Similar estimates of population genetic composition and sex ratio derived from carcasses and faeces of Eurasian otter Lutra lutra
Abstract
Collecting faeces is viewed as a potentially efficient way to sample elusive animals. Nonetheless, any biases in estimates of population composition associated with such sampling remain uncharacterized. The goal of this study was to compare estimates of genetic composition and sex ratio derived from Eurasian otter Lutra lutra spraints (faeces) with estimates derived from carcasses. Twenty per cent of 426 wild-collected spraints from SW England yielded composite genotypes for 7–9 microsatellites and the SRY gene. The expected number of incorrect spraint genotypes was negligible, given the proportions of allele dropout and false allele detection estimated using paired blood and spraint samples of three captive otters. Fifty-two different spraint genotypes were detected and compared with genotypes of 70 otter carcasses from the same area. Carcass and spraint genotypes did not differ significantly in mean number of alleles, mean unbiased heterozygosity or sex ratio, although statistical power to detect all but large differences in sex ratio was low. The genetic compositions of carcass and spraint genotypes were very similar according to confidence intervals of θ and two methods for assigning composite genotypes to groups. A distinct group of approximately 11 carcass and spraint genotypes was detected using the latter methods. The results suggest that spraints can yield unbiased estimates of population genetic composition and sex ratio.
Introduction
DNA typing of faeces could facilitate the characterization of elusive animals (Kohn & Wayne 1997; Taberlet et al. 1999) so it is important to assess any biases of the data that faeces yield. Many mammals use faeces for scent marking, a behaviour often characterized by large differences in individual participation (Sillero-Zubiri & Macdonald 1998; Brashares & Arcese 1999). Collections of mammalian faeces could therefore be prone to representation bias. DNA typing of faeces can yield estimates of population size, genetic composition, mating patterns and sex ratio (Kohn et al. 1995, 1999; Kohn & Wayne 1997; Taberlet et al. 1997; Ernest et al. 2000; Garnier et al. 2001; Lucchini et al. 2002), and theoretical assessments of the effects of genotyping errors and chance matches associated with noninvasive samples on estimates of population size are available (Mills et al. 2000; Waits & Leberg 2000). In contrast, any assessment of bias in estimates of population genetic composition and sex ratio derived from faeces is lacking. Material of the Eurasian otter Lutra lutra can be used to assess such biases. Spraints (faeces) of Eurasian otters are found commonly in riparian habitat (Erlinge 1968), and estimates of population genetic composition and sex ratio derived from otter carcasses (Dallas et al. 1999, 2002; Cassens et al. 2000; Pertoldi et al. 2001) are available.
Genetic characterization of wild otters yields insights about population history (Cassens et al. 2000; Pertoldi et al. 2001; Dallas et al. 2002). Nonetheless, the most accessible material for such studies derives from carcasses, which in the case of the Eurasian otter are mainly road casualties (Philcox et al. 1999; Hauer et al. 2002). Consequently, the sampling used in these studies is opportunistic and mainly limited to areas where otter habitat and roads intersect. In contrast, spraint analysis is widely applicable and is an important means to study otter distribution (Strachan & Jefferies 1996), diet (Heggberget 1993) and exposure to pollutants (Mason & Macdonald 1993). If spraints can yield unbiased samples of otter populations and a sufficiently high proportion of spraints can yield genetic data then designed sampling schemes as well as combined ecological and genetic studies of wild otters would be feasible.
Spraint deposition is unbiased by sex and age class in one coastal, island population of Eurasian otters (Kruuk 1992). Nonetheless, some conservationists believe most Eurasian otter spraints found in riparian habitat to be deposited by males (Strachan & Jefferies 1996: 79). This belief needs verification for two reasons. First, it is based on data obtained from two or three otters using a poor design (single-sex groups; Jenkins 1980; Green et al. 1984). Second, most surveys of otter distribution are based on finding spraints (e.g. Strachan & Jefferies 1996) and the belief implies that such surveys generally fail to detect the sex that contributes theoretically most to population growth.
PCR typing of microsatellites in faecal DNA extracts can yield false genotypes (Taberlet et al. 1997) so it is essential to estimate the proportion of such errors (Taberlet et al. 1999). In addition, chance matches among genotypes obtained from noninvasive samples will usually obscure the true relationship between genotypes and individuals. Chance matches are theoretically most frequent in populations having low levels of genetic polymorphism (Taberlet & Luikart 1999; Mills et al. 2000; Waits & Leberg 2000), such as otters in southern Britain (Dallas et al. 2002).
The goal of the present study was to compare estimates of population genetic composition and sex ratio derived from spraints and otter carcasses from the same area. The proportion of wild-collected spraints yielding composite genotypes, the proportion of false genotypes that spraint DNA extracts can yield, and levels of chance matches in the study population were also estimated.
Materials and methods
DNA extraction and genetic typing
Total DNA was extracted as described previously from otter blood (Dallas et al. 1999) and spraints (Parsons et al. 1999). Spraints were extracted separately using dedicated autoclaved micropipettes, and each batch of extractions included an extraction control. Each spraint was extracted once using approximately 1 mL of material. Spraint DNA extracts were stored at −70 °C.
DNA extracts were typed by the polymerase chain reaction (PCR) for nine otter microsatellites (Lut 435, 457, 615, 701, 715, 717, 832, 833 and 902) using the latest versions of primers (Dallas et al. 2002) and for the male-specific otter SRY gene (Dallas et al. 2000). Typing was performed as described previously (Dallas et al. 1999), except that primers were stored as single-use aliquots of 100 µm at −70 °C and hot-start PCR was used (Kellogg et al. 1994). Each batch of PCR assays included an extraction control, which never yielded strong or consistent PCR products. Spraint DNA extracts were first screened for yield of Lut 717 product then the extracts yielding products were typed for the 10 loci.
Estimation of proportions of PCR errors
Proportions of allele dropout and false allele detection associated with spraint DNA extracts were estimated by comparing the genotypes of DNA extracts of 65 spraints from three captive otters with genotypes of blood DNA extracts from the same otters. The SRY gene had been detected previously without error in 68 spraint DNA extracts that included the 65 samples analysed here (Dallas et al. 2000). Each DNA extract of the blood and spraint samples from captives was typed twice. The limited volumes of spraint DNA extracts remaining after initial tests prevented typing more replicates. Microsatellite genotypes of blood DNA extracts were scored as homozygous when both replicates yielded one fragment and as heterozygous when both replicates yielded the same two fragments. No other type of result was observed. The proportion of allele dropout was estimated as the proportion of all PCR assays of spraint DNA in which a fragment observed in the blood DNA of a given individual was undetected in spraint DNA from the same individual. The proportion of false allele detection was estimated as the proportion of all PCR assays of spraint DNA in which a fragment observed in spraint DNA of a given individual was undetected in the blood sample from the same individual.
Characterization of wild otters in SW England
A total of 426 spraints of wild otters were collected at monthly intervals during May 1997–July 1998 from riparian sites in two areas in SW England (Fig. 1): River Torridge (50°58′ N, 4°10′ W; 81 spraints from 21 sites) and Rivers Brue and Tone (51°1′ N, 3°12′ W; 345 spraints from 127 sites). These areas are in the eastern part of SW England, where 12 microsatellites that include the nine loci analysed here are in Hardy–Weinberg equilibrium (group ESW in Dallas et al. 2002). Not all sites were visited each month. Spraints of fresh appearance were collected mainly during the early morning. Most such spraints would have been deposited during the previous night. Such sampling maximized the proportion of wild-collected spraints that yielded genotypes (J. Dallas, unpublished). Spraints were stored before extraction for up to two months at −20 °C in plastic tubes containing 10 mL of 95% ethanol.
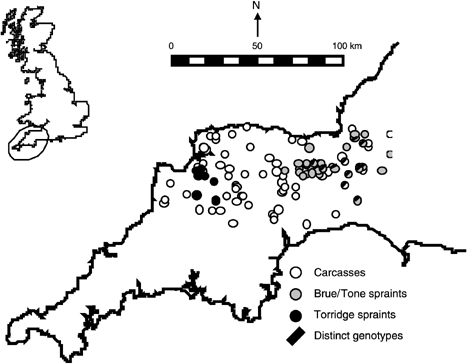
Map of SW England showing the locations of 52 different genotypes of spraints collected on the rivers Brue, Tone and Torridge, and of 70 otter carcasses collected opportunistically. Genotypes indicated as distinct were identified by assignment as described in the text.
Each DNA extract of the wild-collected spraints was typed four times for each locus to reduce errors in genotype identification (Taberlet et al. 1996). Genotypes were scored as homozygous when a single fragment was detected in all four replicates, as heterozygous when the same two fragments were detected at least twice and no other fragments of equal intensity were detected, and as unscored in all other cases. Composite genotypes of wild-collected spraints having three or more unscored microsatellites were not analysed.
Genotypes for the nine microsatellites analysed here are available for 111 otter carcasses collected during 1986–97 in SW England (Dallas et al. 2002). Seventy of these genotypes from the area of spraint sampling were chosen to represent the otter population in this area (Fig. 1). The sex of each carcass had been determined according to external mor-phology and was confirmed by PCR typing for the male-specific otter SRY gene. All composite microsatellite and SRY genotypes of carcasses were complete.
Statistical methods
Allele frequencies, mean numbers of alleles and unbiased heterozygosities were calculated using pop100gene (S. Piry & D. Bourget, Institut National de la Recherche Agronomique, http://www.ensam.inra.fr/URLB/). Values of total numbers of alleles and mean unbiased heterozygosity were tested for significant differences between groups by permutation of composite genotypes (Brookes et al. 1997).
Expected proportions of incorrect genotypes were estimated retrospectively using gemini (Valière et al. 2002), given the proportions of allele dropout and false allele detection estimated using the material from captives and the numbers of PCR replicates and threshold for allele identification used here. The allele frequencies calculated from carcass genotypes were used to simulate a population of 80 individuals. This population size was chosen because it yielded approximately the number of different spraint genotypes observed (52) in 10 simulations of drawing the total number of spraint genotypes observed (84) with replacement. In addition, the minimum number of PCR replicates needed for identification of correct genotypes was estimated by simulating two to eight replicates with a threshold of two observations for allele identification.
Probability of identity (PI) values for unrelated individuals (unbiased version) and full siblings were also calculated using gemini. Numbers of single-locus mismatches among pairs of microsatellite genotypes were calculated using match (P. Palsbøll, University of California–Berkeley, unpublished). One mismatch was counted each time one or both of the alleles at a locus differed between two genotypes.
Departures from Hardy–Weinberg and linkage equilibrium were assessed using genepop 3.3 (Raymond & Rousset 1995). Evidence for genetic differentiation between groups was assessed by two methods available in fstat 2.9.3.2 (Goudet 1995). First, 95% confidence intervals of multilocus values of θ, an unbiased estimator of Wright's FST (Weir & Cockerham 1984) were estimated by bootstrapping single-locus θ-values (15 000 replicates). Second, the significance of the observed value of the multilocus G-test statistic was assessed by comparison with the distribution of values yielded by permutation (10 000 replicates) of alleles among genotypes.
Composite microsatellite genotypes were assigned to groups using structure (Pritchard et al. 2000) and geneclass (Cornuet et al. 1999). structure was used to estimate the proportion of each genotype assigned to each of two postulated groups (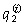 ). The number of replications used for this Bayesian analysis was 20 000 for the burn-in period and 100 000 for the MCMC sampling period, and the analysis was repeated five times. As recommended, no information on the prior identity of genotypes was used. Each genotype yielded two
). The number of replications used for this Bayesian analysis was 20 000 for the burn-in period and 100 000 for the MCMC sampling period, and the analysis was repeated five times. As recommended, no information on the prior identity of genotypes was used. Each genotype yielded two 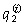 values, one of which provided all the information for that genotype from the analysis. geneclass was used to detect genotypes that were significantly unlikely to belong to their nominal group according to their expected frequency in that group. The Bayesian options for the estimation of allele frequencies and the calculation of expected frequencies of genotypes were used.
values, one of which provided all the information for that genotype from the analysis. geneclass was used to detect genotypes that were significantly unlikely to belong to their nominal group according to their expected frequency in that group. The Bayesian options for the estimation of allele frequencies and the calculation of expected frequencies of genotypes were used.
Differences in sex ratio were tested for significance by Fisher's exact tests using the program struc supplied with genepop 3.3. P-values from multiple tests were assessed for significance by sequential Bonferroni correction.
Results
The proportions of PCR errors in typing spraint DNA extracts for microsatellites, estimated by comparing genotypes of nine microsatellites from three blood and 65 spraint samples of three captive otters, were 0.021 for allele dropout and 0.012 for false alleles. These values are averages for the nine microsatellites, and the single-locus values were similar to each other.
The proportion of otters in SW England whose composite microsatellite genotypes were likely to match was assessed by calculating theoretical probability of identity (PI) values and by empirical observation of the numbers of composite genotypes that matched among different carcasses and the numbers of mismatching loci in cases where composite genotypes mismatched. PI values based on the microsatellite allele frequencies in carcasses (Table 1) in 10 simulated populations each containing 80 individuals were 1.5–2.3 × 10−5 for unrelated individuals and 5.6–6.8 × 10−3 for full siblings. Only two composite genotypes matched (while mismatching for sex) and most (97%) pairs of composite genotypes mismatched at 4–9 loci. Thus, chance matches among composite microsatellite genotypes in the study population would probably have occurred only for some close relatives.
| Locus | Allele (bp) | Carcasses | Spraints |
|---|---|---|---|
| Lut435 | 127 | 0.33 | 0.42 |
| 139 | 0.67 | 0.56 | |
| 143 | 0.02 | ||
| Lut457 | 139 | 0.35 | 0.34 |
| 141 | 0.01 | 0.03 | |
| 143 | 0.16 | 0.15 | |
| 145 | 0.01 | 0.07 | |
| 147 | 0.46 | 0.37 | |
| 149 | 0.01 | ||
| 153 | 0.04 | ||
| Lut615 | 135 | 0.03 | |
| 137 | 0.55 | 0.53 | |
| 139 | 0.43 | 0.35 | |
| 141 | 0.01 | 0.09 | |
| 143 | 0.01 | ||
| Lut701 | 196 | 0.01 | 0.04 |
| 200 | 0.01 | 0.05 | |
| 204 | 0.74 | 0.77 | |
| 208 | 0.24 | 0.14 | |
| Lut715 | 184 | 0.05 | |
| 200 | 0.04 | ||
| 204 | 0.42 | 0.48 | |
| 212 | 0.44 | 0.37 | |
| 216 | 0.14 | 0.06 | |
| Lut717 | 175 | 0.20 | 0.13 |
| 183 | 0.02 | ||
| 187 | 0.03 | ||
| 191 | 0.78 | 0.74 | |
| 195 | 0.02 | 0.04 | |
| 199 | 0.04 | ||
| Lut832 | 182 | 0.01 | 0.02 |
| 186 | 0.21 | 0.22 | |
| 190 | 0.13 | 0.11 | |
| 194 | 0.61 | 0.59 | |
| 198 | 0.04 | 0.07 | |
| Lut833 | 154 | 0.60 | 0.58 |
| 158 | 0.08 | 0.08 | |
| 162 | 0.01 | 0.02 | |
| 166 | 0.31 | 0.27 | |
| 170 | 0.03 | ||
| 174 | 0.02 | ||
| Lut902 | 148 | 0.58 | 0.57 |
| 152 | 0.01 | 0.03 | |
| 160 | 0.03 | ||
| 164 | 0.28 | 0.35 | |
| 168 | 0.10 | 0.05 |
Eighty-four of the 426 wild-collected spraints (20%) yielded genotypes for 7–9 microsatellites and for SRY. Twelve of the Brue/Tone genotypes had missing data for one microsatellite and six had missing data for two microsatellites. Ten of the 15 Torridge spraint genotypes represented different individuals according to pairwise mismatches at three to nine microsatellites and SRY, and 42 of the 69 Brue/Tone spraint genotypes represented different individuals according to pairwise mismatches at 1–9 microsatellites and SRY. No identical genotypes occurred in both the Torridge and Brue/Tone areas. Each composite genotype occurred in an average of 1.5 (Torridge) or 1.6 (Brue/Tone) spraints.
The expected number of false genotypes of wild-collected spraints was estimated retrospectively by simulation. Eighty composite microsatellite genotypes were created using the carcass allele frequencies (Table 1). The total number of spraint genotypes observed (84) was drawn from this population with replacement. PCR typing of each genotype was simulated two to eight times with proportions of allele dropout and false allele identification as estimated using the material from captives. Consensus genotypes were determined using a threshold for allele identification of two observations. This sequence was repeated 10 times. The proportion of correct genotypes was approximately 0.85 for one and two replicates and over 0.99 for three to eight replicates. Thus, the expected number of false spraint genotypes for the number of replicates used here (four) was negligible.
The carcass and spraint genotypes were compared in terms of genetic diversity, genetic composition and sex ratio. Neither group showed significant departure from Hardy–Weinberg equilibrium of microsatellite genotypes (all P-values nonsignificant after Bonferroni correction). No cases of significant departure from pairwise linkage equilibrium were found for carcasses. Only five cases of significant pairwise linkage disequilibrium that were spread over five loci were found for spraints. Carcasses and spraints showed no significant differences in values of mean numbers of alleles and mean unbiased heterozygosity for the nine microsatellites (Table 2). The mean value of θ between carcasses and spraints was nearly zero, and the 95% confidence interval of θ overlapped zero (Table 2). Nonetheless, the permutation test detected a highly significant difference in genetic composition between carcasses and spraints (Table 2).
| Carcasses | Spraints | |
|---|---|---|
| N | 70 | 52 |
| Hea | 0.52 | 0.56 NS |
| nAb | 4.00 | 4.78 NS |
| θc | 0.002 | |
| 95%d CI of θ | −0.002–0.007 | |
| Proportione | 0.0001 | |
| M:Ff | 41:29 | 31:21 NS |
- N, sample size of individuals. Values are means of aunbiased heterozygosity and bnumber of alleles for nine microsatellite loci, cmeans and d95% confidence intervals of θ for comparisons between carcasses and spraints, and ethe proportion of 10 000 permutations yielding a larger value of the G statistic than the observed value for a comparison between carcasses and spraints. Further values are fmale:female ratios. NS indicates that the value or ratio did not differ significantly between carcasses and spraints.
The presence of a genetically distinct group in the carcasses and spraints could have yielded the latter result. This possibility was assessed by assignment of microsatellite genotypes to groups (see Materials and methods). According to structure analyses, the distribution of the proportion of each carcass and spraint genotype assigned to one of two postulated groups (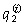 ) was bimodal and extreme. Eleven genotypes yielded low (0.01–0.10)
) was bimodal and extreme. Eleven genotypes yielded low (0.01–0.10) 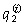 values, two yielded intermediate (0.19 and 0.48) values and 109 yielded high (0.88–1.00) values. The latter group contained 67 of the 70 carcasses and 42 of the 52 spraints. Five repeated analyses yielded very similar
values, two yielded intermediate (0.19 and 0.48) values and 109 yielded high (0.88–1.00) values. The latter group contained 67 of the 70 carcasses and 42 of the 52 spraints. Five repeated analyses yielded very similar 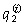 values for the same genotypes. According to geneclass analyses, four carcass genotypes had low probabilities of belonging to carcasses, and seven spraint genotypes had low probabilities of belonging to spraints. The identities of the 11 genotypes categorized as distinct in both analyses were identical, and their locations are shown in Fig. 1.
values for the same genotypes. According to geneclass analyses, four carcass genotypes had low probabilities of belonging to carcasses, and seven spraint genotypes had low probabilities of belonging to spraints. The identities of the 11 genotypes categorized as distinct in both analyses were identical, and their locations are shown in Fig. 1.
The effect of removing the distinct group of 11 genotypes on the genetic differentiation between carcasses and spraints previously identified by permutation tests was assessed. Their removal increased the proportion of permuted datasets having larger values of G than the observed value from 0.0001 to 0.015. Thus, the genetic differentiation between carcasses and spraints identified by permutation appeared to result mainly from the presence of 11 genetically distinct carcass and spraint genotypes.
The carcass and spraint genotypes did not differ significantly in sex ratio (Table 2). The data had low power for such a comparison, however. No configurations of spraints ranging from 40 males:12 females (77%M:23%F) to 21 males:31 females (40%M:60%F) were significantly different from the sex ratio of the 70 carcasses (Fisher's exact tests, P > 0.05).
Discussion
This study found that carcasses and spraints of Eurasian otters from riparian habitat yielded similar estimates of population genetic composition and sex ratio. These results imply that spraint collections could be unbiased with respect to these measures, and that female otters can deposit spraints found in riparian habitat in the same proportion as females occur in carcasses. The sample sizes had low power to detect all but extreme differences in sex ratio. Nonetheless, sex identification using faeces could yield estimates of the minimum numbers of each sex present, provided faeces were also identified to individuals. The proportion of wild-collected spraints yielding genotypes was low, implying that to characterize wild otters genetically using spraints would require large efforts in field sampling and DNA typing.
The wild-collected spraints could have yielded incorrect genotypes owing to PCR typing errors, and chance matches among the genotypes of different individuals could have occurred. The effect of PCR typing errors could have been underestimated for wild-collected spraints if the average period from their deposition to collection was much longer than the period for the spraints from captives. The effect of chance matches could also have been underestimated because observed matches among the genotypes of close relatives can be up to 1000-fold more frequent than the product rule predicts (Donnelly 1995; Taberlet & Luikart 1999; Waits et al. 2001). Nonetheless, the analyses of expected numbers of incorrect genotypes, probabilities of identity and observed mismatches among composite genotypes all suggested that any incorrect or misallocated genotypes of wild-collected spraints were too rare to change the main conclusions of this study.
The origin of the distinct group of 11 genotypes detected by assignment was unlikely to be PCR typing error because the group included carcasses as well as spraints. The distinct genotypes were located in the eastern part of the study area (Fig. 1), and could represent introgression from captive-bred individuals that were released on the river Itchen, which is approximately 120 km east of the Brue/Tone sampling area. The release was carried out during August and September 1993 (G. Roberts, pers. comm.), which is approximately halfway through the period represented by carcasses analysed here. Thus, any effect of introgression on genetic composition is expected to be more evident from recently collected spraints than from the carcasses.
Both genetic composition and sex ratio in carcasses and spraints were similar, especially after the removal of the distinct group of genotypes, implying that spraints could yield unbiased estimates of these measures for otter populations that are hard to sample by other means. The carcasses and spraints could, however, have been equally biased with respect to both measures. The extent to which carcasses that are mainly road kill are intrinsically biased with respect to the true genetic composition and sex ratio of otter populations is unclear. The carcass sex ratio in the present study and the sex ratio in Eurasian otter carcasses from riparian habitat in eastern Germany (Hauer et al. 2002) were very similar, implying either that collections of otter carcasses are unbiased with respect to sex ratio or that any bias is common to most such collections.
The proportion of DNA extracts of wild-collected spraints that yielded a genotype in this study was much lower than found in the first study of DNA typing of otter spraints (Hansen & Jacobsen 1999), and is one of the lowest reported for mammalian faeces (Kohn et al. 1995, 1999; Kohn & Wayne 1997; Taberlet et al. 1997; Ernest et al. 2000; Garnier et al. 2002; Lucchini et al. 2002). The failure of most spraint DNA extracts to yield PCR products was due probably to DNA degradation, not PCR inhibition, because several tests in which spraint DNA extracts yielding no PCR product were added to positive control DNA samples did not abolish PCR yield (J. Dallas, unpublished). The higher proportion of spraints yielding single-locus genotypes for SRY than for the microsatellites could be due to the fragment size of SRY being smaller (70 bp) that the sizes of the microsatellites (127–216 bp). The proportion of extracts of bear faeces in which 246–700 bp fragments of mitochondrial DNA are detectable by PCR is related inversely to fragment size (Wasser et al. 1997). Clearly, if a high proportion of spraint DNA extracts are unlikely to yield reliable genotypes then prescreening extracts by PCR detection of one locus will reduce wasted typing effort. We recommend that in similar studies the locus that is detectable in the highest proportion of extracts be identified in a pilot study and then used to prescreen further extracts.
The proportion of spraints encountered that were collected for this study was 0.1–0.5 (K. Coxon, unpublished) so the proportion of spraints encountered that yielded genotypes was 0.02–0.10. Such low proportions imply that to use spraints for genetic characterization of otters will be inefficient in most circumstances and hardly feasible in recently recolonized areas, where spraint density is typically low (Strachan & Jefferies 1996). We recommend that conservationists who need to know whether spraint DNA typing would be worthwhile should calculate the minimum yield of data given the results found here.
The degree of representation bias associated with animal faeces could differ greatly between species, populations or different times of sampling the same population owing to differences or changes in scent marking behaviour. In the Eurasian otter, for example, rates of spraint deposition within local areas of riparian (Erlinge 1968; Kruuk et al. 1986) and coastal (Kruuk 1992) habitat can be several-fold higher in winter than in summer. Further studies involving more species and ecological contexts are needed for more reliable indications of representation bias associated with animal faeces.
Biases in genetic data associated with animal faeces would presumably be most evident at small spatial and short temporal scales. In this study, the spatial scale was fivefold lower for spraints than for carcasses, and the temporal scale 12-fold lower. Nonetheless, the finding that only 0.02–0.10 of spraints encountered yielded genotypes implied that the spatial and temporal scales of spraint sampling could have been 10–50-fold higher than achievable if spraint availability were the only constraint. Systems characterized by much higher yields of genetic data than otter spraints are therefore needed for a more rigorous assessment of biases associated with collections of animal faeces.
Acknowledgements
We thank Jim Conroy, Don Jefferies, Chris Mason, Martin Rule and Vic Simpson for making available or supplying L. lutra tissue samples and associated data, Mary Rose Lane of the Devon Wildlife Trust, James Williams and several other members of the Somerset Otter Group for collection of otter spraints, Klaus Koepfli for providing the DNA sequence of locus 902 prior to publication and Jim Mallet and Per Palsbøl for making their unpublished programs available. The University of Aberdeen, the Natural Environment Research Council and the Environment Agency supported this work.
References
John Dallas, Freda Marshall, Stuart Piertney and Paul Racey are in the NERC Molecular Genetics in Ecology Initiative, whose purpose is to answer ecological questions using molecular analyses. David Carss and Phil Bacon are in the population ecology section of CEH-Banchory, whose purpose is to resolve ecological conflicts. Karen Coxon is working for a PhD at the University of Exeter, supervised by Paul Chanin, and Tim Sykes works on a variety of conservation-related issues as an employee of the Environment Agency.




