Bacterial diversity in maize rhizospheres: conclusions on the use of genetic profiles based on PCR-amplified partial small subunit rRNA genes in ecological studies
Abstract
A cultivation-independent approach based on polymerase chain reaction (PCR)-amplified partial small subunit rRNA genes and genetic profiling by single-strand conformation polymorphism (SSCP) was used to characterize the bacterial diversity inhabiting the rhizosphere of maize plants grown on an agricultural field. The community structures of two cultivars, a genetically engineered and a nonengineered variety, different herbicide regimes and soil tillage were compared with each other at two sampling dates. SSCP-profiles were generated with DNA from bacterial cell consortia with primers hybridizing to evolutionarily highly conserved rRNA gene regions. On silver-stained gels, each profile consisted of approx. 50 distinguishable bands. Similarity analyses of patterns recorded by digital image analyses could not detect any difference between cultivars or treatments that was greater than the variability between replicates. A total of 54 sequences recovered from different bands were identified and grouped into operational taxonomical units (OTUs). Surprisingly, only five of 40 OTUs contained sequences of both samplings. Three different bands from a profile were selected to test whether this small overlap was due to an incomplete recovery of sequences. From a faint band, two different OTUs were found when 12 clones were analysed, and from two strong bands 24 and 22 OTUs were detected from a total of 26 and 36 clones, respectively. The OTUs belonged to phylogenetically different groups of bacteria. Gene probes that were developed to target different bands of the profiles, however, indicated in Southern blot analyses that patterns between treatments, replicates and samplings, and even from two different growing seasons were highly conserved. Our study demonstrates that community profiles can consist of more sequences than detectable by staining and that gene probes in Southern blot can be a useful control to investigate the composition of microbial communities by genetic profiles.
Introduction
Most microorganisms in nature live in close relationships and communities composed of different organisms. A major objective in microbial ecology is to understand the composition, regulation and sensitivity of such communities in response to perturbation. With the introduction of polymerase chain reaction (PCR) and the increasing attention to molecular ecology it has become important to study the composition of microbial communities in natural habitats independent of cultivation techniques (Pace 1997; Head et al. 1998). Results, based mainly on the diversity of small subunit (SSU) rRNA genes, for bacteria the 16S rRNA genes, clearly indicate that most microbial communities, especially those which are associated with complex ecological habitats, e.g. soils, are composed of a very large number of different organisms (Torsvik et al. 1996). Sequence comparisons of SSU rRNA genes recovered by PCR directly from environmental material show mainly the closest cultivated relatives with less than 100% similarity, thus indicating that the majority of microorganisms have not yet been cultured and identified (Hugenholtz et al. 1998). The database of environmental rRNA gene sequences is still growing, indicating that we are still not approaching the point of saturation at which no further sequences can be found.
Clone libraries of PCR-amplified SSU rRNA genes or ribosomal RNA itself are highly useful in extending our basic understanding of the possible constituents of a microbial community (Stahl 1997) and recent publications show that functional genes may also be discovered and described with a similar strategy (Seow et al. 1997; Henne et al. 2000). The production of clone libraries, however, is time-consuming and limits the number of samples that can be analysed and compared with each other. For many ecological studies the number of samples that can be analysed may be a critical factor, because the natural variability of a community needs to be differentiated from effects that were triggered by, e.g. a changing environmental condition. In this context, genetic profiling, namely denaturing gradient gel electrophoresis (DGGE), has become a widely used method (Muyzer et al. 1998). DGGE converts the diversity of PCR-amplified products of the same size but with a different nucleotide sequence, into profiles composed of bands. The patterns of different samples, e.g. treatments, can be compared with each other at the level of similarity; in addition, the identity of the bands can be determined by DNA-sequencing. As an alternative to DGGE, the same technical strategy and objectives can be achieved by single strand conformation polymorphism (SSCP), a technique originally developed for mutation detection (Hayashi 1991) (Orita et al. 1989) that was previously adapted in our laboratory to microbial community analysis (Peters et al. 2000) (Schwieger & Tebbe 1998). Compared to DGGE, SSCP is more straightforward because it does not require gradient gels and the application of G + C-tailed primers to clamp DNA-single strands (Tebbe et al. 2001).
Even though profiling techniques, such as DGGE or SSCP, have already been used for comparative community analysis, there is still a need to characterize further the quality of information that can be gathered. Open key questions relate to the two critical steps, i.e. (1) the PCR-amplification based on primers hybridizing to evolutionarily conserved gene regions and (2) the actual diversity of products that is amplified from environmental samples (Stahl 1997; von Wintzingerode et al. 1997). Several studies have characterized the first step, e.g. the importance of the choice of primers for detecting defined phylogenetic groups, the limitation of partial sequences for the identification of organisms, or the problem of chimera or other PCR-related artifacts (Suzuki & Giovannoni 1996; Wang & Wang 1996; Schmalenberger et al. 2001). The second step relates to the interpretation of patterns: are different patterns with high similarity really composed of the same sequences and how high is the diversity of PCR-products beyond the threshold of detection by staining?
Here we report on a study in which we compared bacterial communities recovered from rhizospheres of two field grown maize (Zea mays) cultivars, one of them genetically engineered to be resistant against the herbicide Liberty (glufosinate, syn. phosphinothricin). We compared different herbicide regimes and the effect of different soil tillage practices (ploughing vs. nonploughing) on the bacterial community structure. Due to results of a previous study we suspected that the maize rhizospheres would harbour bacterial communities that were quite similar to each other (Schmalenberger & Tebbe 2002). The composition of profiles was characterized by DNA sequencing and the recovered sequences were compared to those gathered from a preceding growing season. We applied the SSCP-technique for profiling and used the highly sensitive silver staining method for band detection. In addition, to study the abundance of specific sequences in different profiles, we extended our currently available methodological repertoire for SSCP by a new protocol for Southern-blot gene probe analyses of community profiles.
Materials and methods
Field site and sampling
The field with a total size of 3.5 ha was located in Braunschweig-Völkenrode. The field was homogeneous regarding its previous agricultural history, its height, water regime and other physico-chemical factors. It was divided into three replicated areas. A replicated area consisted of 36 plots, each with an area of 125 m2. A single plot on a replicated area represented a different agricultural crop or cultivar (maize, sugar beet, wheat) or treatment (herbicide regime, ploughing). The order of plots was in a randomized block design.
In 2000, five different treatments were sampled in context of this study: the maize cultivar Bosphore (Agrevo, Frankfurt, Germany) with no herbicides or treated with conventional herbicides (Artett and Motivell, active compounds: Terbuthylazin and Nicosulphuran); a transgenic derivative of Bosphore (KX8445, transformed with the pat-gene encoding for a l-phosphinothricin, syn. glufosinate, inactivating transacetylase, Agrevo) treated with conventional herbicides or Liberty (active ingredient: l-glufosinate) and Bosphore grown in nonploughed soil treated with conventional herbicides. Sampling was carried out 7 and 35 days after the herbicide treatments, corresponding to 32 days and 60 days after seeding. The shoot heights of the plants at the first sampling date were approx. 25 cm and at the second sampling 140 cm.
Extraction of bacterial cell consortia and DNA
For each sample, a total of eight plants for the first sampling and six plants for second sampling were selected from random positions within each plot and whole plants were transported to the laboratory. Here, roots were dipped into sterile water to remove large adhering soil particles. Fine roots were cut off and used for bacterial cell extraction. For each sample, 8 g (wet weight) of fine roots were suspended in 20 mL sterile saline solution (0.85% NaCl, wt/vol) for 30 min at 4 °C in an overhead shaker (KH, Guwina-Hoffmann) at 20 r.p.m. After removal of the root material, suspensions were divided into two aliquots and each sample was centrifuged at 4100 g for 30 min at 4 °C. Supernatants were discarded and the pellets were stored at −70 °C.
Frozen bacterial cell pellets of rhizosphere extracts were resuspended in 12 mL sterile lysis buffer (0.05 m Tris-HCl, 0.01 m Na2EDTA, 0.05 m NaCl; pH 8.0). Cells were lysed by five cycles of freeze-thawing, each cycle consisting of 5 min freezing in liquid nitrogen and 5 min thawing at 65 °C. Between each step, the suspensions were vortexed for 10 s at the highest setting (VF2, IKA Labortechnik). Subsequently, samples were treated with Proteinase K (Roche) for 60 min at 65 °C in a shaking water bath. DNA was extracted by phenol–chloroform (Sambrook et al. 1989). The DNA was purified further with the Wizard DNA Purification Kit (Promega). For this purpose, 10–30 µL of DNA-solution were loaded onto each column and eluted with 40 µL of 75 °C prewarmed 10 mm Tris-HCl, pH 8.0, according to a protocol of the manufacturer. The DNA concentration in the final solution was in the range of 1–10 ng µL−1.
PCR amplification of partial SSU rRNA genes
Amplifications were processed in a final volume of 100 µL with 5 U Platinum-Taq-Polymerase (Gibco Livetech), onefold PCR-buffer, 1.5 mm MgCl2, 0.5 µm primers, 200 µm of each desoxy-nucleotide (Amersham Pharmacia Biotech) and 2 µL of purified rhizosphere extracted DNA in the thermal cycler Primus 96 (MWG-Biotech). For amplification, we selected primers Com1 and Com2 which hybridize to phylogenetically highly conserved regions of the SSU rRNA gene, corresponding to Escherichia coli position 519–537 and 907–926 (Schwieger & Tebbe 1998; Schmalenberger et al. 2001). The reverse primer was phosphorylated at the 5′ end for single-strand digestion (Schwieger & Tebbe 1998). Primers were synthesized by Gibco Life Technologies. PCR was conducted at 95 °C for 3 min, followed by 35 cycles of 1 min 95 °C, 1 min at 50 °C, 70 s at 72 °C, and a final primer extension for 5 min at 72 °C.
Genetic profiles by single strand conformation polymorphism (SSCP)
PCR-products were purified with the Qiaquick PCR purification kit (Qiagen) as recommended by the manufacturer. Half of the purified PCR product was digested with 10 U lambda-exonuclease (Amersham Pharmacia Biotech) at 37 °C for 45 min according to the single-strand community approach described previously (Schwieger & Tebbe 1998). Proteins were removed by phenol–chloroform extraction, DNA was precipitated, collected and dried (Schwieger & Tebbe 1998). Prior to SSCP, the samples were resuspended in 8 µL of 10 mm Tris-HCl, pH 8.0, and 8 µL of denaturing loading buffer (95% v/v formamide, 10 mm NaOH, 0.025% wt/vol bromophenol blue and 0.025% wt/vol xylene cyanole). Samples were incubated for 2 min at 95 °C and cooled immediately on ice.
For electrophoresis, we diluted a twofold stock solution to 0.6-fold (MDE, FMC Bioproducts) and prepared a gel of 21 cm length in 1 X TBE (Sambrook et al. 1989). A Macrophor sequencing apparatus (Amersham Pharmacia Biotech) was used for SSCP. Gels were run at 20 °C at 8 mA and 400 V for 16 h. DNA in the gels was visualized by silver staining (Bassam et al. 1991).
Extraction, reamplification and sequencing of DNA from silver-stained SSCP-profiles
Selected bands of community profiles were cut out with a sterile razor blade and the single-stranded DNA of these bands was eluted for 3 h at 37 °C and 500 r.p.m. in 50 µL ‘crush and soak’ solution (Sambrook et al. 1989). The eluted DNA was precipitated with ethanol, centrifuged and dried as previously described. The precipitated DNA was finally resuspended in 10 µL of 10 mm Tris-HCl, pH 8.0. The sequences were recovered by PCR with the Com-primers as described above in a 50-µL scale and with a total of 1.25 U of Platinum Taq polymerase (Gibco Life Technologies). PCR products were purified as previously described. Half the products were digested to obtain single stranded DNA (5 U of lambda-exonuclease, Amersham Pharmacia Biotech) to check the correct position of reamplified single bands on SSCP gels. The reamplified DNA molecules recovered from bands of community profiles were used for cloning and sequencing (Peters et al. 2000; Schmalenberger et al. 2001).
Southern blotting of SSCP gels and gene probe hybridizations
In SSCP analyses that were conducted for Southern blot analysis, both thermostatic plate and glass plate were treated with Repel Silane (Amersham Pharmacia Biotech) to allow separation of the acrylamide gel from the glass plates. For transfer of DNA, SSCP-gels were blotted onto positively charged nylon membranes (Hybond N +, Amersham Pharmacia Biotech or Biodyne plus Nylon membranes, respectively) in an electric field for 5 h with 10 mA/cm2, using the Nova Blot Unit (Amersham Pharmacia Biotech) and 0.5 × TBE (Sambrook et al. 1989). Membranes were dried at room temperature and DNA was cross-linked to the membranes with 1.5 J/cm2 in UV light at 266 nm (FluoLink).
Specific probes (Table 1) of 27–28 base length matching inside the SSU rRNA gene position 538–906 (corresponding to the numbering of E. coli) were designed with the ARB software environment (www.arb-home.de) and the oligo 6 software (MBI, Cascade). Probes were ordered at Gibco Life Technologies and labelled with the alkaline phosphatase direct labelling kit (Amerham Pharmacia Biotech) according to a protocol provided by the manufacturer.
| Probe name | SSU rRNA gene target* | No. of hits‡ | Estimated phylogenetic group | Sequence 5′−3′ |
|---|---|---|---|---|
| SPOC621 | 621–649 | 2 | CFB; Sporocytophaga§ | TCT GCA GTA TCA ATG GCA CAT CGA CAG T |
| RUNE655 | 655–683 | 5 | CFB; Runella et al. | CCA TCC ATT CCG GCA GCC TCC AAT TAT T |
| CYTJ625 | 625–652 | 8 | CFB; Bacteroides; C. johnsoniae et al. | AGT CTA ACA GTA TCA ATG GCC GTT CCA |
| BREV816 | 816–845† | 1 | α-Proteobacteria; Brevundimonas et al.§ | CAT GCC TGC CGA CAA CTA GCA CTC ATC G |
| PROC907 | 907–926 | 5858 | Eubacteria | CCG TCA ATT CCT TTG AGT TT |
- * Positions according to E. coli SSU rRNA gene sequence.
- † Base 841 does not exist at sequence 99/28.
- ‡ Number of total hits without a mismatch.
- § Determination of the phylogenetic group based on partial sequences only.
Membranes were placed into glass tubes in a mini hybridization oven (Oncor Appligene) with 20 mL of the hybridization solution according to the manufacturer (RPN3680, Amersham Pharmacia Biotech). Depending on the gene probe and annealing conditions, hybridization temperatures were optimized empirically in the range of 45–50 °C (except probe PROC907 which hybridized at 35 °C). The probe concentration was set to 10 ng/mL and the incubation time was 16 h. Washing procedures were conducted according to the manufacturer with the supplied urea containing buffer 1 and the Tris-base containing buffer 2 (RPN3680, Amersham Pharmacia Biotech). Buffer 1 (50 mL per tube) was applied for 10 min at 10 °C below hybridization temperature. The washing procedure was repeated once. Subsequently the double concentrated buffer 2 was used twice for 10 min and 15 °C (for PROC907: 5 °C) below hybridization temperature to reduce the content of probes which hybridized nonspecifically with the nylon membranes. Finally, buffer 2 was applied twice for 10 min at 30 °C (for PROC907: 25 °C). Afterwards, membranes were incubated with 30 µL cm−2 of the CDP-Star detection reagent for 5 min (Amersham Pharmacia Biotech). Membranes were placed into plastic bags and chemoluminescence was detected with Hyperfilm ECL (Amersham Pharmacia Biotech) incubated for 20 min to 18 h, depending on the expected strength of the hybridization signal, in a film cassette.
DNA sequence analysis
DNA was sequenced to obtain the base compositions in both directions and consensus sequences were generated as described elsewhere (Schmalenberger et al. 2001). For further analysis the consensus sequences were loaded into the ARB Database (http://www.arb-home.de). After alignment of the partial SSU rRNA genes, sequences were integrated with the parsimony iteractive function into an existing phylogenetic tree consisting of more than 10 000 complete SSU rRNA genes. Distance matrices were calculated with a 50% filter for each phylogenetic group.
Nucleotide sequence accession nos
The partial SSU rRNA gene sequences were deposited in GenBank under the following accession nos: AJ431194, AJ431195, AJ431276 to AJ431327 and AJ 437404 to AJ437477.
Results and discussion
Comparison of genetic profiles at the level of pattern similarity
A total of 30 SSCP patterns were generated from the bacterial cell consortia extracted from rhizospheres of maize plants of different age (see Materials and methods). Each of the SSCP patterns consisted of approx. 50 distinguishable bands and all patterns from one sampling date were highly similar (Fig. 1). Some unique bands existed in single profiles but these bands did not occur in all of three replicate samples or in samples from the same field block. Thus, an altered composition of the bacterial community in response to the genetic modification, to herbicides, to soil tillage or to heterogeneities in the field could not be detected. In addition, no significant differences were found when profiles obtained from the two different sampling dates were compared with each other by digital image analysis (data not shown).
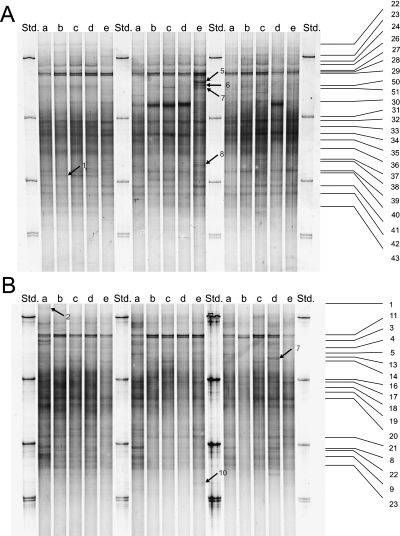
SSCP-profiles of PCR-amplified SSU rRNA partial genes of bacterial cell consortia extracted from maize rhizospheres. Samples were taken 32 days (A) and 60 days (B) after seeding with a nontransgenic (a, b, e) and a transgenic (c, d) cultivar. Plants were grown according to agricultural practice with ploughing (a, b, c, d) or without ploughing (e). Plants were not treated with herbicides (a), treated with conventional herbicides (b, c, e) or treated with glufosinate (d) (see Materials and methods). Std. indicates lanes with SSCP standard consisting from top to bottom of partial SSU rRNA genes amplified from Bacillus licheniformis, Rhizobium trifolii, Flavobacterium johnsoniae and Rhizobium radiobacter (double band). Numbered lines and arrows indicate selected bands for cloning and sequencing.
In a previous study with maize plants grown at the same field site only one year earlier, in 1999, small but significant differences in the composition of the bacterial communities in rhizospheres as a response to plant age (32 days and 70 days after seeding) could be detected (Schmalenberger & Tebbe 2002). As the same set of primers and plants of nearly the same age and from the same field were used in both studies, it is likely that plant-age dependent differences actually exist, but that these differences are close to the threshold of detection with PCR-SSCP, the chosen primer pair, and digital image analysis.
Identification of sequences contributing to the genetic profiles and indications for the size of the rRNA gene pool
In order to characterize the richness of partial rRNA gene sequences contributing to the patterns, we selected bands from different profiles at different positions for further analysis. The positions of the bands that were cloned and sequenced are shown in Fig. 1. We included bands which were consistently observed in the profiles (numbers on the right side of the panel) and those which occurred inconsistently only in single profiles. PCR-reamplification of some eluted bands, i.e. no. 28 and no. 29 from the first sampling (Fig. 1A) and no. 2 and no. 8 from the second sampling (Fig. 1B) resulted in one or two additional bands, as detected by a second SSCP-analysis (no Figure). All these bands were identified by DNA-sequencing. In total, we cloned and sequenced 54 DNA fragments from the SSCP-profiles shown in Fig. 1.
The sequences could be attributed to several phylogenetic groups (Table 2). In accordance with our own previous studies (Schmalenberger et al. 2001; Schmalenberger & Tebbe 2002) and with another study on bacterial communities from maize rhizospheres (Chelius & Triplett 2001) we found that most sequences were related to Proteobacteria from different subgroups and to members of the Cytophaga–Flavobacterium–Bacteroides (CFB) group. The bacterial species Paenibacillus polymyxa (Seldin et al. 1998; von der Weid et al. 2000) and Burkholderia cepacia (Balandreau et al. 2001) can be found as common inhabitants of maize rhizospheres. However, we could not detect rRNA sequences indicating their presence. Variability in microbial community compositions can be explained by soil factors (Miethling et al. 2000) or cultivars (Dalmastri et al. 1999), but also by methodological biases, e.g. caused by the selection of the primers that hybridize in the PCR to evolutionarily conserved regions (universal primers) of the SSU rRNA genes (Schmalenberger et al. 2001), or by varying lysis efficiencies for bacteria with different cell walls (Kuske et al. 1998).
| Phylogenetic group | Clone¶ | Closest relative/accession number | % Similarity‡ | GenBank Accession no. | |
|---|---|---|---|---|---|
| α-Proteobacteria | 2–9 | Rhizosphere soil bacterium | AJ252702 | 95.2 | AJ431326 |
| 1–34 | Mesorhizobium loti | X67230 | 99.7 | AJ431284 | |
| 2–19 | Bradyrhizobium japonicum | AB004807 | 100 | AJ431313 | |
| 1–35 | Bradyrhizobium japonicum | AB004807 | 99.5 | AJ431285 | |
| 1–36 | Bradyrhizobium japonicum | X66024 | 99.5 | AJ431290 | |
| β-Proteobacteria | 1–29b† | Uncultured beta proteobacterium | AF268290 | 99.4 | AJ431301 |
| 2–14 | Uncultured eubacterium | AJ224987 | 99.7 | AJ431310 | |
| 2–3b† | Variovorax sp. | AF214127 | 99.7 | AJ431320 | |
| 2–2 | Comamonas sp. | AF078773 | 100 | AJ431314 | |
| 2–3a† | MTBE-degrading bacterium | AF176594 | 99.4 | AJ431319 | |
| 1–26 | MTBE-degrading bacterium | AF176594 | 99.2 | AJ431296 | |
| 1–27 | Aquabacterium sp. | AF089858 | 98.6 | AJ431297 | |
| 1–60 | MTBE-degrading bacterium | AF176594 | 99.7 | AJ431327 | |
| 2–11 | Aquabacterium sp. | AF089858 | 99.2* | AJ431308 | |
| 1–28a† | Aquabacterium sp. | AF089858 | 99.2* | AJ431298 | |
| 1–28b† | Aquabacterium sp. | AF089858 | 98.3 | AJ431299 | |
| 1–40 | Telluria mixta | X65589 | 99.4 | AJ431294 | |
| β/γ-Proteobacteria | 2–7 | Lysobacter antibioticus | AB019582 | 99.4 | AJ431323 |
| 2–1 | Agricultural soil bacterium | AJ252637 | 100** | AJ431306 | |
| 1–22 | Agricultural soil bacterium | AJ252637 | 100** | AJ431277 | |
| 1–23 | Agricultural soil bacterium | AJ252637 | 99.4 | AJ431278 | |
| γ-Proteobacteria | 1–39 | Agricultural soil bacterium | AJ252658 | 84.2 | AJ431293 |
| 1–43 | Uncultured gamma proteobacterium | AB013257 | 90.1 | AJ431303 | |
| 2–5 | Pseudomonas fluorescens | AJ278814 | 99.7 | AJ431322 | |
| 1–5 | Pseudomonas fluorescens | AJ278813 | 99.7 | AJ431286 | |
| 1–6 | Pseudomonas fluorescens | AJ278813 | 100*** | AJ431287 | |
| 1–50 | Pseudomonas fluorescens | AJ278813 | 100*** | AJ431304 | |
| 1–29c† | Pseudomonas fluorescens | AJ278813 | 100*** | AJ431302 | |
| 1–7 | Pseudomonas fluorescens | AJ278813 | 99.5 | AJ431288 | |
| 2–4 | Pseudomonas thivervalensis | AF100323 | 100 | AJ431321 | |
| 1–8 | Pseudomonas putida | D85999 | 99.2 | AJ431289 | |
| δ-Proteobacteria | 1–31 | Uncultured bacterium | AF142973 | 90.6 | AJ431281 |
| 1–51 | Uncultured bacterium | AF234131 | 87.9 | AJ431305 | |
| 2–13 | Polyangium cellulosum | M94282 | 94.4 | AJ431309 | |
| Cytophaga/ | 2–8a† | Cytophaga sp. | AF260716 | 99.4 | AJ431324 |
| Flavobacterium/ | 2–8b† | Cytophaga sp. | AF260716 | 100 | AJ431325 |
| Bacteroides | 1–37 | Sphingobacterium-like sp. | X89912 | 93.0 | AJ431291 |
| 2–21 | Rhizosphere soil bacterium | AJ252690 | 92.0 | AJ431316 | |
| 1–1 | Rhizosphere soil bacterium | AJ252690 | 94.3 | AJ431276 | |
| 2–22 | Rhizosphere soil bacterium | AJ252690 | 93.1 | AJ431317 | |
| 1–32 | Uncultured bacterium | AF314419 | 97.7 | AJ431282 | |
| 2–18 | Rhizosphere soil bacterium | AJ252694 | 97.4 | AJ431312 | |
| 2–20 | Uncultured bacterium | AF283237 | 97.2 | AJ431315 | |
| 2–10 | Flexibacter sancti | M28057 | 98.6 | AJ431307 | |
| 2–23 | Flavobacterium ferrugineum | M28237 | 91.5 | AJ431318 | |
| High G + C Gram-positive | 2–16 | Uncultured bacterium | AJ277691 | 99.4 | AJ431311 |
| Low G + C Gram-positive | 1–24 | Unidentified eubacterium | AJ222833 | 100 | AJ431279 |
| Chlamydiales/ | 1–29a† | Agricultural soil bacterium | AJ252653 | 99.7 | AJ431300 |
| Verrucomicrobium | 1–41 | Uncultured Verrucomicrobia | AF211287 | 97.8 | AJ431295 |
| Planctomyces | 1–38 | Unidentified planctomycete | U70712 | 88.2 | AJ431292 |
| Holophaga | 1–33 | Eubacteria | Z9571 | 98.3 | AJ431283 |
| 1–30 | Eubacteria | Z95729 | 95.4 | AJ431280 | |
| Archaea | 2–17 | Unidentified archaeon (Crenarchaeota) | U62814 | 97.8 | AJ431194 |
| Plant organelles | 1–42 | Zea mays chloroplast | J01422 | 98.4 | AJ431195 |
- † Isolation and reamplification resulted in two distinct bands which were both sequenced.
- ‡ Similarities were calculated with 50% filters for each phylogenetic group.
- ¶ From SSCP profiles (Fig. 1A = 1_ and Fig. 1B = 2_) isolated distinct bands.
- * to ***: 100% identity with 50% filters, without filters 1–3 bases differences.
Distance matrices were calculated in order to compare the similarity of sequences isolated from our SSCP profiles. In this analysis, we included 30 additional sequences obtained from the same maize cultivars grown on the same field, only 1 year earlier (Schmalenberger & Tebbe 2002), and we placed the sequences into operational taxonomical units (OTUs). In another study, a threshold value of 1% sequence divergence was chosen for the definition of same operational taxonomical unit (OTU) and was found to be useful when SSU rRNA genes were sequenced directly from environmental DNA (Ogino et al. 2001). In our study, we increased the OTU threshold value to 1.5% because our approach incorporated the reamplification of SSCP-gel eluted bands as an additional PCR-step that might have been an additional source of sequence error.
Surprisingly, only five of 40 OTUs were detected at both sampling dates in one year and only two OTUs were found in samples from two different years. The latter OTUs included a member of the α-subgroup of Proteobacteria (AJ308248) and one was from the CFB group (AJ308288). Since all SSCP-patterns from maize rhizosphere were similar, we concluded that there was either an unintended coincidence that different sequences would make up similar patterns, or that there were more sequences contributing to one band than suggested by the sequencing of only a single clone.
How many rDNA-sequences hide behind one band?
To determine the number of possible sequences that make up one single band in an SSCP profile we selected two dominant bands, band nos 32 and 34 from the first sampling date (Fig. 1A), referred to as nos 1–32, 1–34 and one faint band (2–1) (Fig. 1B) from the second sampling date, for further analysis. The band positions are indicated in Fig. 1. The preliminary identifications of these bands are shown in Table 2. Instead of one clone, we sequenced from band 1–32 a total of 26 clones, from 1–34 a total of 36 and from band 2–1 12 clones. We found that in a total of 74 sequences 64 were different. These different sequences could be placed into 50 OTUs. From the faint band (2–1) two OTUs were recovered with one dominant OTU consisting of 11 sequences of which five were identical. The dominant band nos 1–34 consisted of sequences which could be placed into 22 OTUs. Three of these OTUs consisted of four to eight sequences but the others only of one sequence. The diversity of sequences was even higher for band 1–32: only one OTU was represented by three sequences and each of the remaining OTUs consisted only of one sequence.
Sequences from the clone libraries of the bands nos 1–32, 1–34 and 2–1 were computed with the parsimony interactive function into a tree consisting of 62 complete SSU rRNA genes shown in Fig. 2. Sequences obtained from the two strong bands were scattered over a large number of phylogenetic groups, including Proteobacteria of the α-, β-, γ- and δ-subgroups, the CFB-group, Firmicutes with a high G + C content and others. In contrast, sequences recovered from the faint band (no. 2–1) were related closely to each other and could be placed into a deep-rooting branch of the β/γ-subgroup of the Proteobacteria. The closest relatives of these sequences were mainly uncultured bacteria from agricultural soil of a potato field in Germany (AJ252661 and AJ252637; T. Lukow, pers. comm.).
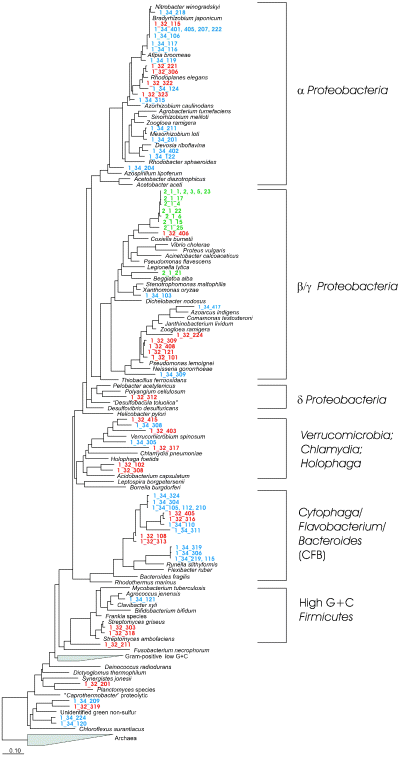
Phylogenetic tree with complete SSU rRNA genes as backbone (Phylip distance tree) and partial sequences recovered and identified from two strong bands of SSCP-profiles, band 1–32 (red) and 1–34 (blue) and one weak SSCP-band, 2–1 (green). For band position in the community profiles, see Fig. 1. The numbers of nucleotides of each partial sequence ranged from 367 to 371.
The high diversity of sequences obtained from the dominant bands was so surprising that we included an additional control experiment with cloned sequences from band 1–34. The vectors with the inserted sequences were used as a template in a PCR reaction with Com-primers. After a single strand digest of the reverse DNA strand, the PCR products were analysed by SSCP with the original community profile as a reference. In fact, all 19 clones checked with this procedure were located in the position of band 1–34 in the community profile (no Figure). This confirmed that several unrelated sequences were found within the same band.
Recent references indicate that also bands in DGGE or TGGE (temperature gradient gel electrophoresis)-community profiles may contain more than one sequence (Gomes et al. 2001; Sekiguchi et al. 2001; Smalla et al. 2001). It is probable that that this phenomenon is much more common than acknowledged at present in the literature. In fact, both methods SSCP and D/TGGE amplify rRNA genes from community-DNA and detect the diversity of PCR products on gels. However, for cloning procedures with D/TGGE, staining is normally performed with ethidium bromide or SYBR green. In particular, ethidium bromide staining is less sensitive than silver staining (Muyzer et al. 1998) and, thus, more PCR-product needs to be loaded onto a gel for pattern generation. The probability that a visible band is composed of different sequences may therefore even be higher with D/TGGE than reported here for SSCP.
Gene probes test the presence of rDNA sequences in different SSCP-profiles for comparative community analyses
Up to this point only bands isolated from single profiles were analysed. In order to characterize the abundance of specific sequences in apparently similar profiles, we decided to develop gene probes for Southern blots with community SSCP-profiles (Table 1). Three probes were selected which were specific for different members of the CFB-group. The probe SPOC621 was derived from the sequence found in band 1–32 (see Table 2), probe RUNE655 was derived from clones of band 1–34, i.e. −115, −219, −306, and −319 (see Fig. 2) and probe CYTJ625 was derived from band 2–8b (Table 2). The probe CYTJ625 also matched to a sequence (AJ308288) that was found in the preceding growing season, in 1999. In addition a probe was included that was designed to be highly specific for a sequence which we had found only in 1999 but not in 2000 (AJ308290) (Table 1). This probe, BREV816 was related to members of the α-Proteobacteria. Finally, a universal gene probe (PROC907, corresponds to primer Com2) was used as a positive control to confirm that at the end of the sequential hybridization procedures blotted DNA from the SSCP profiles was still present on the membranes (Fig. 3E).
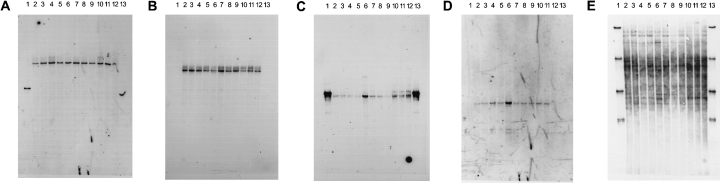
Southern blot analyses of SSCP-profiles from maize rhizospheres. Southern blots were hybridized successively to the following gene probes: SPOC621 (A), RUNE655 (B), CYTJ625 (C), BREV816 (D) and the universal probe PROC907 (E). For probe identities and specificity see Table 1, for conditions of hybridization see Materials and methods. Lanes 1 and 13, species standards (see Fig. 1), lanes 2–5, samples collected in 2000, first sampling (32 days after seeding), lanes 6–9, samples collected in 2000, second sampling (60 days) and lanes 10–12, samples collected in 1999 (35 days). Lanes 2 and 6 nontransgenic maize, no herbicides, lanes 3, 7 and 10, nontransgenic maize with conventional herbicides, lanes 4, 9 and 11, transgenic maize with conventional herbicides and lanes 5, 10 and 12, transgenic maize treated with Liberty (glufosinate containing herbicide).
Probe SPOC621 hybridized with the band from which it had been isolated and it occurred at the same positions in all SSCP patterns that were analysed, including rhizosphere samples from different treatments, from both cultivars and from two growing seasons (Fig. 3A). In addition the probe hybridized weakly with DNA above the positive band, equally for all community patterns that were analysed. The probe also hybridized with Flavobacterium johnsoniae, a member of the CFB group which was a component of our standard mixture flanking the community profiles, even though sequence comparisons suggested five mismatching positions. The positive hybridization signal can be explained by the fact that the amounts of standard DNA were several fold higher for each band than for the community SSCP profiles, amplifying the signal for a weak hybridization. In dot blot analyses with same amounts of reamplified band nos 1–32 and standard DNA, no nonspecific hybridization signals were detected (data not shown).
With probe RUNE655 four additional sequences which would hybridize without any mismatch were found in the database (ARB, see Materials and methods). Gene probes hybridized to the band position from which the sequence was isolated and, in addition, to a band in its vicinity (Fig. 3B). The latter band was at the position of band no. 18 (Fig. 1B), designated as nos 2–18, which had also been identified as a partial sequence belonging to the CFB-group (Table 2). The similarity between the gene probe and that sequence was 100%. As expected from the previous sequence information, probe CYTJ625 hybridized to profiles obtained from both growing seasons (Fig. 3C). A strong hybridization was also observed with F. johnsonae in the standard DNA, which is in accordance to the predicted specificity of that probe. Samples from the previous growing season showed an additional weakly hybridizing band. This pointed to a possible difference between profiles of both years.
Probe BREV816, which was selected to be specific for samples from the 1999 growing season, hybridized with the same intensity to samples from maize rhizospheres collected in 2000. Thus, despite the large number of sequences that were recovered in the context of this study that particular sequence was obviously missed (Fig. 3D).
Conclusions
The results of our studies demonstrate that PCR-amplified community profiles can be composed of many more sequences than detectable by gel staining. PCR with universal primers will amplify a mixture of sequences in different quantities and the resolution of detection will depend on the sensitivity of the selected staining technique. In addition, the resolution of genetic profiles will be affected by the electrophoretic conditions: migration distances or gel properties can limit the number of sequences that are detected. A single band may be composed of several and not of one sequence. Thus, varying band intensities cannot be caused only by higher concentrations of the respective gene in the template DNA (which would be ideal for community analysis), but also by PCR-biases and more than one contributor to a band.
In our study, cloning procedures recovered many more sequences than detectable by staining. It may be argued that direct cloning of PCR-products should be performed first rather than using genetic profiles at all. However, to compare the structure of different microbial communities, the calculation of similarities based on patterns is still appealing, especially if larger numbers of samples need to be analysed. Gene probes and Southern hybridization can serve as valuable control instruments for comparing microbial communities at the level of DNA-sequences with PCR-amplified genetic profiles.
Acknowledgements
We thank Karin Trescher for her excellent technical assistance. This field study was supported by our colleagues from the Institute for Crop and Grassland Science of the FAL, which we gratefully acknowledge. The work was supported by the German Ministry for Education and Research (bmb + f), Projektträger Jülich (project no. 0311740).
References
This work is part of Achim Schmalenberger's PhD thesis which was done in Christoph C. Tebbe's group. Achim Schmalenberger is now a post-doctoral research fellow at BITÖK in Bayreuth, Germany. Christoph C. Tebbe is a group leader at the Institute for Agroecology at the Federal Research Centre for Agriculture, Braunschweig. He is also a lecturer (Privat-Dozent) at the Technical University of Braunschweig. His group focuses on the use of marker genes to study environmental effects on the structure and function of microbial communities in natural habitats and biotechnological processes.




