Extinction-colonization dynamics structure genetic variation of spotted sunfish (Lepomis punctatus) in the Florida Everglades
Abstract
The population genetics of aquatic animals in the Florida Everglades may be strongly influenced by extinction and colonization dynamics. We combined analyses of allozyme and microsatellite loci to test the hypothesis that two levels of population structure are present for spotted sunfish (Pisces: Centrarchidae: Lepomis punctatus) inhabiting the Everglades. We hypothesized that annual cycles of marsh dry-down increase local-scale genetic variation through a process of local extinction and colonization; we hypothesized that barriers to gene flow by levee/canal systems create a second, regional level of genetic variation. In 1996 and 1997, we sampled spotted sunfish from 11 Everglades sites that were distributed in three regions separated by levees. We documented patterns of genetic variation at 7 polymorphic allozyme loci and 5 polymorphic microsatellite loci. Most genetic variation was present among local populations, according to both types of genetic markers. Furthermore, samples from marsh sites were heterogeneous, while those from canals were not. These data supported our hypothesis that dry-down events and local population dynamics in the marsh have a significant effect on population genetic structure of spotted sunfish. We found no support for our hypothesis that water-management structures superimpose a second level of genetic structure on this species, possibly because canals obscure historical structure by facilitating gene flow or because the complete canal system has been in place for fewer than 20 generations of this species. Our data suggests a continent-island (canal-marsh) structure of populations with high gene flow among regions and recurrent mixing in marshes from canal and creek habitats.
Introduction
The genetic structure of a metapopulation may be shaped by the geographical scale and pattern of dispersal leading to colonization following local extinction events (Wade & McCauley 1988; McCauley et al. 1995). For example, colonists from a small number of source sites elevate the level of genetic variation among populations within a metapopulation (propagule-pool colonization). In contrast, when colonists from many sources re-populate a site, local extinction leaves little impact on metapopulation genetic structure (migrant-pool colonization). F-statistics (FST) can be used to distinguish stable entities of a population, identify newly colonized sites and indicate the dominant mode of colonization (Slatkin 1977; Wade & McCauley 1988; Whitlock & McCauley 1990). The genetic structure of a metapopulation and the dominant mode of colonization for a species may depend on the spatial scale and physical structure of the environment being considered.
The effects of habitat fragmentation on population genetic structure can vary widely and may even be concealed or diminished by population-level genealogical relationships that persist when gene flow no longer occurs between two populations. Pannell & Charlesworth (1999) noted that FST is stable to fluctuations in the number of occupied sites, such as in a population that is expanding and contracting over time. They cautioned that FST is relatively insensitive to the mode of colonization if the rate of extinction is less than or equal to the rate of gene flow between populations. Further, FST may be a poor estimate of population divergence because it is sensitive to the total diversity (the denominator) unrelated to the difference between populations. Thus F-statistics may underestimate genetic divergence between populations, especially when allelic diversity is high (Nagylaki 1998; Pannell & Charlesworth 1999).
The Everglades can be characterized as a south flowing river with a braided structure of relatively deep sloughs separated by densely vegetated shallow ridges, and bound by relatively short-hydroperiod wetlands. The slough habitats are routinely fragmented by seasonal dry-down events that temporarily reduce and/or eliminate connections between them (separated by hundreds of metres to kilometres) and concentrate aquatic organisms into deep-water refuges (Loftus & Kushlan 1987; Trexler et al. 2001). Starting before 1900 and culminating in the late 1960s (reviewed in Blake 1980; Light & Dineen 1994), canals that may enhance fish dispersal and levees that may limit or preclude migration by fish have subdivided the ecosystem into regional management units covering hundreds of square kilometres. These two forms of habitat fragmentation, seasonal dry-down events and structural fragmentation, differ in their implications for population genetic structure. Seasonal dry-downs may cause the local extinction of populations, and colonists may re-populate local habitat from a variety of sources. Additionally, dry-downs may encourage mixing by relatively long-range movement of fishes into refuge habitats. Movement of individuals into deep-water refuges from a diversity of marsh sites during a dry-down event may result in population mixing revealed genetically by deviation from Hardy-Weinberg equilibria and deficiency of heterozygotes (Wahlund effect: Hartl & Clark 1997). Structural fragmentation by canals may create corridors that facilitate directional movement, while levee barriers may reduce the dimensionality of movement through the habitat. Thus, structural fragmentation may create either corridors that homogenize populations, or barriers to gene flow facilitating genetic differentiation of subpopulations on either side.
We have used F-statistics to identify the metapopulation genetic structure and distinguish between two modes of colonization (propagule and migrant pool) for a common centrarchid, the spotted sunfish (Lepomis punctatus), found throughout the Florida Everglades. We combined analyses of allozyme and microsatellite loci to test the hypothesis that two levels of population structure may be present for spotted sunfish in the Everglades: one of local variation resulting from extinction and colonization; and a second of regional variation resulting from barriers to gene flow by levees and canals superimposed over the historical pattern of a large unimpeded river. If extinction and colonization are important, relatively greater F-statistics will be observed between sites that are subject to extinction-colonization dynamics compared to sites that are stable. Further, if colonization occurs from many different sources, including some distant from the receiving population (migrant-pool colonization), then local extinctions will have little effect on the population genetic structure within water management units. In the latter scenario, relatively large F-statistics among regions remain plausible. In this case an island model, perhaps at the scale of management units, would be appropriate to describe genetic diversity and the greatest F-statistic would be expected at the regional scale. The greatest F-statistic would be recorded at the local scale within water management units if colonization were from a small number of local sites (propagule-pool colonization) (Slatkin 1977; Wade & McCauley 1988).
Materials and methods
Sample collection
In the Everglades ecosystem, spotted sunfish are relatively large (85 mm adult mean standard length [SL = length from the tip of the snout to the base of the caudal peduncle]), long-lived fish (sexual maturity at approximately 1 year); most Everglades fish are cyprinodontiformes with approximately annual life cycles (Loftus & Kushlan 1987; Trexler et al. 2001). We collected 278 spotted sunfish between 1996 and 1997 from eight canal and three marsh-pond sites distributed throughout Shark River Slough in Everglades National Park (ENP) and Water Conservation Areas 3A (WCA-3A) and 3B (WCA-3B) (Fig. 1; Table 1). Canal and levee constructions separate these three regions of the Everglades, with WCA-3B being completely isolated hydrologically from the rest of the ecosystem by a system of levees completed in 1963. Gates permit a regulated flow of water southward from WCA-3A to ENP. The sample size from each site ranged from 12 to 52, and the fish were obtained by angling and electrofishing. Upon collection, animals were transferred to the laboratory, standard length was measured, and they were stored at −80 °C prior to genetic analyses.
Map of the regions sampled in the Florida Everglades showing the locations of the sample sites. The location of the sampling area is indicated in black on the Florida State map.
| Site | Habitat | Region | Latitude | Longitude | N | SL |
|---|---|---|---|---|---|---|
| L1 | marsh | WCA-3A | 25.51.67 | 80.43.49 | 23 | 84.5 (5.85) |
| L3 | canal | WCA-3A | 25.59.45 | 80.50.35 | 25 | 92.7 (2.51) |
| L11 | canal | WCA-3A | 25.45.70 | 80.49.40 | 25 | 100.0 (4.03) |
| L21 | canal | WCA-3A | 26.06.54 | 80.36.26 | 14 | 93.2 (2.98) |
| L23 | canal | WCA-3A | 26.03.80 | 80.26.30 | 25 | 65.3 (1.74) |
| L25 | canal | WCA-3A | 26.08.82 | 80.34.23 | 26 | 95.1 (1.77) |
| L32 | canal | WCA-3A | 26.08.83 | 80.40.69 | 25 | 85.3 (1.96) |
| L12 | canal | WCA-3B | 25.45.70 | 80.40.40 | 25 | 80.5 (1.88) |
| L33 | canal | WCA-3B | 25.56.52 | 80.26.37 | 12 | 88.2 (5.82) |
| L34 | marsh | ENP | 25.41.42 | 80.45.61 | 19 | 86.4 (3.50) |
| L35 | marsh | ENP | 25.41.32 | 80.45.70 | 12 | 86.0 (5.73) |
Sampling was conducted in the dry season of 1996 to obtain specimens from areas where they were concentrated because of declining water levels. However, an insufficient number of specimens was collected, so additional samples were obtained in the dry season of 1997 and pooled into a single sample with the fish from the previous year. We considered that pooling between the 2 years was appropriate because spotted sunfish live several years, permitting the adult fish from both years to be treated as members of the same cohort. Also, there was no evidence of significant interannual variation in allozyme or microsatellite allele frequencies in these two samples (Trexler et al. 2001).
We estimated the density of spotted sunfish from the marsh habitat adjacent to one of our marsh sampling sites in ENP (L35) from a long-term fish-sampling programme conducted by personnel of the park. These data were obtained by use of a 1-m2 throw trap and approximately 105 samples were collected each year between 1978 and 1997. Fish were collected in February, April, July, October and December (for details of the sampling design see Loftus & Eklund 1994; Trexler et al. 2001). Juveniles of four species of sunfish found in the Everglades are not easily distinguishable (these are L. punctatus, L. marginatus, L. microlophus, and L. macrochirus), though only spotted sunfish and L. marginatus are abundant. We summed the counts of spotted sunfish and unidentified juvenile sunfish to yield a generous estimate of the historical density of spotted sunfish at the sampling site prior to our collection for genetic analysis.
Allozyme analysis
We used starch-gel electrophoresis to document patterns of allozymic variation in spotted sunfish. Whole-tissue extracts were prepared for electrophoresis by homogenization of tissues in approximately 500 µL of grinding buffer (0.025 m Tris pH 7.0, 0.025 m sucrose, 0.005 m mercaptoethanol). We pooled eye, liver and soma clips; preliminary analyses indicated no tissue-specific expression of the proteins, justifying this approach. We screened 32 protein-encoding loci with horizontal starch-gel electrophoresis before selecting 15 resolvable loci to score on all individuals. Of these, seven loci were polymorphic (Table 2). We followed standard techniques described in Selander et al. (1971) and Murphy et al. (1996), with 11% (w/v) starch gels. All allozymes were resolved using a Tris-citrate EDTA buffer at pH 8.0 (Ayala et al. 1972).
| Enzyme | EC no. | N | P | H O |
|---|---|---|---|---|
| Aconitase hydratase (Acoh) | 4.2.1.3 | 3 | 1.0 | 0.52 |
| Aspartate aminotransferase (Aat-1) | 2.6.1.1 | 1 | 0.0 | 0.00 |
| Aspartate aminotransferase (Aat-2) | 2 | 1.0 | 0.27 | |
| Glucose dehydrogenase (Gcdh) | 1.1.1.118 | 3 | 1.0 | 0.09 |
| Isocitrate dehydrogenase (Idh-1) | 1.1.1.42 | 2 | 1.0 | 0.17 |
| L-Lactate dehydrogenase (Ldh-2) | 1.1.1.27 | 2 | 1.0 | 0.00 |
| Malate dehydrogenase (Mdh-2) | 1.1.1.37 | 1 | 0.0 | 0.00 |
| Malate dehydrogenase (Mdh-3) | 1 | 0.0 | 0.00 | |
| Mannose-6-phosphate isomerase (Mpi-1) | 5.3.1.8 | 1 | 0.0 | 0.00 |
| Mannose-6-phosphate isomerase (Mpi-2) | 1 | 0.0 | 0.00 | |
| Phosphoglucomutase (Pgm-1) | 5.4.2.2 | 4 | 1.0 | 0.39 |
| Phosphoglucomutase (Pgm-2) | 1 | 0.0 | 0.00 | |
| Phosphogluconate dehydrogenase (Pgdh-1) | 1.1.1.14 | 1 | 0.0 | 0.00 |
| Phosphogluconate dehydrogenase (Pgdh-2) | 1 | 0.0 | 0.00 | |
| Peptidase val-leu (Pep) | 3.4.-.- | 4 | 1.0 | 0.04 |
| Totals | 0.47 | 0.10 |
Microsatellite DNA analysis
We surveyed nine potential microsatellite-locus primer pairs in spotted sunfish that had been developed in closely related species, Lepomis auritus (DeWoody et al. 1998) and Lepomis macrochirus (Colbourne et al. 1996). From these nine loci, we identified five polymorphic loci that amplified reliably (Table 3). Whole genomic DNA was isolated from muscle tissue by standard phenol-chloroform DNA extraction methods (Hoelzel & Green 1992). The microsatellite loci were amplified using polymerase chain reaction (PCR) in 15 µL volumes, containing 1× buffer, 25 µm MgCl2, 250 µm dNTP, 5 U/µL Taq DNA polymerase (Promega), and a 5 µm primer set. One primer of each pair was end-labelled with a fluorescent dye (6-FAM, NED or HEX; Applied Biosystems). The thermal cycling parameters, modified from Colbourne et al. (1996), were as follows: an initial 1 minute denaturation at 94 °C, followed by 45 cycles of 30 s at 94 °C, 30 s at 55 °C, and 30 s at 72 °C, and a final 10 minute extension at 72 °C. We electrophoresed the amplified samples in 2.5% agarose gel to determine the presence or absence of a product. The amplified products were electrophoresed in 5% denaturing polyacrylamide gels on an ABI 377 automated DNA sequencer. The alleles were sized with respect to electrophoretic mobility compared to a ROX 350 standard and the genotypes were assigned using the genescan (ABI) and genotyper software packages.
| Locus | Dye Marker | Locus Sequence Primer Sequences | N | H O |
|---|---|---|---|---|
| RB7* | 6FAM | not reported | 17 | 0.816 |
| F: 5′–GTGCTAATAAAGGCTACTGTC | ||||
| R: 5′–TGTTCCCTTAATTGTTTTGA | ||||
| RB20* | HEX | not reported | ||
| F: 5′–GGTCTACTGGTAAATGAGGG | 15 | 0.797 | ||
| R: 5′– GTTGGGCTGTCGAGAGTAAAAA | ||||
| Lma 21** | HEX | (TC)19(AC)11 | ||
| F: 5′–CAGCTCAATAGTTCTGTCAGG | 14 | 0.683 | ||
| R: 5′–ACTACTGCTGAAGATATTGTAG | ||||
| Lma 29** | 6FAM | (GT)30 | ||
| F: 5′–CCCTGTTACTTGTGTATTC | 17 | 0.731 | ||
| R: 5′–ATTCAGAGGCAAGCATTATC | ||||
| Lma 87** | NED | (AC)15(A)5 | ||
| F: 5′–ATGACACAGACTCACCATGC | 14 | 0.566 | ||
| R: 5′–CTCCTGCCCATAAATCAGAC |
Statistical methods
We examined patterns of allelic and genotypic diversity in both allozymes and microsatellites. We calculated goodness-of-fit to Hardy–Weinberg expectations at each allozyme and microsatellite locus within each site, and in global tests across loci and across sites, using genepop 3.2 (Raymond & Rousset 1995). When only two or three alleles were observed, we used the complete enumeration method of Louis & Dempster (1987), while we used the Markov chain method to calculate exact P-values for loci that exhibited more than three alleles (Guo & Thompson 1992). Linkage disequilibrium was tested for each pair of allozyme and microsatellite loci within each site using genepop 3.2 (Raymond & Rousset 1995). We used f-stat (Goudet 2001) to estimate gene diversity and allelic richness separately for allozymes and microsatellites. We also tested for differences in gene diversity and allelic richness between allozymes and microsatellites using a Mann–Whitney's U-test with loci as replicates.
We hypothesized that sunfish from marsh sites that experienced seasonal dry-down events that varied in severity might experience significant reductions in their effective population size. The most recent severe drought occurred in 1989–90, when all of ENP and WCA-3B, and most of WCA-3A were dry. Thus, we examined the possible impact of demographic changes from hydrologic fluctuations or habitat fragmentation on allozyme and microsatellite diversity. We estimated deviations from expected heterozygosity and tested for evidence of recent population bottlenecks with bottleneck (Cornuet & Luikart 1996; Piry et al. 1999). This program estimated heterozygosity excesses and deficiencies for each locus, and for each site, and tested significance using a one-tailed Wilcoxon test based on ranks. We used the infinite alleles model for analysis of the allozyme data, and both the stepwise and two-phase mutation models for analysis of microsatellites. In addition, an assignment test was performed according to the direct method (Rannala & Mountain 1997) with the leave-one-out option (geneclass software package; Cornuet et al. 1999). This permitted us to determine the likelihood that each individual's allozyme and microsatellite multilocus genotype belong to the site and/or region from which it was sampled (Paetkau et al. 1995). In order to increase the number of loci and thus the power of the population bottleneck and the assignment tests, allozyme and microsatellite data were combined, and the above analyses were repeated. If local or regional sites are stable and genetically distinct, then recent migrants to a region or a local site might be detected and the source of these migrants could be identified.
To test for isolation by distance and equilibrium of gene flow and drift, we calculated pairwise estimates of FST and RST for allozyme and microsatellite loci, respectively (Slatkin 1993; Hutchison & Templeton 1999). We tested the significance of pairwise correlations between these values and the linear geographical distance separating sites using the Mantel permutation procedure (Mantel 1967) with the Spearman's rank correlation coefficient as the test statistic (genepop 3.2; Raymond & Rousset 1995). Hydrographical distance between sites and linear geographical distance between sites were functionally equal because the Everglades is an expansive river-like wetland (i.e. with few exceptions, fish can move in all directions throughout much of the system). The patterns of the scatterplots were analysed according to Hutchison & Templeton (1999). Residual values from these analyses were classified as within or among regions, or within or among site types (canal or marsh). These values were analysed to test for regional division or site type patterns.
We used f-stat (Goudet 2001) to compare gene diversity and allelic richness separately for allozymes and microsatellites among regions and between marsh and canal sites. We used Weir & Cockerham's (1984) co-ancestry method to hierarchically partition the total observed genetic variation for the allozyme and microsatellite data. These partitions were attributable to variation among individuals within sites (individuals collected in areas < 1 km2) relative to total diversity within their site (θIS), among sites within water-management units relative to the total diversity in that unit (θSP), and among water-management units (regions) relative to the total genetic diversity (θST). This provided estimates of Wright's hierarchical F-statistic of population subdivision based on a random-effects sampling model (Weir 1990). The regions correspond to an area enclosed by levees and canals: WCA-3A, WCA-3B, and ENP (Fig. 1). F-statistic estimates were also calculated separately among all canal sites and among all marsh sites to compare genetic structure estimates between deep-water refuge sites and sites that may be subject to frequent extinction and colonization. The Genetical Data Analysis program (gda 1.0; Lewis & Zaykin 1997) was used to estimate hierarchical F-statistics following Weir & Cockerham (1984), with bootstrapping across loci to estimate 95% confidence intervals.
To make statistical comparisons between the population-genetic patterns detected from allozymes and microsatellites, Weir & Cockerham's (1984) co-ancestry method was employed for both markers. Simulations performed by Ruzzante (1998) suggested that FST and RST perform similarly if mutation has not had enough time to affect allelic divergence of microsatellite loci. We tested for correlations of pairwise FST estimates between sites for allozymes and microsatellites with the Mantel permutation procedure performed as above.
Counts of sunfish collected in 1-m2 samples deviated noticeably from a normal distribution, with many traps yielding zero specimens. Using SAS statistical software, we fit an overdispersed-Poisson linear model with a log linking function to provide estimates of annual density with asymmetrical confidence intervals lower bounded at zero (Littell et al. 1996).
Results
Hardy–Weinberg equilibrium and genetic diversity
Genotypic frequencies of allozymes were more consistent with Hardy-Weinberg predictions than genotypic frequencies of microsatellites. The genotypic frequencies of the allozyme loci were generally consistent with expectations of Hardy-Weinberg equilibrium; testing across loci within each site, we found only one case that did not conform to Hardy-Weinberg equilibrium expectations (L1). Additionally, we found evidence of heterozygote deficiency (f < 0) at 3 of the 7 polymorphic loci examined for that site (Table 4). Several of the microsatellite loci did not conform to Hardy-Weinberg predictions. Testing within each site indicated that genotypic frequencies within three of the sites were inconsistent with Hardy-Weinberg predictions (L1, L11, and L32). Additionally, there was significant heterozygote deficiency detected for some loci within those sites, though different loci were deficient in heterozygotes among sites (Table 5). There was some evidence for linkage disequilibrium in allozymes (Gcdh & Pep) and in microsatellites (Lma29 & Lma87).
| Collection Site | Locus | Totals across loci | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Acon | Aat-2 | Gcdh | Idh-1 | Ldh-2 | Pgm-1 | Pep | |||
| L1 | N | 23 | 23 | 23 | 13 | 23 | 16 | 23 | |
| A | 2 | 2 | 3 | 2 | 2 | 3 | 3 | ||
| H E | 0.476 | 0.231 | 0.565 | 0.269 | 0.474 | 0.283 | 0.482 | ||
| R | 2.000 | 1.992 | 2.992 | 2.000 | 2.000 | 2.932 | 2.777 | ||
| f | −0.004 | –0.128 | 0.692 | −0.143 | 1.000 | −0.103 | 0.730 | 0.402 | |
| HW | 1.000 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 | 0.000 | |
| L3- | N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| A | 3 | 2 | 2 | 2 | 1 | 3 | 2 | ||
| H E | 0.588 | 0.302 | 0.040 | 0.150 | 0.000 | 0.223 | 0.040 | ||
| R | 2.993 | 1.999 | 1.480 | 1.935 | 1.000 | 2.735 | 1.480 | ||
| f | −0.089 | 0.072 | 0.000 | −0.067 | — | −0.079 | 0.000 | −0.043 | |
| HW | 0.376 | 1.000 | — | 1.000 | — | 1.000 | — | 0.982 | |
| L11 | N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| A | 3 | 2 | 1 | 2 | 1 | 3 | 1 | ||
| H E | 0.538 | 0.327 | 0.000 | 0.150 | 0.000 | 0.279 | 0.000 | ||
| R | 2.969 | 1.999 | 1.000 | 1.935 | 1.000 | 2.473 | 1.000 | ||
| f | 0.034 | 0.020 | NA | –0.067 | — | −0.146 | NA | −0.020 | |
| HW | 0.886 | 1.000 | — | 1.000 | — | 1.000 | — | 1.000 | |
| L12 | N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| A | 3 | 2 | 2 | 2 | 1 | 3 | 1 | ||
| H E | 0.587 | 0.245 | 0.078 | 0.078 | 0.000 | 0.681 | 0.000 | ||
| R | 2.986 | 1.993 | 1.735 | 1.735 | 1.000 | 3.000 | 1.000 | ||
| f | −0.295 | −0.143 | −0.021 | −0.021 | — | 0.119 | NA | −0.078 | |
| HW | 0.051 | 1.000 | 1.000 | 1.000 | — | 0.326 | — | 0.612 | |
| L21 | N | 14 | 14 | 14 | 14 | 14 | 14 | 14 | |
| A | 3 | 2 | 2 | 2 | 1 | 3 | 2 | ||
| H E | 0.527 | 0.308 | 0.137 | 0.253 | 0.000 | 0.467 | 0.071 | ||
| R | 2.857 | 2.000 | 1.984 | 2.000 | 1.000 | 2.857 | 1.857 | ||
| f | 0.323 | 0.304 | –0.040 | –0.130 | — | −0.376 | 0.000 | 0.028 | |
| HW | 0.238 | 0.348 | 1.000 | 1.000 | — | 0.468 | — | 0.772 | |
| L23 | N | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |
| A | 3 | 2 | 2 | 2 | 1 | 4 | 3 | ||
| H E | 0.423 | 0.245 | 0.115 | 0.115 | 0.000 | 0.603 | 0.079 | ||
| R | 2.480 | 1.993 | 1.867 | 1.867 | 1.000 | 3.466 | 1.960 | ||
| f | 0.053 | −0.143 | −0.043 | −0.043 | — | 0.006 | −0.011 | −0.013 | |
| HW | 1.000 | 1.000 | 1.000 | 1.000 | — | 0.647 | 1.000 | 1.000 | |
| L25 | N | 23 | 26 | 26 | 26 | 26 | 21 | 26 | |
| A | 2 | 2 | 2 | 2 | 1 | 3 | 1 | ||
| H E | 0.451 | 0.265 | 0.111 | 0.111 | 0.000 | 0.479 | 0.000 | ||
| R | 2.000 | 1.996 | 1.852 | 1.852 | 1.000 | 2.822 | 1.000 | ||
| f | 0.132 | −0.163 | −0.042 | −0.042 | — | −0.393 | NA | −0.128 | |
| HW | 0.642 | 1.000 | 1.000 | 1.000 | — | 0.126 | — | 0.889 | |
| L32 | N | 23 | 25 | 25 | 25 | 25 | 25 | 24 | |
| A | 3 | 2 | 1 | 2 | 1 | 2 | 3 | ||
| H E | 0.611 | 0.245 | 0.000 | 0.150 | 0.000 | 0.040 | 0.082 | ||
| R | 2.992 | 1.993 | 1.000 | 1.935 | 1.000 | 1.480 | 2.000 | ||
| f | 0.074 | 0.143 | NA | 0.067 | — | 0.000 | 0.011 | 0.000 | |
| HW | 0.158 | 1.000 | — | 1.000 | — | — | 1.000 | 0.884 | |
| L33 | N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| A | 3 | 2 | 2 | 2 | 1 | 4 | 1 | ||
| H E | 0.591 | 0.159 | 0.288 | 0.348 | 0.000 | 0.420 | 0.000 | ||
| R | 3.000 | 2.000 | 2.000 | 2.000 | 1.000 | 4.000 | 1.000 | ||
| f | 0.295 | −0.048 | −0.158 | 0.283 | — | 0.189 | NA | 0.078 | |
| HW | 0.169 | 1.000 | 1.000 | 0.403 | — | 1.000 | — | 0.865 | |
| L34 | N | 17 | 19 | 19 | 17 | 19 | 18 | 19 | |
| A | 3 | 2 | 2 | 2 | 1 | 1 | 1 | ||
| H E | 0.465 | 0.430 | 0.102 | 0.114 | 0.000 | 0.000 | 0.000 | ||
| R | 2.706 | 2.000 | 1.871 | 1.920 | 1.000 | 1.000 | 1.000 | ||
| f | −0.265 | 0.633 | −0.029 | −0.032 | — | NA | NA | 0.128 | |
| HW | 0.082 | 0.012 | 1.000 | 1.000 | — | — | — | 0.086 | |
| L35 | N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| A | 2 | 2 | 2 | 2 | 1 | 3 | 1 | ||
| H E | 0.462 | 0.288 | 0.167 | 0.288 | 0.000 | 0.595 | 0.000 | ||
| R | 2.000 | 2.000 | 2.000 | 2.000 | 1.000 | 3.000 | 1.000 | ||
| f | −0.082 | −0.158 | 1.000 | −0.158 | — | 0.019 | NA | 0.027 | |
| HW | 1.000 | 1.000 | 0.043 | 1.000 | — | 1.000 | — | 0.792 | |
| Total all sites | N | 179 | 186 | 186 | 174 | 184 | 173 | 185 | |
| A | 3 | 2 | 3 | 2 | 2 | 4 | 4 | ||
| H E | 0.520 | 0.277 | 0.146 | 0.184 | 0.043 | 0.367 | 0.069 | ||
| R | 2.808 | 1.988 | 2.223 | 1.994 | 1.994 | 2.974 | 1.805 | ||
| f | 0.006 | 0.026 | 0.396 | −0.034 | 1.000 | 0.078 | 0.556 | 0.126 | |
| HW | 0.342 | 0.975 | 0.007 | 1.000 | 1.000 | 0.965 | 0.003 | 0.000 | |
| Collection Site | Locus | All loci | |||||
|---|---|---|---|---|---|---|---|
| RB7 | Lma21 | Lma29 | RB20 | Lma87 | |||
| L1 | N | 22 | 23 | 23 | 20 | 23 | |
| A | 13 | 7 | 11 | 11 | 5 | ||
| H E | 0.918 | 0.791 | 0.848 | 0.850 | 0.610 | ||
| R | 9.025 | 5.561 | 7.472 | 7.152 | 4.004 | ||
| f | 0.257 | 0.120 | 0.282 | 0.176 | 0.073 | 0.190 | |
| HW | 0.000 | 0.001 | 0.000 | 0.000 | 0.208 | 0.000 | |
| L3 | N | 13 | 13 | 13 | 13 | 13 | |
| A | 10 | 5 | 8 | 12 | 6 | ||
| H E | 0.897 | 0.718 | 0.849 | 0.923 | 0.785 | ||
| R | 8.209 | 4.463 | 7.104 | 9.551 | 5.416 | ||
| f | −0.029 | −0.393 | −0. 177 | 0 | 0.020 | −0.106 | |
| HW | 0.854 | 0.024 | 0.906 | 0.574 | 0.597 | 0.432 | |
| L11 | N | 31 | 31 | 31 | 30 | 31 | |
| A | 13 | 8 | 12 | 12 | 7 | ||
| H E | 0.887 | 0.711 | 0.891 | 0.923 | 0.751 | ||
| R | 8.111 | 4.659 | 8.344 | 9.059 | 4.967 | ||
| f | −0.019 | 0.092 | 0.095 | 0.350 | 0.485 | 0.197 | |
| HW | 0.184 | 0.114 | 0.271 | 0.000 | 0.000 | 0.000 | |
| L12 | N | 45 | 40 | 45 | 43 | 45 | |
| A | 15 | 10 | 16 | 14 | 9 | ||
| H E | 0.908 | 0.765 | 0.885 | 0.909 | 0.743 | ||
| R | 8.887 | 5.967 | 8.436 | 8.725 | 5.273 | ||
| f | 0.045 | 0.150 | 0.071 | 0.027 | 0.073 | 0.071 | |
| HW | 0.117 | 0.006 | 0.213 | 0.252 | 0.519 | 0.018 | |
| L21 | N | 23 | 22 | 23 | 22 | 21 | |
| A | 12 | 9 | 11 | 15 | 8 | ||
| H E | 0.914 | 0.766 | 0.813 | 0.935 | 0.785 | ||
| R | 8.888 | 6.079 | 6.998 | 10.173 | 5.976 | ||
| f | 0.144 | −0.008 | −0.016 | 0.076 | 0.150 | 0.072 | |
| HW | 0.428 | 0.441 | 0.938 | 0.250 | 0.231 | 0.517 | |
| L23 | N | 12 | 11 | 8 | 10 | 12 | |
| A | 11 | 4 | 9 | 7 | 7 | ||
| H E | 0.932 | 0.745 | 0.911 | 0.900 | 0.790 | ||
| R | 9.347 | 3.985 | 9.000 | 6.754 | 5.653 | ||
| f | 0.106 | −0.098 | 0.039 | 0.444 | 0.194 | 0.143 | |
| HW | 0.131 | 0.026 | 0.735 | 0.001 | 0.331 | 0.002 | |
| L25 | N | 34 | 33 | 33 | 26 | 33 | |
| A | 14 | 7 | 15 | 13 | 6 | ||
| H E | 0.906 | 0.746 | 0.910 | 0.927 | 0.694 | ||
| R | 8.957 | 4.587 | 9.410 | 9.583 | 4.042 | ||
| f | 0.156 | 0.107 | 0.034 | 0.087 | 0.257 | 0.122 | |
| HW | 0.108 | 0.329 | 0.787 | 0.048 | 0.295 | 0.114 | |
| L32 | N | 50 | 43 | 51 | 30 | 44 | |
| A | 14 | 9 | 12 | 14 | 10 | ||
| H E | 0.905 | 0.744 | 0.900 | 0.918 | 0.754 | ||
| R | 8.719 | 4.792 | 8.329 | 9.387 | 5.134 | ||
| f | 0.116 | 0.000 | 0.390 | 0.129 | 0.187 | 0.169 | |
| HW | 0.009 | 0.009 | 0.000 | 0.094 | 0.006 | 0.000 | |
| L33 | N | 21 | 20 | 19 | 19 | 21 | |
| A | 11 | 5 | 9 | 14 | 6 | ||
| H E | 0.910 | 0.761 | 0.865 | 0.920 | 0.733 | ||
| R | 8.646 | 4.325 | 7.029 | 9.357 | 5.172 | ||
| f | 0.005 | 0.737 | 0.514 | −0.087 | 0.545 | 0.317 | |
| HW | 0.157 | 0.000 | 0.000 | 0.888 | 0.000 | 0.000 | |
| L34 | N | 14 | 12 | 14 | 14 | 14 | |
| A | 9 | 4 | 12 | 12 | 8 | ||
| H E | 0.860 | 0.705 | 0.923 | 0.912 | 0.810 | ||
| R | 7.568 | 3.666 | 9.426 | 9.124 | 6.505 | ||
| f | 0.003 | −0.301 | 0.149 | 0.139 | 0.207 | 0.053 | |
| HW | 0.491 | 0.354 | 0.361 | 0.195 | 0.253 | 0.317 | |
| L35 | N | 12 | 12 | 10 | 12 | 11 | |
| A | 9 | 4 | 7 | 11 | 5 | ||
| H E | 0.890 | 0.784 | 0.867 | 0.917 | 0.800 | ||
| R | 7.898 | 3.997 | 6.730 | 9.225 | 4.920 | ||
| f | 0.157 | 0.362 | −0.154 | 0.000 | −0.023 | 0.064 | |
| HW | 0.097 | 0.163 | 0.971 | 0.571 | 0.739 | 0.436 | |
| Total all Populations | N | 305 | 279 | 294 | 257 | 289 | |
| A | 17 | 14 | 17 | 15 | 14 | ||
| H E | 0.902 | 0.749 | 0.878 | 0.913 | 0.750 | ||
| R | 8.78 | 5.20 | 8.51 | 9.11 | 5.30 | ||
| f | 0.131 | 0.091 | 0.161 | 0.152 | 0.226 | 0.143 | |
| HW | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
We observed moderate variation at the 7 polymorphic allozymes surveyed (7 of the 15 loci examined were polymorphic = 46.7%, average heterozygosity of loci that were polymorphic = 0.25; Table 2). The number of alleles per polymorphic locus across all sites ranged from 2 (Ldh, Idh-1) to 4 (Pgm-1 and Pep). We observed considerable variation in the five microsatellite loci studied (Table 5). The number of alleles per locus ranged from 14 (Lma21 and Lma87) to 17 (RB7 and Lma29; Table 5). The observed heterozygosity, averaged over all sites, ranged from 0.57 (Lma87) to 0.82 (RB7) (Table 3).
Compared to allozymes, microsatellites had a greater average gene diversity and allelic richness (Mann-Whitney's U = 75.00; P < 0.001). Matrices of allozyme and microsatellite pairwise FST estimates were significantly correlated (Mantel test, r = 0.571, P = 0.009), indicating concordance between the two types of molecular markers.
Recent bottleneck test
There were no indications that any of the studied sites experienced a recent, significant bottleneck. There was one site (L1) where the number of allozyme loci showing heterozygote excess was significantly greater than that expected from the allele diversity. Under the expectations of both models of mutation, there were no sites where the number of microsatellite loci showing heterozygote excesses was significantly greater than expected from the allele diversity. After applying a Bonferroni correction for multiple tests, none of these observations could be considered statistically significant (α = 0.05).
Assignment test
The assignment test performed with the allozyme data did not reliably assign individuals to their site or region of sampling origin. Only 57 of 231 (24.7%) were correctly assigned to the site from which they were sampled, and 80 of 231 (34.6%) correctly assigned to WCA-3A, WCA-3B or ENP. Following King et al. (2001), we found that the inclusion of all allozyme loci produced the greatest assignment accuracy. The assignment test performed with the microsatellite data did not reliably assign individuals to their site or region of sampling origin. Only 48 of 283 (17.0%) were correctly assigned to the site from which they were sampled, and 163 of 283 (57.6%) were correctly assigned to WCA-3A, WCA-3B or ENP. The inclusion and exclusion of microsatellite loci did not alter the assignment accuracy. Similarly, the assignment test performed with a pooled data set of allozymes and microsatellites did not reliably assign individuals to their site or region of sampling origin. Only 58 of 231 (25.1%) were correctly assigned to the site from which they were sampled, and 114 of 231 (49.4%) were correctly assigned to WCA-3A, WCA-3B, or ENP. This suggests that there is not a unique multilocus genotypic distribution for the allozyme or microsatellite loci that described a site or region. This could result from routine mixing within and/or between the regions. The assignment test assumes Hardy-Weinberg equilibrium, which was partially violated by the microsatellite data.
Isolation by distance
We found no correlations between pairwise estimates of geographical distance and FST from allozymes or FST and RST from microsatellite loci, further indicating a lack of spatially explicit genetic structure (Fig. 2). Furthermore, residual values calculated from these regressions did not reveal any significant patterns of deviation associated with region or site type. The overall pattern of the scatterplots was consistent between allozymes and microsatellites.
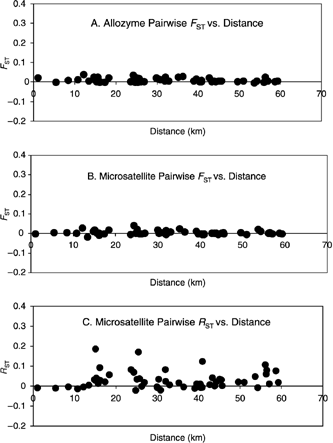
Relationship between geographical distance and (A) θST for the allozyme data (B) θST for the microsatellite data, and (C) RST for the microsatellite data.
Hierarchical genetic diversity
Gene diversity and allelic richness were not significantly different between WCA-3A, WCA-3B and ENP (P > 0.05) for allozymes and microsatellites. They were not significantly different between marsh and canal sites for allozymes (P > 0.05). However, gene diversity and allelic richness were significantly different between marsh and canal sites for microsatellites (gene diversity; P = 0.022: allelic richness; P = 0.009). Marsh sites had, on average, less gene diversity and allelic richness than canal sites.
Allozymes and microsatellites revealed consistent and significant genetic structure among sites within regions (Table 6). When considered separately, sites within WCA-3A displayed significant genetic structure, but sites in WCA-3B and ENP did not (i.e. bootstrap confidence intervals cross zero). The F-statistic estimates were an order of magnitude larger for allozymes than for microsatellites. This is consistent with expectations of F-statistic estimates calculated from low to moderately variable loci compared to highly variable loci (reviewed in Hedrick 1999). Canal sites were not genetically differentiated, whereas marsh sites were significantly heterogeneous (confidence interval of θPT marsh did not cross zero). Based on a t-test re-sampling across loci with 1000 replicates, marsh sites were more heterogeneous than canal sites (HO: θPT canals = θPT marsh; P = 0.115 for microsatelites and P = 0.109 for allozymes; Re-sampling test combining both data sets P = 0.08).
| Microsatellite data | Allozyme data | |||
|---|---|---|---|---|
| θSP | θPT | θSP | θPT | |
| All Sites | 0.0052* | −0.0001 | 0.0745* | −0.0068 |
| Canal | NA | 0.0021 | NA | 0.0485 |
| Marsh | NA | 0.0229* | NA | 0.1446* |
- * indicates 95% confidence interval does not cross zero.
Spotted sunfish population dynamics
Spotted sunfish density in the marsh habitat near our ENP sampling sites fluctuated between approximately 0.5/m2 to indistinguishable from zero over the 18 years prior to our study (Fig. 3a). No specimens of spotted sunfish (or of indistinguishable juveniles of the co-occurring congeners) were collected in 1988 or 1989, and very few specimens were collected in 1990. These latter low-density years overlapped with a relatively extreme 2-year drought event in 1989 and 1990. A caveat is required for the low estimate in 1988; that is the only year of the 2-year record where sampling events were missed but water levels were above the ground surface; no samples were collected in April or May of that year.
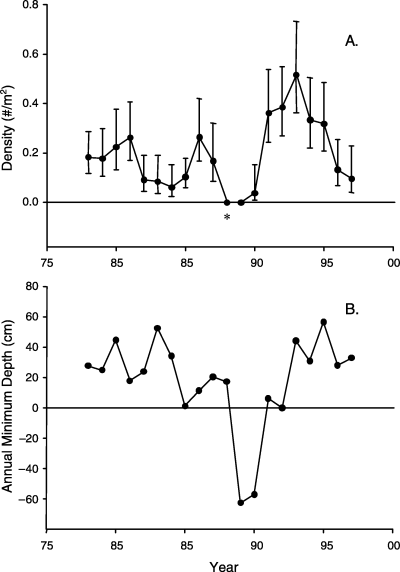
Long-term record of density of spotted sunfish in marsh habitat adjacent to site L35 in ENP. (A) The average density of spotted sunfish (including all indistinguishable juvenile sunfish) in a marsh habitat with 95% confidence intervals estimated on an annual basis from 1978 to 1997. Note that no spotted sunfish were collected in 1988 or 1989, and very few in 1990. Asterisk indicates year when sampling was missed for 2 of the 5 months. (B) Annual minimum water depth at the collection site between 1978 and 1997.
Discussion
The spotted sunfish data were not consistent with use of regional-scale island models (at the spatial scale of water management units or greater) to describe the population structure for this species. The data suggested that spotted sunfish populations could be described as metapopulations that are not at equilibrium between gene flow and genetic drift. We propose that local-scale population dynamics have a significant effect on the population genetic structure of spotted sunfish in the Everglades. We found no evidence of additional regional-scale variation. There are three possible sources of the lack of regional structure: historical population structure has been lost because of the homogenizing effects of canals as conduits for dispersal; the ecosystem historically had high gene flow and the canals and levees have not altered that situation; or, no genetic structure existed historically and, though the modern management system now limits gene flow, inadequate time has passed (approximately 20 generations of spotted sunfish) for the large regional populations to differentiate. Below, we elaborate on these conclusions.
Hardy-Weinberg equilibrium
Some sites were deficient in the frequency of heterozygotes, especially for microsatellite loci. Heterozygote deficiencies can arise from inbreeding, underdominant selection, mixing of genetically differentiated populations at a site (spatial Wahlund effect), or mixing of cohorts (temporal Wahlund effect) (Hartl & Clark 1997). The concordance we observed between allozyme and microsatellite markers (pairwise FST Mantel test) suggests that gene flow and genetic drift were the major causes of any observed patterns of population differentiation (cf., Estoup et al. 1998; Ross et al. 1999). The absence of widespread patterns of heterozygote deficiency suggests that Wahlund effects are not principal factors in the genetic structuring of local populations of spotted sunfish.
The coefficient of variation (CV) of body size of individuals collected from site L1 was much greater than that of any other site sampled (CVL1 = 8.91; CVOTHER SITES = 2.76). The average size was not significantly different among sites with the exception of site L23 (Table 1). Although definitive discrimination between spatial and temporal Wahlund effects is beyond the power of our data, we pooled individuals into 1.0 cm size classes across all sites (6 size classes total, sample sizes within each class ranged from 14 to 39). We were unable to create size-based cohorts within sites because of low sample size. We re-tested for consistency with Hardy-Weinberg expectations within size classes for the microsatellites, and found only one locus (M87) that significantly deviated from expectations and only one of the cohorts was inconsistent with predicted frequencies (size class 6, 10.0 + cm, a mixture of larger/older classes). This suggests that temporal Wahlund effects should be considered in future studies and highlights the possibility that temporal variation and genetic differentiation between sites may be transient in time (Tessier & Bernatchez 1999). Genetic structure could be modified each generation by the addition of new cohorts and the mixing of old cohorts, depending on the connectivity of sites throughout the Everglades marsh. Thus, unique genetic signatures within sites may be masked by temporal variation (Whitlock & McCauley 1990; David et al. 1997); however, this does not preclude the possible effects of spatial mixing. It is interesting to note that pooling among sites within regions or among regions did not improve the fit of the data to Hardy-Weinberg expectations.
Patterns of genetic diversity
Allozyme and microsatellite loci differed in the amount of genetic diversity they showed, and in the patterns of gene diversity and allelic richness among sites. Highly variable microsatellite loci are likely more sensitive to these measures of genetic differentiation (Hedrick 1999). Both markers showed no evidence for recent population bottlenecks, and assignment tests did not reliably assign individuals to the site or region from which they were sampled. The assignment test failed to identify a unique genetic signal for regions and sites within regions. Thus, colonization of the marsh habitat after the dry season is not likely the result of a very small number of individuals that survive through the dry season in a deep-water refuge or colonize from canals to populate the site through in situ reproduction. Rather, the data indicate that a moderate to large immigrant population of spotted sunfish probably colonizes marsh sites. Also, a recent severe drought in 1989–90 did not cause a detectable bottleneck in those marsh sites, or subsequent colonization of the marsh sites from canal sites has masked the effects of the bottleneck.
We observed no evidence for isolation by distance in either allozymes or microsatellites, conforming to Hutchison & Templeton's (1999) case II. This pattern suggests that populations of spotted sunfish are not in equilibrium with respect to gene flow and genetic drift, possibly because of a recent colonization event from a relatively homogeneous source population. This conclusion is supported by the hierarchical population structure analyses, which did not reveal population structure for allozymes or microsatellites at the regional scale. Small but significant population structure and genetic differences were detected at the local level, which were likely the result of extinction and colonization effects at local spatial scales.
Local scale extinction-colonization dynamics of fish are tightly coupled with the natural hydrologic cycles of the Everglades (Trexler et al. 2001). We illustrated that the pattern with a 20-year record of spotted sunfish density in the marshes adjacent to one of our sites used for sampling genetic variation. Approximately 6 years prior to our study, the density of spotted sunfish dropped very low (indistinguishable from zero) for 1 to 2 years (1989 and 1990, we exclude the 1988 result because of low sample size). In 1990, alligator ponds in the vicinity of this marsh-sampling site dried, in addition to the marsh, because ground water depths dropped below 60 cm from the ground surface (Fig. 3b). We believe that this pattern of population dynamics is linked to our finding of modest population structure among marsh sites, but not among canal sites for spotted sunfish. This pattern is present even though the average distance between the marsh sites (16.8 km) was less than (P = 0.104) the average distance between canal sites (30.2 km). Such a pattern of population genetic structure is expected when a group of recently colonized populations is compared to populations that are not subject to extinction and colonization turnover (Whitlock & McCauley 1990). Additionally, the low F-statistics and small difference between canal and marsh F-statistics suggests that spotted sunfish metapopulations are intermediate along a gradient between propagule pool and migrant pool patterns (McCauley et al. 1995). One might also expect this result if ‘routine’ migration has diluted the effects of a population crash on patterns of genetic variation, possibly as in our study where the crash was noted in 1990, six or more years prior to our collections.
Our data suggest that canal sites yield a homogeneous pool of colonists for marsh sites that dry routinely. We hypothesize that marsh sites are colonized by a modest number of individuals from such sources representing a subset of the total genetic diversity present there, but not diminishing genetic variation enough to be detected statistically as a ‘bottleneck’. We propose that these colonization events create a modest genetic signature in the resulting marsh populations that may be short lived. Thus, a continent-island (canal-marsh) population structure best describes spotted sunfish genetics in the Everglades.
Genetic variation of Everglades spotted sunfish may not be at equilibrium at the regional level, in addition to the local dynamics already discussed. We do not know the historical population structure of sunfishes in the Everglades, but the regional habitat structure we sampled is a relatively recent addition to the Everglades ecosystem. The Tamiami Canal, separating WCA-3A and ENP, was originally constructed in 1926, and WCA-3A was enclosed by levees in 1963, thus the present regional habitat structure has been in place for 35–80 years. A minimum generation time for spotted sunfish is 2 years, so that 18–40 generations have passed since the regional structure was superimposed on the ecosystem. If the historical population was unstructured and the canals and levees form barriers to gene flow, the contemporary populations may not be at equilibrium with these changes. Genetic differentiation by drift, even with complete isolation (which is only possible for WCA-3B), is unlikely to have occurred in this short time without strong limits to dispersal and severe population bottlenecks (e.g. Arnaud et al. 2001).
The Everglades’ natural hydropattern has been disrupted by recent water management practices. Regions of the Everglades that were historically flooded only a fraction of the year, are now flooded for prolonged periods of time, while other areas are dry much longer each year than they were historically (Fennema et al. 1994). Also, historical patterns of sheet flow (flow of water across the wetland landscape driven by rainfall events) have largely been replaced with pulsed flow tied to management needs, resulting in lacustrine (southern WCA-3A) or stagnant conditions (WCA-3B) at some locations (Light & Dineen 1994; DeAngelis et al. 1997). Hydrologic patterns may affect the efficiency of colonization and exploitation of marsh habitats that become seasonally available to aquatic species in the Everglades. Our data suggest that patterns of periodic dry-down may create recurrent mixing events for spotted sunfish and, in this way, water management decisions may shape patterns of genetic diversity of this species in the contemporary Everglades ecosystem.
Acknowledgements
We thank Walter DeLoach, Adrian Jelenzsky, Brook Shamblin, Tom Turner, and Travis Tuten who helped collect fish for this project. Tom Turner also helped with the initial analysis of allozymes and Kathleen McElroy helped with organizing the figures. We thank Bill Loftus and Ronnie Best of the US Geological Survey for supporting this project and the comments of three anonymous reviewers that improved the presentation of this work. Bill Loftus and Everglades National Park provided long-term estimates of spotted sunfish density. The work was funded by cooperative agreement CA 1445-CA09-95–0112, Subagreement no. 1, between the U.S.G.S and Florida International University.
References
Thom McElroy, Karen Kandl, and Joel Trexler are interested in the role of population dynamics in shaping population genetic structure of aquatic organisms in fluctuating environments. This work is part of our larger project on the metacommunity dynamics of aquatic organisms in the Florida Everglades. Janette Garcia assisted with microsatellite analysis in developing her senior undergraduate thesis at Florida International University.




