Haplotype (mtDNA) diversity of brown trout Salmo trutta in tributaries of the Austrian Danube: massive introgression of Atlantic basin fish — by man or nature?
Abstract
Mitochondrial haplotype diversity in 27 populations of brown trout, Salmo trutta L., in Austria was investigated by sequencing the 5′ end of the mitochondrial DNA (mtDNA) control region. Although all populations are within the Danube drainage, 44% of all individuals carried Atlantic basin haplotypes. It is argued that the presence of these haplotypes in Austria primarily reflects introgression stemming from the stocking of hatchery-reared fish. However, several lines of evidence suggest that some natural colonization from Atlantic lineages may have contributed to the present haplotype diversity. Nonetheless, the more diverse Danubian clade is represented by regionally distinct haplotype diversity that should be protected from the continued introduction of domesticated strains of exogenous fish.
Introduction
Screening of genetic variation in brown trout, Salmo trutta L., has been extensively applied towards evaluating introgression of hatchery strains into wild populations (Hansen & Loeschcke 1994; Arias et al. 1995; Largiadér & Scholl 1995; García-Marín et al. 1999a). Genetic markers revealing distinct geographical patterns have also been used to infer the presence of glacial refugia and postglacial gene flow (Bernatchez et al. 1992; García-Marín et al. 1999b; Weiss et al. 2000).
Although brown trout is the most popular sport fish in Austria, the country remains one of the few regions where no systematic studies of genetic variation have been made. Austrian rivers drain almost exclusively into the Danube basin. The ‘Danubian clade’ or ‘grouping’ for brown trout was first described in Bernatchez et al. (1992) based on mitochondrial DNA (mtDNA) control region sequence variation. This study included five individuals from an Austrian river (the Gurk) but all carried the common Atlantic clade (At1) haplotype. Presumably on this basis, the authors speculated that perhaps the Atlantic clade had an ancient origin in this region, but stocking of hatchery fish was not ruled out as the cause. We note that the Gurk River receives obligatory introductions of hatchery-reared fish.
Recent experimental studies have demonstrated the poor performance of hatchery fish in Austrian streams as well as negative demographic effects on resident populations (Weiss & Kummer 1998; Weiss & Schmutz 1999a,b). There is growing interest in adapting current management practices to a more conservation-orientated approach, and stocking is seen as a threat to the genetic integrity of wild populations. This study provides the first broad-scale screening of mtDNA variation in brown trout in Austria and uses this genetic characterization to address the question of the origin of the Atlantic lineage in the upper Danube. These aims are deemed important to those wishing to formulate a conservation strategy for brown trout fisheries in Austria and should improve our understanding of brown trout phylogeography in central Europe.
Materials and methods
Brown trout (n = 600) were collected from 27 populations, including three hatcheries. Due to the fragmented and privatized nature of most Austrian fisheries and rearing facilities, as well as the long tradition of stocking, it is impossible to obtain objective or useful information concerning what has been stocked historically. It is additionally questionable what use such information would be in evaluating broad-scale patterns of genetic diversity, as annual survival of hatchery-reared brown trout in Austria is low and variable (1–19%) (Weiss & Schmutz 1999a), their movement is unpredictable (Weiss & Kummer 1998 and citations therein) and the genetic history of stocking material is unknown. Nonetheless, our aim was to sample natural populations, to the degree possible, and thus we chose river stretches which, according to the party presently holding fishing rights, were not recently (1–5 years) stocked or currently open to fishing (and thus not subject to obligatory stocking); multiple year classes including juveniles were included to minimize or prevent sampling newly released fish.
Whole genomic DNA was isolated from ethanol-preserved tissue with a high salt-extraction technique (Miller et al. 1988) and the complete mtDNA control region was amplified with primers H20 and L19 using conditions described in Bernatchez et al. (1992). Polymerase chain reaction (PCR) product was purified, quantified, and sequenced on an ABI-377 automated sequencer with 6% polyacrylamide gels following procedures outlined in Weiss et al. (2000), including double-stranded sequencing of at least two individuals of each haplotype and replicated DNA extractions, PCR and sequencing of rare haplotypes. All sequences were aligned by hand using the sequence navigator software. A few populations were first screened with restriction enzymes (TseI and/or AseI) which distinguish Atlantic from Danubian haplotypes. This protocol was abandoned, however, after other Atlantic-clade haplotypes were found on the Iberian Peninsula (Weiss et al. 2000) and we hypothesized that similar informative variants might occur in central Europe.
Analysis
Genetic variation within each population was quantified by haplotype diversity (h) and nucleotide diversity (π; Nei 1987). Haplotype diversity within and among populations was evaluated using an analysis of molecular variance (amova; Excoffier et al. 1992) in the program arlequin 1.1 (Schneider et al. 1997). Natural populations were grouped according to hydrological or physiogeographic regions (Fig. 1) and a series of hierarchical amova models was used to infer the historical causes for present geographical patterns. Samples were grouped by: (i) previously glaciated vs. unglaciated regions (van Husen 1987); (ii) rivers north and south of the Danube; (iii) rivers on northern vs. southern slopes of the Alps; and (iv) six physiogeogrpahic regions (essentially drainage basins). To infer the potentially differing historical processes responsible for the spread of each clade, the analysis of each model was repeated using Danubian-clade haplotypes only. Pairwise difference matrices were used as a distance measure in all models, and significance was determined by permuting haplotypes (10 000 times) across populations. The significance of population diversity indices (h and π) was tested across dichotomous geographical categories using Student’s t-tests.
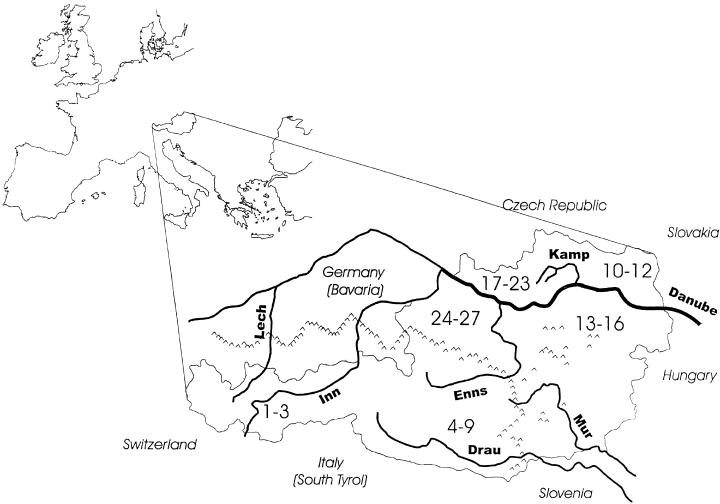
An overview of the Austrian section of the Danube and some of the major tributary systems sampled in this study. The Mur River meets the Drau before it reaches the Danube at river kilometer 1367 in Croatia. Small symbols approximate the forward edge of glacial tongues (about 20 000 years bp) according to the 1 : 500 000 map of van Husen (1987). Numbers in bold refer to populations and are grouped by the regions used in one amova model and listed in Table 1.
Results
Twelve haplotypes (GenBank accession nos AF321990–AF322001) were resolved from 516 sequences and an additional 84 individuals were assigned to the Atlantic clade based on restriction enzyme digests. The majority of individuals overall (54%), and 64% of the natural populations were unambiguously assigned to the Danubian grouping (analysis not shown). Except for one brook trout, Salvelinus fontinalis haplotype, and a marbled trout, Salmo trutta marmoratus, all remaining individuals (44%, n = 261) carried Atlantic-clade haplotypes. Among these, only four of the sequenced individuals revealed a haplotype other than the common north-Atlantic variant (At1). Therefore, the remaining 84 individuals were assigned, for convenience, to the At1 haplotype. Thus, the At1 haplotype was the most frequent (44%) overall; 35% in natural populations and 89% in hatchery stocks (Table 1). At1 occurred in all riverine populations (1–79%) but was absent in the two Alpine lakes. According to historical records these lakes were originally free of fish, being stocked in the nineteenth century (Anrasersee) or during the ruling period of Maximilian I (1486–1519) (Gossenkoellersee).
| Population | Haplotype At1 | At10 | At11 | Da1 | Da2 | Da3 | Da9 | Da22 | Da23 | Da24 | Ma2 | * | Total | % At1 | h | π | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The Tyrol | |||||||||||||||||
| Hornbach | 1 | 12(4) | 0 | 0 | 11 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 40% | 0.674 | 0.007 |
| Gossenkoellersee | 2 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0% | 0 | 0 |
| Hatchery | 3 | 22(16) | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 73% | 0.405 | 0.005 |
| Total (or mean) | 34(20) | 0 | 0 | 36 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 77 | 44% | 0.359 | 0.004 | |
| Styria & Carinthia & E. Tyrol | |||||||||||||||||
| Faisternitzbach | 4 | 6(5) | 0 | 0 | 1 | 15 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 23 | 26% | 0.526 | 0.006 |
| Panikbach | 5 | 5(3) | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32 | 16% | 0.272 | 0.004 |
| Drau | 6 | 5 | 0 | 0 | 20 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 26 | 19% | 0.385 | 0.004 |
| Kristeinbach | 7 | 7 | 0 | 0 | 8 | 0 | 0 | 8 | 0 | 0 | 0 | 9 | 2 | 34 | 21% | 0.772 | 0.012 |
| Anrasersee | 8 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0% | 0 | 0 |
| Hatchery | 9 | 28(20) | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 90% | 0.181 | 0.002 |
| Total (or mean) | 51(28) | 0 | 0 | 78 | 18 | 0 | 9 | 1 | 0 | 0 | 9 | 2 | 168 | 30% | 0.356 | 0.005 | |
| Lower Austria (North of the Danube) | |||||||||||||||||
| Kamp (Rosenburg) | 10 | 16(15) | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 73% | 0.455 | 0.006 |
| Kamp (Schönbach) | 11 | 8 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 8 | 0 | 0 | 0 | 21 | 38% | 0.714 | 0.009 |
| Lohnbach | 12 | 2 | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 6 | 0 | 0 | 0 | 14 | 14% | 0.758 | 0.007 |
| Total (or mean) | 26(15) | 0 | 0 | 7 | 0 | 7 | 2 | 1 | 14 | 0 | 0 | 0 | 57 | 46% | 0.642 | 0.007 | |
| (South of the Danube) | |||||||||||||||||
| Schwarzenberg | 13 | 2 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 25% | 0.607 | 0.006 |
| Adlitzbach | 14 | 5 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 71% | 0.476 | 0.006 |
| Piesting(Zellenbach) | 15 | 23(17) | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 79% | 0.360 | 0.005 |
| Lunzerseebach | 16 | 3 | 0 | 0 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 25% | 0.621 | 0.006 |
| Total (or mean) | 33(17) | 0 | 0 | 14 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 | 59% | 0.516 | 0.006 | |
| Upper Austria & Salzburg (North of the Danube) | |||||||||||||||||
| Kleine Mühl | 17 | 8 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 42% | 0.515 | 0.007 |
| Kollerschlägerbach | 18 | 13 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 65% | 0.511 | 0.006 |
| Sauedterbach | 19 | 7 | 0 | 0 | 11 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 35% | 0.600 | 0.007 |
| Viehbach | 20 | 15 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 20 | 75% | 0.416 | 0.005 |
| Hummelmühlbach | 21 | 2 | 1 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 11% | 0.292 | 0.004 |
| Flanitz | 22 | 12 | 0 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 60% | 0.568 | 0.006 |
| Waldaist | 23 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 18 | 72% | 0.425 | 0.006 |
| Total (or mean) | 70 | 1 | 2 | 54 | 3 | 0 | 0 | 1 | 0 | 5 | 0 | 0 | 136 | 51% | 0.475 | 0.006 | |
| (South of the Danube) | |||||||||||||||||
| Weisenbach | 24 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 17 | 41% | 0.515 | 0.007 |
| Haslingbach | 25 | 4(4) | 0 | 0 | 3 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 18 | 22% | 0.582 | 0.006 |
| Blühnbach | 26 | 1 | 1 | 0 | 15 | 15 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 36 | 3% | 0.657 | 0.004 |
| Hatchery | 27 | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 100% | 0 | 0 |
| Total (or mean) | 47(4) | 1 | 0 | 18 | 15 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 106 | 54% | 0.438 | 0.004 | |
| Total | 261(84) | 2 | 2 | 207 | 52 | 7 | 11 | 28 | 14 | 5 | 9 | 2 | 600 | 44% | 0.455 | 0.005 | |
| % of total | 44% | < 1% | < 1% | 35% | 9% | 2% | 5% | 2% | 1% | 2% | < 1% | < 1% |
The mean frequency of At1 was significantly higher in unglaciated (45%) vs. glaciated regions (24%) (t = −2.093, d.f. = 22, P = 0.048) as well as on northern (42%) vs. southern (16%) slopes of the Alps (t = 3.408, P = 0.003). This trend held for populations north (46%) vs. south (32%) of the Danube but was not statistically significant. Genetic diversity (h and π) was not significantly different in these dichotomous comparisons but was heterogeneous across regions, with populations in Lower Austria, north of the Danube, displaying the highest values (h = 0.642, π = 0.007; Table 1).
Of seven Danubian haplotypes found, four (Da1, Da2, Da3 and Da9) were previously described (Bernatchez et al. 1992; Bernatchez & Osinov 1995; Osinov & Bernatchez 1996). Da1 was the most frequent (35%) and broadly distributed (22 of 27 populations) whereas the other three occurred infrequently (1–9%) and in restricted geographical areas.
The within-population variance component of the amova (ΦST) was highly significant in all models (Table 2). The component (ΦSC) representing variance among populations within groups was also significant, with negligible variance among groups (ΦCT) (Table 2). When considering only Danubian haplotypes, ΦSC values were considerably higher in the three large-scale structuring models, with corresponding reductions in ΦST. The fourth (regional) model displayed the highest ΦCT value (0.1924) of the analysis (Table 2).
| N/S Danube | N/S Alps | Glacial | Regions | |||||
|---|---|---|---|---|---|---|---|---|
| Structure | All | Da only | All | Da only | All | Da only | All | Da only |
| ΦST | 0.3037** (70) | 0.5542** (44) | 0.3048** (70) | 0.5429** (45) | 0.3104** (69) | 0.5455** (45) | 0.2894** (71) | 0.5674** (43) |
| ΦSC | 0.2558** (24) | 0.5525** (55) | 0.2622** (23) | 0.5620** (55) | 0.2462** (23) | 0.618** (58) | 0.2295** (21) | 0.4644** (38) |
| ΦCT | 0.0643* (06) | 0.0039 (< 1) | 0.0577 (08) | −0.0435 (< 1) | 0.0852* (09) | −0.0371 (–) | 0.0787 (08) | 0.1924* (19) |
- ΦST, within-population variance; ΦSC, among-population, within-groups variance; ΦCT, among-groups variance.
- * P < 0.05;
- ** P < 0.001.
- N/S Danube = populations north (n = 10) vs. south (n = 14) of the Danube; N/S Alps = populations north (n = 19) vs. south (n = 5) of the Alps; Glacial = populations located in previously glaciated (n = 9) vs. unglaciated (n = 15) regions; and Regions = the six physiogeographic regions as outlined in Table 1. Shown are the phi-statistics and in parentheses the percentage variation explained within each model. For each model, two analyses were performed; one including all brown trout haplotypes in the data set, and one for Danubian-clade haplotypes only.
Discussion
The high frequency of At1 in Austria is not surprising considering that it is found at high frequencies in hatchery populations (73, 90 and 100%), that stocking is obligatory in waters open to angling and presuming that At1 predominates in commercially available hatchery strains. While mtDNA can only indirectly serve as an indicator of introgression, we assume introgression as our sampling protocol excluded or at least minimized sampling hatchery fish. Furthermore, there is little reason to suppose mating barriers or strong assortive mating between these lineages as introgression has been demonstrated in the uppermost drainage (Largiadér & Scholl 1995; Riffle et al. 1995) and interbreeding of hatchery and wild fish of diverse lineages has been repeatedly demonstrated (Taggart & Ferguson 1986; Largiadér & Scholl 1996; Poteaux et al. 1998). Thus, the question is not whether introgression between Atlantic and Danubian lineages exists but rather its geographical extent and whether we can distinguish between introgression occurring in recent decades from that which may have occurred in the ancient past. We suggest that this question can only be approached by evaluating recombination at one or more well-described nuclear markers, or through phylogeographic inference. Since no adequate markers have been described for the former, we chose the latter approach to evaluate the likelihood of natural and/or anthropogenically caused colonization.
Distribution of Danubian-clade haplotypes
The Danubian clade in the upper Danube lacks large-scale geographical structure (i.e. north/south) as only the regional amova model shows significant among-group variance (ΦCT; Table 2), resulting from the occurrence of rare haplotypes (Da3, Da9 and Da23) in the Kamp drainage. Palaeohydrological evidence describes a connection between the Kamp/Danube/March river region and a system of inland lakes that connected the Vienna basin, the Hungarian lowlands and the Black–Caspian–Aral sea region in the mid-to-late Pliocene (Fink 1966). Such a system would have provided an avenue for colonization, or harboured the ancestral pool of Danubian haplotypes which is now vicariantly distributed.
Elsewhere north of the Danube, one population (Waldaist) yielded a private haplotype (Da24) and except for Da22, which shows high frequencies in two proximate populations in Upper Austria (south of the Danube), the more frequently occurring Danubian haplotypes are broadly distributed and show little geographical pattern. Thus, this clade reveals both distinct patterns of regional colonization marked by some haplotypes as well as broad-scale homogeneity, presumably the result of the postglacial spread of the most common haplotypes.
Distribution, spread and origin of Atlantic-clade haplotypes
The significantly higher frequency of Atlantic-clade haplotypes in unglaciated vs. glaciated regions, as well as on northern vs. southern slopes of the Alps, appears to conflict with a purely anthropogenic introduction, as there is little reason to suppose higher use or survival of hatchery-reared fish in the north. Stocking in Austria is obligatory and ubiquitous and experimental data do not support higher survival of hatchery-reared fish in the more hydrologically and thermally variable systems in the north (Weiss & Kummer 1998; Weiss & Schmutz 1999a).
If the Atlantic-clade naturally colonized the upper Danube one might expect some haplotype diversity, reflecting heterogeneous origins, and/or mutation during its residence. The occurrence of two rare Atlantic-clade haplotypes (At10 and At11) in the northern populations is congruent with this expectation (Table 1), though the presence of these at some time in the past in hatchery stocks, or their natural occurrence elsewhere in the upper Danube where sampling may have been inadequate, cannot be excluded.
Considering the diversity of Atlantic-clade haplotypes within the south-west Atlantic (Weiss et al. 2000), we assume that this clade arose there having split from a common ancestor subsequent to the isolation of the Mediterranean and spread north early in the Pleistocene or late in the Pliocene. Following this scenario, even if Atlantic-clade brown trout arrived naturally into the upper Danube, they have done so via a north-to-south colonization route, concordant with our results of significant genetic structure across north/south geographical features.
Conclusions
In contrast to the more diverse Danubian grouping of brown trout, Atlantic-basin fish in the upper Danube represent a more limited and derived gene pool in this region. The predominant mechanism of introduction has been the release of hatchery-reared fish but some level of natural colonization may have occurred in the relatively recent (late to post-Pleistocene) past. Nonetheless, significant phylogeographic structure of the Danubian clade can still be found in the upper Danube and at least some isolated populations (Blühnbach, Lohnbach, Gossenkollersee and Anrasersee) are dominated or fixed for Danubian-clade haplotypes. Continued stocking of allochthonous strains of brown trout will erode the genetic integrity of natural populations in this region. As introduced fish have been shown to be both economically and ecologically inefficient (Weiss & Schmutz 1999a,b) we strongly recommend that their use in a sustainable and conservation-orientated management strategy in the upper Danube be reconsidered.
Acknowledgements
This project was financed by the Austrian Science Foundation (FWF; contract P11629-GEN), and the Austrian National Bank (Jubiläumsfonds 7671). During the preparation of this manuscript S. Weiss was financed by a postdoctoral grant from the Fundação para a Ciência e a Tecnologia (Praxis XXI/BPD/22052/99). We thank all those private parties who either gave permission or assisted with sampling, with special thanks to Reinhard Haunschmid (Institut für Gewässerökologie, Fischereibiologie und Seenkunde, Scharfling) for providing critical material from the Mühlviertel region of Austria and Michaela Bodner of the World Wildlife Fund for providing access and assistance in the Kamp drainage.
References
S. Weiss has diverse interests in the ecology, evolution and conservation management of freshwater fishes and is currently involved in several phylogeographic studies of salmonid fishes; the laboratory work for this study was carried out in the population genetics group of C. Schlötterer. H. Waidbacher is an associate professor specializing in applied fisheries ecology in the Austrian Danube and M. Jungwirth is a full professor and department head with interests in the management, ecology and restoration of freshwater fisheries in Austria.




