Impaired granulocyte formylpeptide-induced superoxide generation in chronically ill, malnourished, elderly patients
Abstract
Abstract. Cederholm T, Gyllenhammar H (Huddinge University Hospital, Huddinge, Sweden). Impaired granulocyte formylpeptide-induced superoxide generation in chronically ill, malnourished, elderly patients. J Intern Med 1999; 245: 475–482.
Objective. Protein-energy malnutrition (PEM), often occurring in patients with chronic disease, is associated with a decreased capacity to combat infections. In this study we assessed polymorphonuclear neutrophil (PMN) superoxide anion (·O2–) formation in elderly PEM patients with various chronic diseases.
Design and subjects. Nineteen patients (75 ± 1 years), with body mass index 17.1 ± 0.4, and 19 age-matched healthy controls were included. Fourteen patients and 14 controls were re-examined in a 3 month follow-up.
Setting. A service of internal medicine at a university-affiliated hospital.
Interventions. Eight patients were prescribed a dietary liquid supplementation during the observation period.
Main outcome measures. Superoxide production in PMN induced by fMLP (a receptor ligand) and phorbol myristate acetate (PMA), which acts directly on protein kinase C.
Results. fMLP-induced superoxide generation in the malnourished patients was 55 ± 5% of that of the controls. However, the patients retained their capacity, 108 ± 6% of control PMN generation, to respond to PMA. In those who received formula supplementation, fMLP-generated ·O2– production levels were 48 ± 8 and 73 ± 13% (P = 0.12) of those of controls at the start and after 3 months, respectively. Corresponding figures in those who were not prescribed supplementation were 57 ± 8 and 64 ± 4% (P = 0.55).
Conclusion. Possibly contributing to reduced host defence, receptor ligand-induced PMN generation of ·O2– is significantly lower in chronically ill, elderly patients with PEM than in age-matched healthy controls.
Introduction
Protein-energy malnutrition (PEM) is commonly encountered in chronically ill, elderly patients. Clinically, PEM has been associated with increased susceptibility to bacterial and fungal infections [1, 2] and altered inflammatory responses [3, 4]. On the cellular level, reports have focused on impaired cellular immunity, such as defects in T lymphocyte [5, 6], killer cell [7] and macrophage [6, 8] functions. However, amongst the inflammatory cells, the polymorphonuclear neutrophil leucocyte (PMN) is of crucial importance for the host defence against bacterial and fungal infections (reviewed in [9]). In this respect, the production of superoxide anions (·O2–) by the NADPH-oxidase in activated PMNs is of particular interest, illustrated by the profound susceptibility to infections in patients with the rare inherited disorder chronic granulomatous disease, where the NADPH-oxidase is deficient and ·O2– cannot be produced [10]. We have, in previous reports, shown that low levels of essential fatty acids (EFAs) in rats induced impaired bacterial killing by phagocytes [11] and, in chronically ill humans, was associated with impaired delayed skin hypersensitivity [12]. Furthermore, we have reported that PMNs from rats with dietarily induced EFA deficiency show a profound decrease in their production of ·O2– on stimulation with the surface receptor- and Ca2+-dependent PMN agonist f-met-leu-phe (fMLP), a synthetic analogue of bacterial oligopeptides produced in vivo during infections [13].
The aim of this study was to evaluate PMN ·O2– production in malnourished, chronically ill patients without known malignant disorders. Although reversal of malnutrition is non-controversial, the importance of this aspect of the patient’s condition may not be fully recognized. Therefore, we also related PMN ·O2– production to the outcome of the nutritional status during an observation period of 3 months.
Patients and methods
Subjects
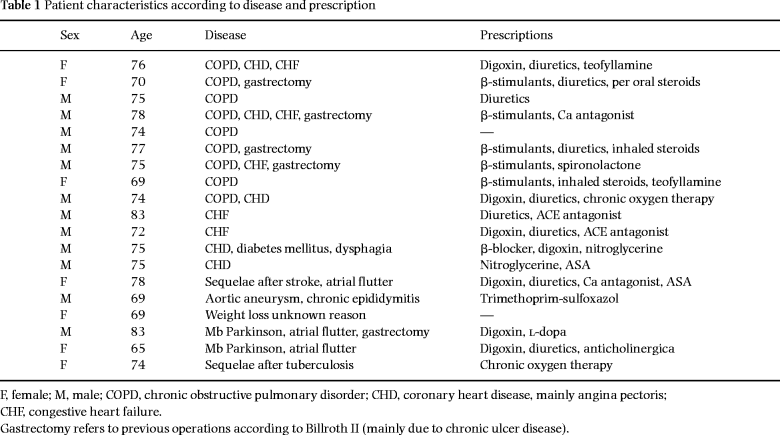
The details of the patients and study design have been reported previously [14]. Briefly, 19 patients, mean age 75 ± 1 years, with PEM but without known or suspected malignant or chronic inflammatory diseases were entered into the study. Twelve of the patients were male. Nineteen age- and sex-matched healthy and well-nourished persons were voluntarily recruited from a local association of retired citizens to serve as controls (age 72 ± 1 years). The patients were selected as being capable of a meaningful cooperation. Two-thirds of the patients were recruited at discharge from the Department of Medicine, Stockholm Söder Hospital, and the remaining six were from the outpatient clinic. Those recruited as in-patients were, in general, examined 1 week after discharge. All patients were ambulatory and capable of a self-maintained life at inclusion. Table 1 shows the various disorders from which the patients suffered and their major prescriptions. Most of the patients displayed chronic cardiac or pulmonary diseases. Twelve of the subjects had, within the preceding 2 months, suffered from pneumonia, bronchitis or urinary tract infection that had been treated by antibiotics. Except for one patient with chronic epididymitis, none showed physical signs of infection or were under antibiotic treatment at entry into the study. A written informed consent was obtained from all subjects at the time of entry into the study. The study was approved by the local ethics committee and conformed to the Helsinki declaration.
Follow-up
The study was designed to evaluate PMN superoxide production related to nutritional status at entry and at 3 months after entry. At the start of the study period, all patients received written and verbal information about the anticipated importance of improving their nutritional status and were given nutritional counselling. Ten patients were prescribed oral supplementation equivalent to 400 kcal (1.7 MJ) and 40 g of protein daily in the form of two 200 mL packages of Fortimel® (Nutricia, Zoetermeer, The Netherlands). Consent to participate in the random supplement administration was obtained from 13 of the 19 patients. Four patients died shortly after inclusion due to their underlying illness (chronic obstructive pulmonary disorder (COPD), COPD/congestive heart failure (CHF)/gastrectomy, COPD/coronary heart disease and CHF), and one was later excluded because of severe herpes zoster. The latter patient and one of those who died belonged to the group that had been prescribed supplementation. Re-examination was undertaken in the remaining 14 patients (eight of whom had been prescribed the supplement) and their corresponding controls 3 months after inclusion. All patients were followed on an ambulatory basis, and those who were prescribed nutritional supplementation were encouraged to follow the prescription by repeated phone calls.
Nutritional assessment
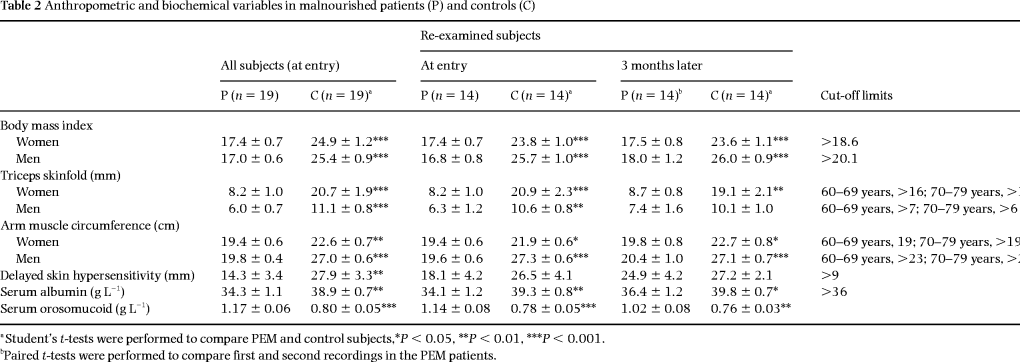
The nutritional status was determined, as described previously [15], by assessing body mass index [BMI; weight (kg)/height (m2)], triceps skin fold thickness by a Harpenden® caliper [16] and arm muscle circumference [16], serum albumin and delayed cutaneous hypersensitivity reaction by means of injections of extracts from Candida, PPD and mumps [17]. The size of the largest induration was registered 48–72 h later and expressed as the sum of the greatest diameter and the greatest perpendicular length [17]. In general, the malnourished patients exhibited at least three subnormal nutrition variables; the cut-off limits have been described previously [15] and are summarized in Table 2. Serum concentrations of orosomucoid were analysed in order to detect an ongoing acute-phase reaction (reference value < 1.1 g L–1).
Chemicals
Cytochrome C (bovine heart, type III), N-formyl-methionyl-leucyl-phenylalanine (fMLP) and phorbol myristate acetate (PMA) were from Sigma Chemical Co. (St Louis, MO); superoxide dismutase (SOD) was from Boehringer Mannheim (Mannheim, Germany); Percoll was from Pharmacia Fine Chemicals (Uppsala, Sweden); and endotoxin-free Hanks balanced salt solution (HBSS) and doubly distilled water were from Gibco (Paisley, Scotland). All other chemicals were also of the highest purity and quality commercially obtainable.
PMN preparation
Polymorphonuclear neutrophil leucocytes were obtained from venous blood from patients and their matched controls by a one-step discontinuous Percoll gradient centrifugation as described previously [18]. The purified PMNs (> 98% purity and viability) were washed twice before lysis of contaminating erythrocytes with ice-cold NH4Cl (0.155 mol L–1) and resuspended in HBSS at pH 7.4 at 2.5 × 106 cells mL–1. There were no differences in cell viability or in the purity of PMN preparations between patients and controls (data not shown). The number of PMNs retrieved after separation varied between 80 × 106 and 160 × 106, but there were no consistent or statistically significant differences between the patient and the control populations (data not shown). Assessment of the viability of PMNs was performed with trypan blue exclusion.
Measurement of superoxide anion production
The SOD-inhibitable cytochrome C reduction method in a real-time continuous assay, as described previously [19], was employed. Briefly, PMNs at 1.25 × 106 mL–1 in HBSS were activated with LTB4, fMLP (both at 0.1 µmol L–1) or PMA (1 µmol L–1) in the presence of 100 µmol L–1 cytochrome C, with or without 30 µg mL–1 SOD. The reaction was followed continuously at 37°C in a Perkin Elmer Lambda 7 spectrophotometer at 550 nm for 12 min (LTB4 and fMLP) or 30–60 min (PMA). Readings were taken every 12 s for LTB4 and fMLP or every 30 s for PMA. For calculations, the SOD-inhibitable reduction of cytochrome C was used, corrected for spontaneous reduction, and an ε = 21.1 (mmol L–1)–1 cm–1 was used [19]. Cells from patients and their matched controls were always assessed in duplicate and in parallel experiments on the same day to minimize differences in pre-experiment conditions. The same individuals who served as controls at the first assessment were always used as controls at the second point of measurement.
Statistics
Data are presented as mean ± SE. Student’s t-test was performed when comparing patients and controls, whereas Students’ two-tailed t-test for paired samples was used to evaluate changes over time. Pearson’s correlation coefficient was determined in correlation analyses. The calculations were performed using the BMDP-SOLO statistical software (BMDP Statistical Software, Inc., Los Angeles, CA).
Results
The patients were severely malnourished at entry into the study ( Table 2). For example, BMI measurements in the patients were 68% of corresponding measurements in the controls. Moreover, as reported previously [14], the patients showed an increase in serum orosomucoid concentrations and slightly lower serum albumin concentrations compared with controls both at entry into the study and at the 3-month follow-up ( Table 2). Blood leucocyte counts were in the normal range for both patients and controls, being 6.5 ± 0.6 and 5.9 ± 0.5 × 109 L–1, respectively.
The spontaneous, i.e. unstimulated, production of ·O2– from patient and control PMNs did not differ significantly at entry into the study or at the 3-month follow-up (data not shown). When PMNs were stimulated with PMA, acting by direct activation of protein kinase C, patient and control cells responded similarly with a pronounced and extended production of ·O2– both at entry and at follow-up ( Fig. 1). There were no significant differences in kinetic properties of the responses (data not shown). As the resolution in the assay for PMA stimulation in this protocol is 30 s, minor differences in lag-time could not be discovered, but at 2 min after addition of stimulus all samples showed an initiated response (data not shown).
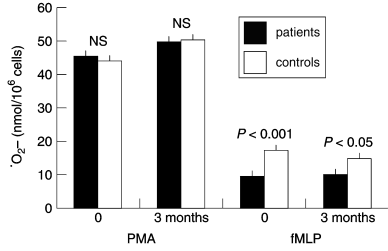
Superoxide ·O2– generation in granulocytes, stimulated by phorbol myristate acetate (PMA) or f-met-leu-phe (fMLP), from protein-energy malnourished patients and well-nourished healthy elderly controls at entry (0) and after 3 months.
Next, we studied the response to fMLP, acting by attachment to cell-surface receptors and by a G-protein and Ca2+-dependent mechanism [9]. Contrary to what was found upon PMN activation with PMA, the patient PMNs produced significantly less superoxide than the control PMNs in response to fMLP at both time-points ( Fig. 1). At entry, the patient PMN response initiated by fMLP was 55 ± 5% of the corresponding control response, i.e. 9.6 ± 1.3 and 17.5 ± 1.4 nmol ·O2– per 106 PMNs (P < 0.001), respectively. The kinetic properties of this response were similar for patients and controls (data not shown). The fMLP-induced superoxide production showed significant correlations with the nutritional variables body mass index (r = 0.5, P < 0.01) ( Fig. 2), arm muscle circumference (r = 0.51, P < 0.01) and serum albumin (r = 0.45, P < 0.05). Moreover, a negative correlation with the acute-phase reactant, serum orosomucoid, was noted (r = – 0.36, P < 0.05). Superoxide levels did not correlate (r = 0.13) with delayed cutaneous hypersensitivity, the latter reflecting cellular immunity.
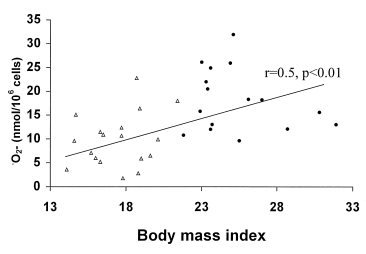
The relationship between body mass index and fMLP-induced PMN generation of superoxide anion (·O2–) in malnourished patients (▵) and well-nourished (●) elderly subjects.
Stimulation of PMNs with LTB4, also activating PMNs by attachment to a specific cell surface receptor, but producing a less pronounced activation of the oxidative metabolism compared with fMLP or PMA [19], was assessed on four patient/control pairs, at entry into the study only. In accordance with what was found upon fMLP activation, the patient PMNs produced less superoxide than the control PMNs: 0.93 ± 0.08 vs. 1.45 ± 0.12 nmol per 106 PMNs (P < 0.05), respectively.
Finally, we assessed the effect on superoxide anion formation from patient PMNs of a peroral dietary liquid additive (Fortimel®), prescribed for 3 months. Body weight changes were the same in the supplemented and unsupplemented patients: +1.4 ± 0.5 and +1.7 ± 1.8 kg (NS), respectively. In both groups, ·O2– formation tended to increase. For the 14 patients taken together, rates of PMA-induced ·O2– formation were 44.8 ± 2.3 and 49.9 ± 1.4 nmol ·O2– per 106 PMNs at entry and at follow-up, respectively (P = 0.055), and when generated by fMLP, corresponding figures were 8.2 ± 1.1 and 10.1 ± 1.4 nmol ·O2– per 106 cells (P = 0.1). In those who received formula supplementation, levels of fMLP-induced superoxide generation were 48 ± 8 and 73 ± 13% (P = 0.12) of those of controls at the start and after 3 months, respectively. Corresponding figures in those who were not prescribed supplementation were 59 ± 8 and 64 ± 4% (P = 0.55). Levels of fMLP-induced ·O2– generation in supplemented and unsupplemented patients were 161 ± 46 and 120 ± 18% ( Fig. 3) of baseline values, respectively (P = 0.12). There were no correlations between body weight changes (kg) and alterations in fMLP-induced (r = 0.13) or PMA-induced (r = 0.2) superoxide production amongst the patients during the intervention period. Neither were changes in serum orosomucoid concentrations significantly related to changes in fMLP-induced (r = 0.32) or PMA-induced (r = 0.38, P = 0.2) ·O2– generation.
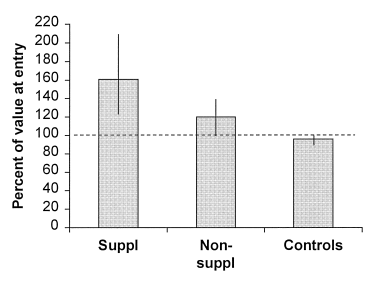
Granulocyte fMLP-induced generation of superoxide anion at follow-up, expressed as a percentage of generation at entry, in supplemented and non-supplemented malnourished patients and controls.
Discussion
In this study we have demonstrated a lower superoxide formation by PMNs from chronically ill patients with PEM than by PMNs from age-matched but well-fed healthy controls , when induced by surface-receptor-dependent stimuli (LTB4 and fMLP). In contrast, the PMN response to PMA, acting directly on protein kinase C, was intact in the patients. On a subcellular level, this argues for a defect in the stimulus–response relation localized to the proximal part of the cell-activation pathway, i.e. proximal to activation of protein kinase C, but the present data do not permit an exact localization of the defect. Although direct parallels cannot be made with investigations in animal cells, we found in previous studies on rat and monkey PMNs [11, 13, 20], with a similar response pattern, that the number and affinity of fMLP receptors were not affected by the dietary derangement. Defining the part of the subcellular transduction mechanism for cell activation that is affected by PEM requires further studies. The findings of impaired fMLP-induced superoxide generation are consistent with our earlier findings in rats [13] and monkeys [20] with dietarily induced EFA deficiency. Whether EFA concentrations have any influence on the PMN ·O2– generation in the present set of patients is not known. However, we have previously reported low serum levels of EFA in a comparable group of malnourished elderly patients [12]. The current results also conform with other reports on decreased PMN functions in PEM, including functions such as bacterial killing [21], PMN migratory capacity [22] and phagocytosis [23].
The nutritional intervention in this study was modest, but exceeded what is currently the practice in most patients. Contrary to expectation, in our study the weight gain was the same irrespective of prescription of dietary supplementation. This suggests a possible placebo effect, perhaps derived from the verbal and written information given to the unsupplemented patients about their inadequate nutritional status. Taken together, the 14 patients showed a strong tendency for improvement of their ·O2– generating capacity during the observation period. In contrast to the six unsupplemented patients, PMN from those eight subjects who were supplemented tended to generate more ·O2– on fMLP stimulation. It cannot be deduced from the current data whether the tendency for increased ·O2– production is conferred by an improved nutritional status or a possible improvement in the underlying disease. The identical weight gain in supplemented and non-supplemented patients may in part explain the non-significant difference in ·O2– production between the two groups. It has been shown that skeletal muscle function improves upon nutritional intervention in malnourished patients independent of alterations in anthropometry [24]. The restitution of energy metabolism is suggested to confer such improvements in muscle [25]. The same might hold true for PMN. Previous studies on leucocytes isolated from malnourished children have shown reduced E. coli bactericidal capacity, which was considerably improved after nutritional treatment [26]. Moreover, PMNs from depleted children have displayed reduced phagocytosis-induced hydrogen peroxide (H2O2) production, which likewise was restored to normal after treatment [27].
Several factors other than malnutrition might affect the patient’s PMN responses, such as medication and concurrent diseases. When examining the medication that the patients received during the study period, no consistent pattern was found which could associate medication with PMN functional capacity. The effect of diseases on PMN function is not clear, since highly variable results are reported when examining in vitro PMN function in various disorders [26–29]. Our finding of a negative correlation between superoxide generation and the acute-phase reactant serum orosomucoid suggests that neutrophil ·O2– production might be adversely affected by factors associated with inflammatory activity. Serum orosomucoid levels were elevated both at entry and at follow-up. In a previous study, we tried to elucidate possible mechanisms for the persisting inflammation found in these malnourished patients [14]. However, we were not able to relate bacterial infections, serum endotoxin levels or serum expression of antinuclear antibodies to the inflammatory state of the patients. Pro-inflammatory cytokines may interfere with oxygen radical production [8], and since the activity of such cytokines is often deranged in many diseases [30, 31], such factors may account for the variable findings. In a recent report, utilizing the same patients as in the current study, we documented increased serum levels of interleukin-6 (IL-6) and significantly increased monocyte production of IL-1β and IL-6 upon in vitro activation with lipopolysaccharide [14]. However, regression analysis was not able to detect any correlation between the previously reported cytokine data and PMN ·O2– production (data not shown). It is conceivable that the reduced PMN ·O2– production that we observed was the combined effect of protein-energy malnutrition and the underlying disease of the patient. There are no possible ways to separate these two factors when studying a population of elderly patients recruited from internal medicine services. Starvation not related to concurrent disease is rare in affluent societies.
We conclude that, amongst several alterations in host defence found in chronically ill patients with PEM, there is a decreased superoxide anion formation by PMN upon physiologically relevant receptor agonists. The dietary intervention performed in this study on a small number of subjects tended to improve the response to fMLP. Even if a more pronounced dietary intervention could conceivably be more effective, the current data do not allow for conclusions to be drawn as to the efficacy of supporting energy and nutrient intake in order to ameliorate host defence mechanisms in chronically ill, malnourished, elderly patients. Based on the intact response to PMA, there seems to be a retained activity in the superoxide anion-forming NADPH-oxidase, which indicates the potential to increase PMN ·O2– production in such elderly patients.
Acknowledgements
We thank Professor Jan Palmblad for helpful discussions. Mrs Anette Landström and Pia Spångberg are gratefully acknowledged for skilful technical assistance. This work was supported by grants from the Swedish Medical Research Council (grant 19X-05991), the funds of the Karolinska Institute, Tore Nilson’s Fund, the funds of the Swedish Medical Association, the Swedish Heart and Lung Fund, King Gustaf V Fund, the Börje Dahlin Fund, the Inga-Britt and Arne Lundberg Fund, the Swedish Association Against Rheumatism, P. & A. Hedlund’s Fund and Nutricia Nordica AB.
References
Received 19 January 1998; accepted 16 October 1998.