G to A transition at position 2372 of the pnp sequence (Regnier et al., 1987). This mutation changes the GGT codon for Gly-570 to the GAT codon encoding Asp. The Gly-570 is the most highly conserved residue of the KH domain, present in all reported PNPases. Conceivably, the substitution of this glycine by aspartate alters the structure of the KH domain and affects PNPase function in a significant way.
The pnp-71 mutation decreases pgal-sib1-galK mRNA expression
To investigate how galactokinase expression from pMS1 is affected in the PNPase71 mutant, we examined the status of galK mRNA by Northern blot. The blot of total RNA using the anti-galK 5′ probe showed a fourfold decrease in the steady-state level of the pgal-sib1-galK mRNA in the pnp-71 mutant compared with the wild-type strain (Fig. 3B). In contrast, the parallel blot using the anti-sib probe showed only a 1.5-fold reduction in the pgal-tI(sib1 ) mRNA steady-state level in the same mutant. This result suggests that there is neither a significant decrease in transcription initiation at pgal, nor a significant increase in transcription termination at the tI (sib1 ) terminator.
As mentioned before, PNPase participates in the decay of RNA I, the antisense repressor that normally downregulates the copy number of ColE1-type plasmids (Lin-Chao and Cohen, 1991; Sarkar, 1997). As pMS1 is a ColE1-derived plasmid, the reduced expression of galK mRNA by pMS1 could be attributed to a decrease in plasmid copy number caused by the pnp-71 mutation. However, a quantitative measurement of the relative plasmid content showed that, on the contrary, the pnp-71 mutation increased the copy number of pMS1 as well as pBR322 about twofold when compared with the wild-type strain (data not shown). This is consistent with the observation reported below that the PNPase activity is elevated in the pnp-71 mutant, which should cause a decrease in RNA I and, consequently, an increase in plasmid replication. Incidentally, the effect of the pnp-71 mutation on galK expression is dependent on the presence of the sib1 sequence upstream of galK ; the anti-galK blot showed that the level of galK mRNA expressed from pKG1800 is not reduced by pnp-71. In fact, there is an increase in the half-life of the full-length message, and the degradation products show an increase in half-life as well (from a value of less than 1 min to ≈2.1 min; compare line pnp with line pnp-71 in 2Fig. 2A).
The G-570D substitution does not increase the intrinsic phosphate exchange activity of purified PNPase enzyme
To investigate whether the increased ADP polymerization displayed by PNPase71 mutant is caused by an enhanced catalytic property or not, we compared the phosphate exchange activity of PNPase purified from the wild-type cell and the mutant. The phosphate exchange reaction is an excellent indicator of the intrinsic proficiency of the polymerization and the phosphorolytic activities of PNPase. This results from the fact that both reactions are involved in the mechanism of replacement of the β-phosphate of nucleoside diphosphate in the presence of inorganic phosphate. The wild-type PNPase and PNPase71 enzymes were purified to homogeneity (Fig. 5A), and the exchange reaction specific activity was measured as described in the Experimental procedures. While the wild-type enzyme showed a specific activity of 3438, the G-570D mutant showed a specific activity of 2276. In other words, the mutant enzyme was about 66% as active as the wild type. This result is clearly inconsistent with the notion that an approximate fourfold increase in PNPase activity in the crude extract of the pnp-71 mutant is a consequence of an increase in the intrinsic catalytic activity. Also inconsistent with this conjecture is our observation that both the ADP polymerization activity and the poly(A) phosphorolysis activity of the purified mutant enzyme (as described in Experimental procedures ) show no significant differences compared with the wild-type enzyme (data not shown). Interestingly, when the ADP polymerization activity of the purified enzymes was analysed by electrophoresis in non-denaturing polyacrylamide gels, the PNPase71 mutant enzyme exhibited the same reduced migration, as was observed in crude extracts (data not shown). This suggests that the mutant enzyme has an altered aggregation property compared with the wild-type enzyme, a possibility that remains to be investigated in future work.
The G-570D substitution causes an increased accumulation of the PNPase α-subunit
We next raised anti-PNPase α-subunit antiserum and used it to quantify the PNPase α-subunit in crude extracts by both slot-blot and Western blot analyses. As shown in 5Fig. 5B, the pnp-71 mutant contains a significantly greater amount of the α-subunit compared with the wild-type cell. Note that no α-subunit of 77 kDa size was detected in the extract prepared from GF5322, the strain that contains the pnp ::Tn5 allele; instead, a shorter product was detected that corresponds roughly with the expected size of the truncated α-subunit produced by Tn5 insertion. In order to quantify the α-subunit content accurately, varying amounts of pure wild-type α-subunit were subjected to slot-blot analysis generating a linear dose response. Under this condition of measurement, signals produced by extracts from wild type and the mutant were compared with that displayed by known amounts of pure α-subunit. From these results, we estimate that there is at least sevenfold more α-subunit in extracts coming from the mutant than that from the wild type. Thus, the G-570D substitution has caused an increased accumulation of the PNPase α-subunit, which must result from either increased synthesis or reduced degradation.
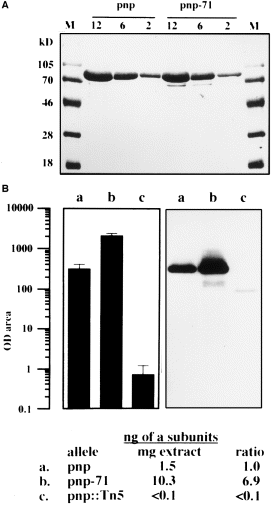
. A. PNPase and PNPase71 SDS–PAGE. Appropriate dilutions of each enzyme were loaded and electrophoresed in a 10.0% SDS–polyacrylamide gel. Proteins were stained with Coomassie blue (see Experimental procedures ). Numbers indicate μg of protein loaded per lane. B. PNPase immunodetection in crude extracts. PNPase α-subunits were detected in a Western blot of 2 μg lane−1 of E. coli extracts, as described in Experimental procedures. Lanes (a, pnp; b, pnp-71; and c, pnp ::Tn5 ) correspond to crude extracts of GF5321, MU950 and GF5322 bacteria. OD area μg−1 data of chemiluminescent records are the average of six independent determinations. In this assay, 1.0 OD area is equal to ≈5 pg of pure α-subunits of PNPase. Ratio corresponds to ng of α-subunits in pnp-71 or pnp ::Tn5 divided by ng of α-subunits in pnp. For pnp ::Tn5, < 0.1 is equal to 0.0034 ng of α-subunits μg−1 extract, and < 0.1 ratio is equal to 0.0022 (see Experimental procedures ).
The PNPase71 mutant is defective in autoregulation
We next investigated directly whether the increased steady-state level of PNPase α-subunit in the pnp-71 mutant results from defective autoregulation. As mentioned before, PNPase acts at a post-transcriptional level to repress its own synthesis. This autorepression is conveniently monitored using a pnp ::lacZ protein fusion reporter engineered in phage lambda (Robert-Le Meur and Portier, 1992). Thus, a Δlac strain GF5321, which contains the pnp ::lacZ fusion in prophage lambda, expresses the hybrid β-galactosidase at a significantly reduced level compared with the pnp ::Tn5 derivative GF5322. To investigate the influence of pnp-71 on autoregulation, we transduced Zgi:Tn10 pnp-71 to GF5321, and then compared β-galactosidase expressed from this strain (MU950) with that from the isogenic pnp and pnp ::Tn5 strains. 6Figure 6A shows that, unlike the wild-type PNPase, which represses the level of hybrid β-galactosidase 21-fold, the product of the pnp-71 allele repressed β-galactosidase by only threefold. Thus, the PNPase71 mutant α-subunit has a significant defect in autorepression.
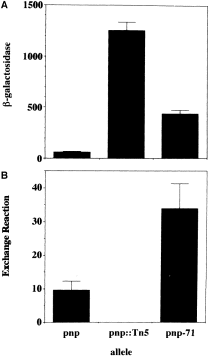
. Enzymatic activities in E. coli crude extracts. A. Values of β-galactosidase activity correspond to units min−1 mg−1 extract. B. Values of PNPase activity correspond to μmol of phosphate exchanged h−1 mg−1 extract. In both assays, figures are the average of nine independent determinations (see Experimental procedures ).
Discussion
Since the discovery of polynucleotide phosphorylase (Grunberg-Manago and Ochoa, 1955), extensive biochemical and genetic studies have been devoted to characterize this enzymatic activity and to reveal its function in E. coli (Grunberg-Manago, 1963; Reiner, 1969; Godefroy-Colburn and Grunberg-Manago, 1972; Portier, 1980; Littauer and Soreq, 1982; Yancey and Kushner, 1990). In this work, we have isolated a PNPase mutant of E. coli, pnp-71, that shows several intriguing properties: (i) the mutant enzyme increases the sib1-dependent galK mRNA decay from the pMS1 plasmid; (ii) the mutant enzyme shows an altered electrophoretic mobility in native gels, indicative of a novel complex; and (iii) the mutant enzyme accumulates in greater abundance in the cell because of a defect in autogenous control. We have shown that all of these phenotypes of the mutant are caused by a single amino acid substitution in the catalytic subunit of PNPase.
The pnp-71 mutation is caused by a G to A transition, which changes a glycine residue to aspartate in the most strongly conserved motif of the KH domain that potentially plays a role in both RNA–protein and protein–protein interactions (see Gibson et al., 1993; Musco et al., 1996). Although the PNPase71 mutant was selected for its ability to reduce galK expression from the pMS1 vector and, indeed, it enhances the decay of the pgal-sib1-galK transcript (Fig. 2C), the half-life of the pgal-galK mRNA expressed from the pKG1800 vector is increased in the mutant to a level that is similar to that observed in the pnp ::Tn5 background (Fig. 2A). This apparently paradoxical result raises the possibility that the pgal-galK and the pgal-sib1-galK mRNAs decay via two distinct mechanisms. The α-subunit of PNPase is a component of the E. coli mRNA degradosome, a multienzymatic complex that also contains the endoribonuclease RNAse E (Carpousis et al., 1994; Py et al., 1994; 1996; Miczak et al., 1996; Blum et al., 1997). There is additional evidence suggesting an association between PNPase and RNase III in the cell (Swatantra et al., 1982). As the phage lambda sib structure is normally processed by RNase III, a complex of RNase III and PNPase may recognize the message containing sib1. Our results showed that the pgal-tI(sib1 )-galK readthrough transcript and the pgal-tI(sib1 ) terminated transcript are degraded more efficiently in the pnp-71 background than in wild type. On the other hand, the pgal-galK mRNA is stabilized in the pnp-71 mutant, similar to the fate of this mRNA in a strain devoid of PNPase activity. These results could be explained simply by a decrease in the affinity of the PNPase71 mutant degradosome complex for mRNAs such as pgal-galK that are relatively stable as a result of their intrinsic genetic structure.
A more interesting explanation for the differential degradation of the two different RNA species with and without sib1 in the pnp-71 mutant is summarized in the model presented in Fig. 7A. According to this model, the PNPase catalytic subunit exists in an equilibrium between free subunits and the one associated in the degradosome. The subunits assembled in the higher order complex would participate in a combined endo/exonucleolytic pathway responsible for the degradation of the pgal-sib1-galK mRNA that could generate the pgal-tI(sib1 ) transcripts by exonucleolytic trimming rather than by endonucleolytic processing. A further postulate in the model is that the free PNPase subunits (or a different complex) participates in the exonucleolytic degradation of the pgal-galK mRNA devoid of the sib1 structure. According to these postulates, the pnp-71 mutation shifts the equilibrium towards the formation of the degradosome complex. Thus, a depletion of the free PNPase subunit would stabilize the galK mRNA made from pgal, while an abundant supply of the higher order complex would cause the sib1-containing transcripts to be degraded rapidly. Another intriguing possibility that can be tested is that the PNPase71 increases its association with RNase III in a way that increases the affinity of the complex for sib1, thereby enhancing the processing of the mutant sib1 hairpin.
Perhaps the most striking observation reported in this paper is that the pnp-71 mutation causes an altered electrophoretic mobility of PNPase present in crude cell extracts. Pure PNPase is isolated from E. coli as a homotrimer of the α-subunit (Portier, 1975). Clearly, the altered molecular behaviour of the PNPase71 mutant enzyme could be attributable to a higher order oligomer. However, this altered oligomeric state cannot be attributed to a simple aggregation caused by the higher amount of the mutant enzyme in the crude extract: a high level of the wild-type PNPase expressed from a high-copy plasmid vector increases the ADP polymerization activity but not the altered mobility observed with the mutant (Fig. 4A, lanes 8, and 9). Furthermore, the co-expression of both wild type and the mutant enzyme in the cell leads to the production of PNPase complexes with intermediate mobility (Fig. 4A, lane 7). As the pnp-71 mutation does not change the mobility of the PNPase α-subunit in SDS–PAGE, the observed effect in the native gel must be attributed to an altered structure of the α-subunit oligomer, albeit because of a different oligomeric state or an increased affinity of the mutant PNPase oligomer for another component(s) in the cell. It is noteworthy that, in the case of the human protein FMR1, the KH domain has been shown to be necessary for the formation of homomeric complexes, as well as heteromeric complexes with other FMR1 homologues, FXR1 and FXR2 (Zhang et al., 1995). Although much further work will be needed to address the physical properties of our mutant PNPase fully, the altered gel mobility in itself supports the model presented above. It is tempting to speculate that the KH domain of PNPase is involved in a protein–protein interaction that enables the PNPase catalytic subunit to form the degradosome complex or a complex with RNAse III. The pnp-71 mutation alters the KH domain such that the complex is formed more avidly. Consequently, the mutant enzyme acts more efficiently on RNA substrates containing structures that specifically mark these mRNAs for facilitated degradation by the degradosome complex. Notably, the pnp mRNA is processed by RNAse III. It will be important to determine whether pnp-71 augments the decay of its own mRNA.
Another striking attribute of the pnp-71 mutant is its defect in autogenous regulation. Previous work by Robert-Le Meur and Portier (1992) has suggested that this autoregulation might involve translational repression by the same enzyme, rather than self-degradation of the mRNA. According to this model, the enzyme binds its own messenger around the Shine–Dalgarno sequence, but only after specific determinants have been exposed by RNase III-mediated processing of the mRNA. Previous to this work, all the mutations that diminish PNPase autorepression are located in the ribosome loading site of the PNPase mRNA, and none has been isolated in the cistron of the enzyme, with the exception of the pnp ::Tn5 allele, which is clearly defective in both catalysis as well as autorepression (Robert-Le Meur and Portier, 1994). If PNPase indeed interacts directly with its own messenger, a remarkable aspect of this mechanism of autoregulation would be the specific affinity of the enzyme for the processed messenger. Although one report claims a sequence-specific binding of PNPase to a double-stranded DNA sequence isolated from rat (Zhang et al., 1998), to our knowledge a preferential binding of PNPase for a specific RNA sequence has yet to be documented.
One interesting possibility for the autoregulation mechanism is that the α-subunit acquires a specific affinity for its own mRNA by interaction with another protein in vivo. If the pnp-71 mutation increases a protein–protein interaction that favours the assembly of the mutant PNPase in the higher order degradosome complex, it might at the same time reduce the amount of the repressor complex that binds pnp mRNA and hence cause a loss of autorepression. Alternatively, if PNPase does bind the pnp mRNA directly, the reduced pool of the free PNPase α-subunit might be sufficient for a loss of autogenous control. An equally appealing possibility is that the specific affinity of PNPase α-subunit for the pnp mRNA is directly affected by alteration of the KH domain, which has been proposed to be involved variously in RNA binding. These possibilities are experimentally testable.
As mentioned before, the G-570 residue represents the most highly conserved amino acid in all known bacterial PNPases (Clarke and Dowds, 1994; Fleischmann et al., 1995; Jones and Bibb, 1996; Luttinger et al., 1996) (Fig. 7B). Although no other mutations have been reported in the PNPase KH domain, a glycine replacement similar to the one selected in pnp-71 has indeed been isolated in the unique KH domain of the gld-1 gene of Caenorhabditis elegans (Jones and Schedl, 1995). The gld-1 KH domain mutation abolishes the essential function of gdl-1 in directing oogenesis and also the tumour suppressor activity of this germline-specific factor. It has also been shown that changing the Gly-X-X-Gly loop sequence in the KH domain of the human protein hnRNPK abolishes its in vitro poly(C)-binding capacity (Siomi et al., 1994). The fact that the pnp-71 mutation does not greatly affect the intrinsic catalytic properties of PNPase [the ADP polymerization or poly(A) phosphorolysis, each of which is a measure of RNA binding by PNPase] supports our view that the G-570D mutation affects protein–protein interaction. Whether such a defect in turn affects the recognition of a specific RNA sequence remains to be determined.
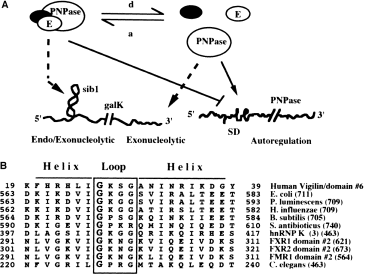
. A. The equilibrium model for PNPase 71. For autoregulation inside the bacteria, the α-subunits of the polynucleotide phosphorylase may exist free or associated with other proteins such as the components of the degradosome. In contrast to the free enzyme, the associated PNPase is unable to repress the expression of its RNAse III-processed mRNA. In the pnp-71 mutant, the change in the KH domain affects the equilibrium and favours the ‘a’ reaction. In the case of the endo/exo versus exonucleolytic activities, according to this model, when aggregated with other enzymes such as RNase E, PNPase participates in endo/exo activities that degrade structured RNA. On the other hand, when the α-subunits of PNPase are dissociated, the enzyme exhibits phosphorolytic activities preferentially on unstructured RNA. In the case of the PNPase71, the mutation in the KH domain might increase the stability of the complex; consequently, the endo/exonucleolytic activity would be increased in the mutant (see text). E represents RNAse E; SD, Shine–Dalgarno signal. ‘a’ indicates the association reaction of PNPase with other proteins; ‘d’ indicates the reverse or dissociation reaction. The black circle represents any other component of the degradosome or another unknown protein. B. Amino acid alignment of selected sequences of KH domains. The alignment was performed using the MPsrch program (S. S. Sturrock and J. F. Collins, Biocomputing Research Unit, University of Edinburgh, UK) and the PNPase maxi KH domain. The structural predictions are delimited according to the three-dimensional structure of human vigilin domain number 6, as described by Musco et al. (1996). Shown in the alignment are the amino acid sequences of the α-loop-α motifs from PNPase from E. coli (Regnier et al., 1987), P. luminescens (Clarke and Dowds, 1994), H. influenzae (Fleischmann et al., 1995), B. subtilis (Luttinger et al., 1996), S. antibioticus (Jones and Bibb, 1996) and the KH motifs of human hnRNP K domain number 3 (Siomi et al., 1993), human FXR1 domain number 2 (Siomi et al., 1995), human FXR2 domain number 2 (Zhang et al., 1995), FMR1 domain number 2 (Verkerk et al., 1993) and GLD-1 from C. elegans (Jones and Schedl, 1995). The box indicates the loop; helices are marked with a top line; an enlarged G in the loop indicates the conserved glycine residue mutated in the pnp-71 allele. Numbers to the side of the sequences indicate the location of the motif in the amino acid sequence except for the vigilin domain number 6, where the number indicates the position in the Maxi KH domain (Musco et al., 1996). The numbers in parenthesis indicate the total number of residues for each protein.
Experimental procedures
Bacterial strains, phages and plasmids
The E. coli strains used in this work were derivatives of S165 Δgal165−(KTE )υ− (Shapiro and Adhya, 1969) or N99 sup°strA galK2 (NIH collection) and were constructed as indicated in the text. Cells were transformed with recombinant DNA according to Davies et al. (1980). Transducing lysates were prepared according to Silhavy et al. (1984). SGM71 bacteria were infected with a P1vir lysate prepared by infection of a Zgi:Tn10 nusA1 strain in which the nusA1 mutation is about 60% linked to TetR. We selected SGM71 TetR-nusA transductants at 37°C. Seven out of seven transductants analysed displayed the altered PNPase activity in polyacrylamide gel analysis. The SGM71 Zgi:Tn10 nusA strain was used in further transduction experiments. This strain contained the presumptive PNPase71 mutation linked to the Tn10 transposon. The PNPase71 phenotype was displayed by 26% out of N99 tetracycline-resistant transductants. We selected a candidate strain N99 Zgi:Tn10 pnp-71, which tested positive for PNPase71 protein activity by gel analysis.
Plasmid pKG1800 is a pBR322 derivative bearing a pgal–galK fusion (McKenney et al., 1981). pUS6 is a derivative of pKG1800 containing the 0.23 kbp sib region (co-ordinates 27724–27479 of λ genome) between the promoter and the galK gene (Schmeissner et al., 1984b). pMS1 is derived from pUS6; it contains the sib1 mutation at position 27573 (Montañez et al., 1986). pGB2 is a pSC101 derivative (Churchward et al., 1984). pCAS11 and pCAS21 are pGB2 derivatives containing the Sal I–BamHI pnp and pnp-71 PCR products respectively. pYN115 contains a fragment of the E. coli chromosome, containing the pnp wild-type operon (Nakamura and Mizusawa, 1985), and plasmid pCJ11, carrying the PCR-amplified pnp wild-type operon (García-Mena, 1992), is a pUC19 derivative (Yanisch-Perron et al., 1985). The plasmid pAJKB encoding the anti-galK 5′ probe was constructed by cloning the 105 bp EcoRV–NarI fragment of the galK gene into the EcoRV–AccI sites of pSP72 (Promega). The N99 strain carrying pCJ11 was used for purification of PNPase wild type, while the N99 pnp-71 pCAS21 strain was used for the purification of the PNPase71 mutant. GF5321 (argG6, argE3, his4, ΔlacX74, rpsL ) is a lysogenic strain of phage λGF2 containing a pnp ::lacZ fusion. GF5322 is an isogenic strain containing the pnp ::Tn5 allele (Robert-Le Meur and Portier, 1992). MU950 is a Zgi:Tn10 pnp-71 derivative of GF5321 obtained by transduction.
Growth conditions and nitrosoguanidine mutagenesis
Media were prepared as described by Sambrook et al. (1989). When necessary, antibiotic concentrations were tetracycline 12.5 μg ml−1, ampicillin 50 μg ml−1, kanamycin 25 μg ml−1 and spectinomycin 50 μg ml−1. For mutant selection, S165 cells were grown on LB-Amp plates containing 0.6% galactose (LB-Amp-Gal). The mutagenesis procedure was a modification of Burns et al. (1987).
Escherichia coli extract preparation
LB 32°C overnight cultures (5 ml), in appropriate conditions for each strain, were diluted using fresh LB to an OD600 of 0.1. Samples were removed at 0.2, 0.3 and 0.4 OD600, pelleted and resuspended to an OD600 of 1.0 in 100 mM Tris base, pH 8.0, 400 mM NaCl and 8 mM β-mercaptoethanol. Cells were sonicated at 0°C. Debris was removed by centrifugation, and the supernatant was dialysed at 4°C using Spectrapor membrane tubing (retention range > 3500), against 50 mM Tris base, pH 8.0, 250 mM NaCl, 8.4 mM β-mercaptoethanol and 0.1 mM EDTA, pH 8.0. Extracts were aliquoted and stored at −80°C.
Enzymatic activities in crude extracts
Galactokinase assays were made in tryptone broth according to an adapted procedure (Wilson and Hogness, 1966). Calculation of specific activity was determined as follows: specific activity = ([c.p.m. − blank]/input) × 1/(15 min assay) × 1/(ml extract used) × 1/[actual OD resuspended cells (≈1.0)] × 0.03 ml reaction volume × 1000. The ADP polymerization activity of polynucleotide phosphorylase was studied in native polyacrylamide gels, according to Thang et al. (1967). The exchange phosphate reaction activity was measured according to Portier (1980). Activity units are expressed as μmol of [32P]-UDP synthesized h−1 ml−1. The β-galactosidase activity of the pnp ::lacZ fusion was assayed according to a method adapted from Sambrook et al. (1989). The optical densities of the reactions were measured in Microtest III tissue culture plate Falcon 3072, using a Bio-Rad model 450 microplate reader filter with a 405 nm filter, and a 490 nm reference filter. Units of β-galactosidase min−1 mg−1 extract = [A405/(min reaction) (ml extract) (mg protein ml−1 extract)] × 1000.
DNA amplification and manipulation
DNA-modifying enzymes were purchased and used according to protocols from New England Biolabs. Plasmid clones were screened by restriction analysis of plasmids prepared by the alkaline SDS method (Birnboim and Doly, 1979). Amplification of the wild-type and mutant 2462 bp pnp gene DNA segments was performed by the DNA PCR method (Saiki et al., 1985) using a thermocycler from Coy Instruments and Vent DNA polymerase. Two 22-mer oligonucleotides were used: primer-1 5′-CTG GGT CGA CGT CGC TAA TTC T-3′; and primer-2 5′-CGT CCG GAT CCC GGT TGC TAA C-3′ (Operon Technologies). For the cloning and sequencing of PNPase-amplified DNA fragments, the pGB2 vector was digested at the AseI site, and samples were BamHI or Sal I digested. Sal I–BamHI-digested PCR pnp or pnp-71 DNA fragments were ligated to 2.0 kbp AseI–spcR–BamHI and 2.2 kbp Sal I–rep–AseI fragments, preventing transcriptional readthrough coming from spcR to the pnp genes. For DNA sequencing, pCAS11 and pCAS21 were double digested with BsiWI and BanI. The 478 bp BanI–BsiWI fragments were isolated, Klenow blunted and ligated to SmaI-linearized, CIP-dephosphorylated pUC19 vector. Sequencing was determined by the dideoxy method (Tabor and Richardson, 1987), using procedure and Sequenase v2 from US Biochemicals.
Galactokinase mRNA half-life measurement
Total RNA was isolated using a modified method (Sarmientos et al., 1983). Cells were grown to an OD600 of 0.4. At time zero, transcription was inhibited using rifampicin to a final concentration of 1 mg ml−1, and aliquots were taken at appropriate times. RNA probes were synthesized using reagents and protocol from Promega using [α-32P]-UTP (Dupont NEG-007H). The anti-galK 5′ probe of 148 bases was transcribed with SP6 RNA polymerase using the EcoRV-linearized pAJKB vector. The anti-sib probe of 149 bases has been described previously (Cisneros et al., 1996). Northern blots of total RNA on Hybond-N membranes (Amersham) were hybridized with the anti-galK 5′ and anti-sib riboprobes. The signal of selected bands was estimated by OD quantification of autoradiographic films under linear conditions. The half-lives were calculated by least-squares analysis of a semi-logarithmic plot of each mRNA's OD as a function of time (Belasco and Brawerman, 1993).
Measurement of plasmid content
The plasmid copy number was estimated comparatively in the different strains used in the isolation of total RNA as follows: 1.0 ml cultures adjusted to an OD550 of 2.0 from each strain carrying the plasmid under test were mixed with 0.2 ml adjusted to an OD550 of 2.0 of bacteria carrying a control vector. Miniprep plasmid isolation was performed quantitatively by the alkaline SDS method (Birnboim and Doly, 1979), and appropriate aliquots were digested with EcoRI. Reaction mixtures were resolved in 1% agarose, and the bands of linearized DNA stained with ethidium bromide were quantified by densitometry. Values were normalized using the plasmid control in each lane.
Enzyme preparation and catalytic activities
PNPase was purified by a procedure reported by Portier (1975) with some modifications. The last steps of purification included adsorption on a Pharmacia MonoQ HR 5/5 FPLC column, equilibrated with 0.05 M NaCl, 0.02 M Tris base, pH 7.5, and elution using a linear 0.05–0.70 M NaCl gradient. Approximately 16 mg of wild-type α-subunit was obtained from 19 g of N99 pCJ11 pellet, while ≈8 mg of PNPase71 α-subunit was obtained from the same amount of N99 pnp-71 pCAS21 pellet. The SDS–PAGE gel in 5Fig. 5A shows that both enzymes had the same size and equivalent levels of purification. The enzymatic activities of exchange phosphate, phosphorolysis and polymerization were measured as described by Portier (1980).
Protein electrophoresis and quantification
Extracts or enzymes were separated in 10%, or 7.5%, 0.1% SDS–polyacrylamide gels. Prestained size markers were Gibco BRL 6041 LA. When necessary, gels were stained according to Laemmli (1970). Protein concentration was determined according to Bradford (1976), using BSA as standard.
Immunodetection of PNPase
The PNPase α-subunits in E. coli extracts were detected in Western blots on Millipore Immobilon-P, using chemiluminescent detection with CSPD substrate and goat anti-rabbit IgG according to the manufacturer's instructions (Tropix). Slot-blot PNPase signals of known amounts of pure α-subunit and E. coli extracts were recorded on X-Omat KXK-1 Kodak films. Signals were quantified by densitometry using a Bio-Rad 620 video densitometer. Densitometric areas were expressed per μg of extract. Appropriate conditions were used to obtain a linear relationship between signal recorded and amount of pure α-subunit or α-subunit in extracts. Anti-PNPase antiserum (Duncroft) was prepared by immunization of rabbit with wild-type α-subunit purified in this work. Serum was treated with N99 pnp ::Tn5 acetone powder to remove non-specific antibodies (Harlow and Lane, 1988).
Acknowledgements
We are grateful to J. DeVito, M. L. Muñoz, M. Robert-Le Meur and B. Savelli for their help in various phases of the work, and to R. Bermudez, D. Court, M. Gottesman and M. Grunberg-Manago for helpful discussion. This work was supported by NIH grant GM28946 (to A.D.) and grant CEE CI1-0790-M (to C.M.). J.G.M. was supported in part by fellowships from COSNET, CONACyT, CIBMyC and IUBMB.




