The spectrum of hepatobiliary disease in primary hypogammaglobulinaemia
Abstract.
Objective. To study the prevalence of hepatobiliary disease in a clinically and immunologically well-characterized group of 88 adult Norwegian patients with primary hypogammaglobulinaemia.
Subjects. Eighty-eight patients with primary hypogammaglobulinaemia were followed and signs and symptoms of liver disease were recorded. The patients were examined clinically and radiologically on a regular basis with liver biopsies performed when indicated. All patients were tested for hepatitis C virus (HCV) RNA, hepatitis G virus (HGV) RNA and hepatitis B virus (HBsAg).
Results. Twenty-one patients were HCV RNA-positive, all having signs of chronic liver disease. Only four patients were HGV RNA-positive, of whom two were also HCV RNA-positive. Amongst the 67 HCV RNA-negative patients, 26 had signs of chronic liver disease, including two who were HGV RNA-positive. HCV RNA-negative patients with liver disease had received intravenous immune globulin substitution more frequently, had a longer history of any form of immune globulin substitution and had a greater incidence of common variable immunodeficiency than patients without signs of liver disease. In most cases (21 of 26 patients) the liver disease was relatively mild. Three patients had granulomatous liver disease, with a relatively aggressive course in all three.
Conclusion. Hepatobiliary disease is a frequent complication in primary hypogammaglobulinaemia. Liver disease in HCV RNA-negative patients usually has a mild course. HGV does not seem to be a major cause of chronic liver disease in these patients.
Introduction
Patients with primary hypogammaglobulinaemia may have a variety of organ manifestations. Most infections occur as a direct consequence of their immunodeficiency state. In addition to their susceptibility to commonly occurring infections, the patients are also at special risk for exposure to infectious agents through intravenous substitution therapy with contaminated immune globulin. Transmission of hepatitis C virus (HCV) infection through several intravenous immune globulin (IVIG) preparations has been reported [1–4] and incidence and prevalence of viral hepatitis amongst these patients exposed to virus-contaminated immune globulin are high [3]. We have previously described the prevalence and clinical course of HCV infection in patients with primary hypogammaglobulinaemia and have reported that the clinical course is severe, resulting in development of liver cirrhosis in a considerable proportion of patients [3]. Several factors may contribute to this: the humoral deficiency itself may be of importance for the accelerated disease course; the presence of other hepatobiliary diseases in this patient group may contribute to the serious prognosis of chronic HCV infection; and, finally, coexistence with other viral infections or autoimmune disorders affecting the liver may render the liver more susceptible to the HCV infection.
The prevalence and clinical importance of liver diseases other than chronic HCV infection have not been studied in detail amongst patients with primary hypogammaglobulinaemia. In the present paper, we describe the spectrum of hepatobiliary disease manifestations in a large group of well-characterized Norwegian patients with primary hypogammaglobulinaemia, including the prevalence and possible importance of hepatitis G virus (HGV) infection.
Methods
Patients
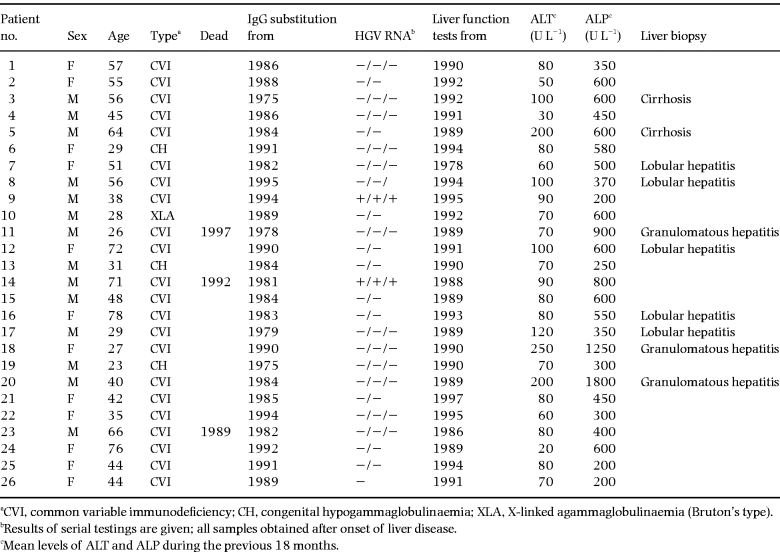
The patient group comprised 88 patients with primary hypogammaglobulinaemia. The diagnosis of primary hypogammaglobulinaemia was based on established criteria [5, 6] and the classification was performed as described elsewhere [7]: seven patients had X-linked agammaglobulinaemia (Bruton’s type), 54 had common variable immunodeficiency (CVI) and two patients had the hyper-IgM syndrome (HIM) with very low levels of IgG and IgA; 25 patients had congenital hypogammaglobulinaemia (CH).
Congenital hypogammaglobulinaemia includes patients with clinical manifestations before the age of 2 years, but with no known cases in the family suggesting an X-linked inheritance. We have introduced the CH group to enable us to relate our findings to precise diagnostic groups, and to avoid inclusion of XLA cases without family history in the CVI group. The categorization has been published previously [3, 7]. Analysis of Btk mutations has not been used routinely.
All 88 patients had IgG levels below 2.0 g L–1 before IgG substitution was initiated. The median age of the patients at the last follow-up was 44 (range 14–76) years and there were 51 males and 37 females.
Only two of the patients had used drugs intravenously, whilst three patients had tattoos. Five patients had received transfusions of erythrocytes or blood platelets. All patients were Caucasians.
All patients were seen on a regular basis at the Section of Clinical Immunology and Infectious Diseases, Department of Medicine A, National Hospital, Oslo, for several years (median follow-up 8.2 years, range 1.6–28). A complete biochemical liver status, including serum concentrations of alanine amino transaminase (ALT), alkaline phosphatase (ALP), γ-glutamyl transferase (gGT), bilirubin, albumin and coagulation factors (NT, Normotest, Nycomed, Oslo, Norway), was performed at least twice yearly in all patients. Isoenzymes for ALP were analysed in patients with significantly elevated levels of this enzyme. Patients were classified as having abnormal liver tests if one or more of ALT or ALP and gGT was elevated (> 1.5 times upper reference limit) in at least three subsequent samples obtained over a period of > 6 months. Upper reference limits were: ALT: males, 50 U L–1; females, 35 U L–1; ALP: 195 U L–1; and gGT: males, 80 U L–1; females, 50 U L–1. The history of immune globulin substitution was recorded. All patients had been substituted with immune globulins for several years (median 12.4 years, range 1.6–43). Thirty-one patients received one or more of four different commercial preparations of IVIG. For the last 9 years, the great majority of patients had been substituted with standard immune globulin administered subcutaneously [8]. Ultrasound examination of the abdomen, including the liver and the spleen, with Doppler duplex examination of the hepatic vessels was performed in all patients. In most patients, a CT scan of the abdomen was also performed. In patients with symptomatic liver disease and/or elevation of ALP and gGT, endoscopic retrograde cholangiopancreaticography (ERCP) was performed. In patients with significantly elevated biochemical liver function tests, liver biopsies were performed. The biopsies were evaluated by the same pathologist (TH).
Hepatic insufficiency has been defined as clinical signs of severe liver disease – ascites, portal hypertension, jaundice – or biochemical tests indicative of severely reduced liver function, i.e. albumin < 30 g L–1, thrombocytes < 70 × 109 L–1, NT < 50%, and exclusion of non-hepatic causes of abnormalities.
Due to the high prevalence of HCV infection amongst these patients, the total patient population was divided into three groups: (i) patients who were HCV RNA-positive with PCR methodology; (ii) patients with biochemical and/or clinical signs of chronic liver and/or biliary disease but who were HCV RNA-negative and HBsAg-negative (non-B, non-C liver disease patients); and (iii) non-liver disease patients with consistently normal liver function tests who were HCV RNA-negative. Liver function tests were recorded when patients were not afflicted by acute or severe infections which might influence the liver function tests.
Investigation of viral hepatitis
Blood samples for detection of HCV RNA, HGV RNA and HBsAg were obtained prior to antiviral therapy (interferon or interferon and ribavirin combination therapy). Blood samples from all patients were available for analysis. Serial blood samples (two to three serum samples obtained with intervals > 1 year) were analysed in the majority of the patients (62 patients). In the remaining 26 patients, only one serum sample was available. All serum samples were stored at –70°C. The blood samples were obtained during the period 1989–97, a period when IVIG preparations were used in only very few of our patients. Blood samples were obtained after immune globulin substitution therapy was started in all patients.
HBsAg and hepatitis D virus were analysed using commercially available kits (Auszyme; Abbott and Hepanostika HDV, Organon Teknika, respectively). The method used for HCV RNA by PCR has been published previously [3]. HGV RNA was detected by a PCR method, GBV-C LCx (Abbott) with primers from the 5′ non-coding region of the genome [9].
Results
Immune globulin substitution history
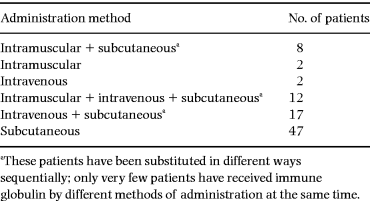
The history of immune globulin substitution is outlined in Table 1. Nearly 50% of the patients (41/88) received IgG substitution by more than one method of administration; 47 patients received immune globulins through subcutaneous infusions of standard immune globulin alone. The majority of the 41 patients who received several types of IgG substitution therapy were treated with IVIG preparations (n = 31). Of these, 23 patients were substituted with an immune globulin later demonstrated to be contaminated with HCV (Gammonativ, KabiVitrum) during the period 1982–86. Intravenous infusions of immune globulins were mainly performed during the period 1980–88.
Viral hepatitis status
A total of 21 (24%) patients were HCV RNA-positive. The clinical course in 18 of these patients has been reported previously [3]; one of these patients was also HBsAg- and HDV-positive. Patients found to be HCV RNA-positive in one serum sample were retested in samples obtained > 1 year prior to or following the positive sample and found to be consistently positive (all samples tested prior to antiviral therapy). The great majority (20 of 21) of HCV RNA-positive patients had received a contaminated immune globulin preparation as previously reported [3].
Four patients were found to be HGV RNA-positive by PCR. They were consistently positive in multiple serum samples obtained over a period of more than 3 years. Two of the HGV RNA-positive patients were also HCV RNA-positive and both had received the HCV-contaminated immune globulin mentioned previously [3]– both for a relatively short period (8 and 10 months, respectively). One of the two HGV RNA-positive, HCV RNA-negative patients had been substituted with the contaminated immune globulin. Only one patient (mentioned above) out of the total of 88 was HBsAg-positive.
Liver function tests
All HCV RNA-positive patients had pathological liver function tests. These patients developed pathological liver tests within 1 month to 8 years (median 28 months) after exposure to the contaminated immune globulin. The distribution of patients according to HCV and HGV RNA status and presence of liver disease is outlined in Table 2.
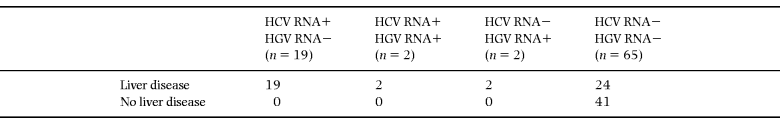
Liver function tests were found to be persistently abnormal in 26 of 67 patients who were HCV RNA-negative (non-B, non-C liver disease patients). As can be seen from Table 3, most of the 26 non-B, non-C liver disease patients had increased levels of both ALT and ALP. In all these patients, ALT and/or ALP and gGT were elevated on several occasions until the last examination or the patient’s death. Two patients developed severe signs of cirrhosis with hepatic insufficiency, with portal hypertension, ascites and coagulopathy. The median duration of their liver disease was 68 (range 22–126) months. Only one of the patients with abnormal liver function tests had a high intake of alcohol. Two of the patients had received transfusions with erythrocytes, both more than 10 years ago, both being HCV and HGV RNA-negative. Three of the non-B, non-C liver disease patients died during the observation period. None of the deaths could be ascribed to liver disease.
Ultrasound and endoscopic findings
Ultrasound examination demonstrated hepatomegaly in 14 patients, of whom five had biochemical and/or clinical signs of liver disease. Splenomegaly was similarly seen in 35 patients, of whom 16 had signs of liver disease. ERCP was performed in eight patients, with normal findings in five of these. Cholangiographic signs compatible with cirrhosis were found in two patients, whilst a picture compatible with sclerosing cholangitis was found in one patient.
Characteristics of patient groups
From Table 4 it can be seen that the patients in the HCV group were younger, but had a longer history of immune globulin substitution, than the other groups. All of these patients except one (20/21) had a history of substitution with an IVIG preparation known to be contaminated. Amongst the HCV RNA-positive patients, there were seven with CVI, six with XLA, six with CH and two with the HIM syndrome.
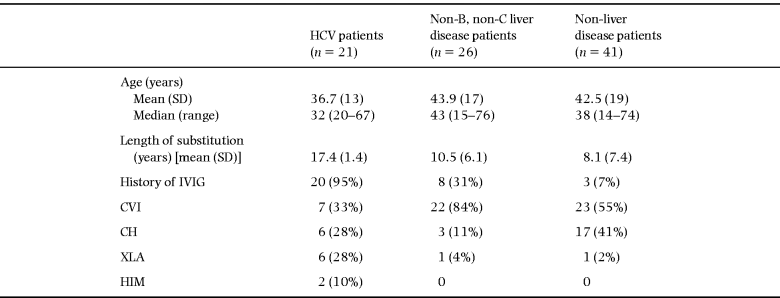
The remaining 67 patients were both HCV RNA- and HBsAg-negative. Of these, 26 had biochemical signs of chronic hepatobiliary disease. In 24 out of 26, this appeared after the initiation of immune globulin substitution. The median period of time from start of immune globulin substitution in any form to the first abnormal liver function test was 36 (range 3–82) months. Amongst the 26 non-B, non-C liver disease patients, the presence of hepatobiliary disease was associated with a higher frequency of previous IVIG substitution (8/26) and they had a significantly longer history of any immune globulin substitution than non-liver disease patients. Presence of hepatobiliary disease other than HCV was not associated with any specific immune globulin preparation, and non-B, non-C liver disease patients were slightly older (not significantly) than non-liver disease patients. Amongst the 26 non-B, non-C liver disease patients, most (84%) had CVI, whilst 55% of the non-liver disease patients had CVI.
Amongst the HGV RNA-positive patients, there were three CVI patients and one with CH. All four HGV RNA-positive patients had signs of chronic liver disease, both the two who were HCV RNA-positive and the two who were HCV RNA-negative. The two patients coinfected with HCV and HGV had a more severe liver disease than the two patients infected with HGV alone.
Liver histology
Liver biopsies were available from 10 of the 26 non-B, non-C liver disease patients. Three patients had undergone more than two liver biopsies. Three of the liver biopsies showed chronic, granulomatous hepatitis. Two of these patients had multi-organ granulomatous inflammation. All three patients with granulomatous inflammation of the liver had a relatively aggressive course of their liver disease. The histological changes in the liver were graded as moderate in all three patients. Two patients had bioptic findings compatible with cirrhosis of unknown aetiology. In the remaining five patients, including the patient with cholangiographic findings compatible with sclerosing cholangitis, a histological picture with lobular inflammation, lack of portal inflammation and normal biliary ducts was seen. The microscopic appearance of these biopsies was comparable to those found in the HCV RNA-positive patients [3], leading the pathologist to suggest a viral aetiology. In the three patients with serial liver biopsies available, signs of progression were observed during a period of 3–6 years.
Discussion
We have previously demonstrated both a high prevalence and a severe clinical course of hepatitis C virus infection amongst patients with primary hypogammaglobulinaemia [3]. All of our patients contracted their HCV infection more than 10 years ago. In these patients the liver disease represents a major clinical problem, and the clinical course appears to be particularly severe in patients with CVI.
Patients who received substitution therapy with blood-derived products such as immune globulins may also have been exposed to other known and unknown hepatotropic viruses. Hepatitis G virus has been shown to be associated with blood transfusion [10, 11]. The presence of HGV amongst recipients of blood products, such as haemophiliacs and patients with hypogammaglobulinaemia, has been reported [12–14]. The role of HGV in acute and chronic liver disease in immunocompetent patients has been studied extensively [15–18]. However, in most such studies, HGV seems to be an innocent bystander rather than a causative factor for liver disease [15–18]. However, even if clinically unimportant in immunocompetent individuals, little is known about the possible impact of HGV infection in immunocompromised patients. In view of our observations of the prevalence and clinical course of HCV infection in this patient group [3], similar data on HGV infection are of considerable interest. Prevalence of HGV RNA amongst our patients is lower than that found amongst recipients of other blood-derived products [12, 14]. The presence of HGV RNA was not associated with specific extrahepatic complications.
From other epidemiological and post-transfusion studies with HCV and HGV [9, 12], one might expect that patients with primary hypogammaglobulinaemia infected with HCV through a contaminated immune globulin would run a high risk of also having contracted HGV.
Most of our patients exposed to IVIG preparations received such therapy more than 5 years ago, whilst the blood samples examined were collected within the last 5 years and do not permit any conclusion concerning the incidence of a possible acute HGV infection in these patients. A high clearance rate of acute HGV infection might explain the low prevalence of present HGV infection. Alternatively, our findings may be due to the fact that preparations contaminated with HCV did not contain sufficient amounts of HGV to infect the recipient. This may be due to the greater lability and lower infectivity of HGV as compared with HCV [12]. The finding that HGV is of little importance in the development of chronic liver disease in patients with primary hypogammaglobulinaemia is in accordance with a recent report by Morris et al. [14].
Hepatobiliary disease other than chronic HCV seems to be strongly associated with CVI, previous IVIG treatment and duration of immune globulin substitution. Together with the fact that almost all patients with hepatobiliary disease had normal liver function tests prior to the immune globulin treatment, this suggests that IgG substitution may directly or indirectly play a role in the development of liver disease. One possible explanation, also suggested by the bioptic findings, is infection with one or more hepatotropic viruses leading to their non-B, non-C, non-G liver disease through IVIG therapy. However, another interpretation of our data is that development of chronic liver disease after initiation of substitution therapy indicates that such disease is mainly of an autoimmune nature as the patient should have been protected against most infectious complications during this period.
The history of immune globulin therapy is essential in interpreting these data. The majority of our patients received immune globulin substitution therapy aiming at serum IgG levels within the reference ranges throughout their history of such therapy. In a minority of the patients, incomplete immune globulin substitution therapy for some years may have rendered the patient susceptible to infectious complications which might have led to chronic viral disease. The details of the patients’ history of immune globulin substitution therapy during the period 10–20 years ago, which are necessary to assess whether the patients were incompletely substituted for a shorter or longer period, are rather inaccurate. We have thus not found it possible to perform a further classification as to history of immune globulin substitution therapy.
The great majority of the patients demonstrating non-B, non-C liver disease started substitution therapy after 1980. During this period, the great majority of the patients were followed in our department and IgG levels were controlled on a very regular basis. We might thus presume that these patients were adequately treated throughout their history of immune globulin substitution.
The strong association with CVI is interesting as these patients are prone to autoimmune complications of various types [19, 20]. Triggering of autoimmune inflammatory processes by hepatotropic viruses is one possible mechanism by which such chronic liver disease may develop. This appears to be the case with HCV infection [21].
In three non-B, non-C liver disease patients, granulomatous inflammation was demonstrated in liver biopsies. Two of these patients also had granulomas in other organs (skin, lungs and eyes) and the liver disease is probably part of a multi-organ granulomatous disease. Such granulomatous complications with features resembling sarcoidosis are well known in CVI [22–24]. One might speculate that these granulomatous complications, including the liver disease, are caused by unknown infectious agents.
In large series of primary hypogammaglobulinaemia patients published [19, 22], hepatobiliary diseases have been described, but not studied in detail. In our relatively large and well-characterized group of patients, we have found a high prevalence of such disease. Even though most patients with non-B, non-C hepatobiliary disease have only minor symptoms, this still remains a major cause of morbidity amongst the patients. Further studies of aetiology and pathogenesis are therefore warranted.
Acknowledgements
We are indebted to Bjørg-Guri Gutigard and Bodil Lunden for excellent technical assistance.
References
Received 3 April 1998; accepted 16 September 1998.