Investigation of media formulations promoting differential antigen expression by Photobacterium damsela ssp. piscicida and recognition by sea bass, Dicentrarchus labrax (L.), immune sera
Abstract
Photobacterium damsela ssp. piscicida (Phdp) isolates were grown in various bacteriological media, in eukaryotic cell culture media and in the presence of fish cells (resembling some aspects of in vivo growth environments). Bacterial cells, extracellular products (ECPs) and crude capsular polysaccharide were isolated and analysed by electrophoresis and Western blot using sea bass sera. Growth in bacteriological media conserved the synthesis of cell and extracellular components when these were compared with those prepared under near-in vivo growth conditions. In fact, synthesis of a larger range of cell components was induced after growth in bacteriological media. Certain media based on yeast extract and peptones from various sources and a specific salt formulation induced the synthesis of novel cell components at approximately 21.3 and 14 kDa. These antigens were recognized by sea bass sera collected after natural pasteurellosis outbreaks and other sea bass sera raised against live or inactivated Phdp cells. The ECPs of the pathogen were not good immunogens in their soluble form despite various treatments prior to immunization. The results are discussed with respect to vaccine development.
Introduction
Bacteria are not static entities but active biological systems. They interact with their environment and reflect the conditions under which they are forced to survive and multiply by alterations expressed both phenotypically and antigenically (Brown & Williams 1985). Accordingly, Photobacterium damsela ssp. piscicida (Phdp), a temperate marine fish pathogen, has to adjust its biological activities in order to succeed in growing in the internal environment of the fish host, the marine environment or in laboratory conditions. These adjustments can involve the bacterial cell, e.g. the response to the lack of iron, expressed as production of iron-regulated outer membrane proteins (Magarinos, Romalde, Lemos, Barja & Toranzo 1994; Bakopoulos, Adams & Richards 1997a). They could also involve the various extracellular products (ECPs) that the pathogen produces in order to facilitate the abstraction of nutrients from the surrounding environment for growth or for successful penetration and survival inside the host. The effects of the different environments on the physiology and metabolism of bacteria have been well documented in the literature both for Phdp and for other pathogens (Neilands 1982; Neelam, Thompson, Price, Tatner, Adams, Ellis & Stevens 1995).
It is important to be aware of such antigenic alterations between various growth conditions, especially in vaccine development. The laboratory media used to culture the pathogen in vitro must induce protective antigens (Ags) for the vaccine to be effective.
Previous studies have demonstrated that Phdp is a fairly homogeneous bacterial species antigenically, especially the European isolates (Bakopoulos, Adams & Richards 1997b; (Bakopoulos, Volpatti, Adams, Galleotti & Richards 1997c). However, despite the various pasteurellosis vaccines currently available in the market (based on formalin-inactivated whole Phdp cells), data from the field regarding the protection conferred to susceptible fish species such as yellowtail, Seriola quinqueradiata (Temminck & Schlegel), sea bass, gilthead sea bream, Sparus aurata (L.), and others against the disease are contradictory (Kusuda & Kawai 1998; Le Breton 1999). One of the many reasons for this may be the lack of expression of appropriate protective antigens by the Phdp bacterial cells grown in vitro for inclusion in these vaccines. Unfortunately, there is no published data on the differences that may exist for Phdp cells and ECPs produced in vivo compared with those produced in vitro.
In this study Phdp isolates were cultured in various media resembling in vivo conditions and a range of novel bacteriological media. Cell, ECPs and crude capsular polysaccharide antigens were analysed and compared using electrophoresis. Furthermore, the antigens recognized by the fish host were analysed by Western blot using polyclonal sera from immunized and naturally infected sea bass.
Materials and methods
Bacteria
The Phdp strain 003 (isolated in 1999 from sea bream in Italy) was used. The strain was stored at −70 °C on cryobeads (Cryoprotect®, Heywood, Lancs, UK).
Chemicals and equipment
Brain heart infusion broth (BHIB) and yeast extract were purchased from Oxoid, Hampshire, UK. Bacterial and fish peptone were purchased from Difco, Detroit, IL, USA. All the salts, carbohydrates and chemicals were purchased from Sigma, St Louis, MO, USA. Disposable filters and nitrocellulose membranes were purchased from Schleicher & Schuell, Dassel, Germany. Leibovitz medium (L15), Dulbecco's modified Eagle's medium (DMEM) and foetal calf serum (FCS) were purchased from Gibco BRL, Paisley, UK. Anti-mouse IgG-biotin and streptavidin-HRP were purchased from SAPU, Lanarkshire, UK. Centrifugation was performed with a Biofuge 28RS (Hereus Sepatech, Hanau, Germany) apparatus and samples were kept when required in a Forma Scientific (Marietta, OH, USA) −70 °C freezer. Colorimetric measurements were performed with an Ultrospec 2000 (Pharmacia Biotech, Cambridge, UK) spectrophotometer.
Fish
Selonda Aquaculture S.A., Greece, provided sea bass of 300 g with no previous disease history. These fish were non-vaccinated. On arrival fish were acclimatized for at least 2 weeks before any experimentation. They were fed with a commercial diet at 0.5% body weight per day. These fish were kept in a closed re-circulating aerated seawater system at 22±1 °C with a total capacity of 10 m3 of water. Bacterial loads were kept low by treatment of the water using two UV-C lamps of 55 W each. Water in the system was exchanged every hour. Salinity was kept at 35‰ and pH was 7.87±0.5. Ammonia, nitrite and nitrate levels were monitored frequently.
Growth media and conditions
Bacteria were grown in the following cell lines and biological material: striped bass larvae (SBL) cells, rainbow trout gonad (RTG) cells, sea bass red blood cells (RBCs), sea bass head kidney cells (HKCs), sea bass spleen cells (SCs), Leibovitz L-15 + 10% FCS and DMEM + 10% FCS. The growth media and conditions that were used comprised components regularly used for the maintenance and culture of fish cells (Munoz, Esteban & Meseguer 1999).
The head kidney and spleen from naive sea bass were aseptically removed and cells were isolated on a Petri dish flooded with DMEM + 1% penicillin/streptomycin + 10% FCS + 10 IU mL−1 heparin. The HKCs and SCs were then suspended in the latter medium and washed twice. Blood was collected with heparinized syringes and RBCs were isolated after centrifugation for 10 min at 100 g. The RBCs were also washed twice with DMEM + additives as described above. The HKCs, SCs and RBCs were washed once more with the same medium without the addition of penicillin/streptomycin and finally similar cell biomasses were resuspended in 30 mL of DMEM or L15 with or without 10% FCS or heat-inactivated (HI) sea bass serum.
Cultures of Phdp 003 in BHIB in the late log phase of growth were inoculated into the fish cell lines (SBL, RTG) and the media containing sea bass cells. Incubation lasted 48 h at 22 °C. At the end of this period, the cultures were centrifuged for 10 min at 150 g to spin down eukaryotic cells and processed as described below.
Growth in the bacteriological culture media was performed at 19–21 °C for a period determined from the bacterial growth in each medium (usually 24–48 h). These media and their abbreviations are listed in Table 1.
| Medium | Abbreviation |
|---|---|
| BHIB + 2% NaCl | BHIB |
| YPEa + saltsb + 1% NaCl | YO |
| YPE + salts + 1% NaCl + 0.175 mM EDDAa + 2% glucose | YOEG |
| (2×) YPE + salts + 1% NaCl | 2× YO |
| (2×) YPE + salts + 1% NaCl + 0.175 mM EDDAa + 2% glucose | 2× YOEG |
| Medium 1a + 2% NaCl | M1 |
| Medium 2a + 2% NaCl | M2 |
| YFPa + salts + 1% NaCl | YFO |
| YFP + salts + 1% NaCl + 0.175 mM EDDA + 2% glucose | YFOEG |
| (2×) YFP + salts + 1% NaCl | 2× YFO |
| (2×) YFP + salts + 1% NaCl + 0.175 mM EDDA + 2% glucose | 2× YFOEG |
- a YPE: yeast extract peptone; EDDA: ethylene diamine diacetic acid; Medium 1 and 2: supplemented by Aquatic Vaccines Ltd; YFP: yeast extract fish peptone. Yeast extract was added at a concentration of 5 g L−1. The peptones were added at 10 g L−1. The 2× media contained 10 g L−1 of yeast extract and 20 g L−1 of peptones.
- b Salts: the salt formulation was composed of potassium phosphate, iron sulphate, manganese sulphate, magnesium sulphate and calcium chloride. This formulation was modified for the preparation of iron limited media by the abstraction of iron compounds.
- Note: detailed information on Medium 1 and 2 as well as the salt formulation cannot be provided due to current industrial use and pending patent applications.
Processing
Bacterial cells were collected after centrifugation at 300 g for 1 h at 4 °C. These were washed twice with phosphate buffered saline (PBS), pH 7.2, and finally resuspended in PBS at a final concentration of 108 cells mL−1 (OD610 = 1). A portion of the suspension was centrifuged and the resultant bacterial pellet was resuspended in electrophoresis sample buffer (ESB) ( Nomura & Aoki 1985) and stored at −70 °C for electrophoresis and Western blot (WB) analyses.
The culture supernatants were filter sterilized through 0.45 μm disposable filters. The protein content was measured with the Bradford dye method (BioRad kit, BIORAD, Hercules, CA, USA), following the instructions of the manufacturer. A portion was mixed with ESB (1:1 volume) and stored at −70 °C for use in electrophoresis and WB analyses.
Crude capsular polysaccharide (cCPS) was isolated from bacterial cells following the method of Bonet, Magarinos, Romalde, Simon-Pujol, Toranzo & Congregado (1994) with only minor modifications (Karamanos, personal communication). Briefly, bacterial cells were harvested by centrifugation, vortexed with sterile glass beads in 1 N NaOH and centrifuged. The supernatants were decanted and their pH was brought to 7. The resultant solution was concentrated using dialysis membranes and polyethylene glycol at 4 °C. Finally, the concentrate was dialysed against PBS, pH 7.2, for 24 h at 4 °C, with three changes of buffer. The CPS content was measured with the phenol-sulphuric acid method using a glucose standard as follows: 0.25 mL sample mixed with 0.15 mL of 5% phenol and 1 mL of 18 N H2SO4. The absorbance was measured at 485 nm after 20 min. The protein content was measured with the Bradford dye method (BioRad kit), following the instructions of the manufacturer. The samples were stored at −70 °C.
Production of antibodies (Abs) against Phdp
The immunological probes used are given in Table 2. All the sea bass sera raised against Phdp cells were produced after intraperitoneal (i.p.) injection of 1 mL of a bacterial solution containing 108 cells mL−1, unless otherwise stated. All the sea bass sera raised against ECPs were prepared after i.p. injection of fish with a solution of 1–2 mL of either 40 or 60 μg mL−1 of ECP protein for non-inactivated and inactivated ECPs, respectively, unless otherwise stated. Sera were isolated from blood allowed to clot at 4 °C overnight. Blood was collected after 11–12 days at 20±1 °C post-immunization as it has been shown that the response is directed against a wider range of antigens at this time (Bakopoulos & Dimitriadis 1999). Samples from each immunization were pooled and stored frozen.
| Probe no. | Probe description |
|---|---|
| I | Sea bass anti-live Phdp 003 cells |
| II | Sea bass anti-Phdp 003 HI cells grown in YO |
| III | Sea bass anti-Phdp 003 HI cells grown in YOEG |
| IV | Sea bass serum against Phdp 003 ECPs produced in vivo |
| V | Sea bass serum isolated after a natural pasteurellosis outbreak |
| VI | Sea bass anti-Phdp 003 FI cells grown in YOEG |
| VII | Sea bass anti-Phdp 003 neat ECPs prepared in 2× YFO |
| VIII | Sea bass anti-Phdp 003 HI neat ECPs prepared in YOEG |
| IX | Sea bass anti-Phdp 003 neat ECPs prepared in YOEG |
| X | Sea bass anti-Phdp 003 PMSF-treated neat ECPs prepared in YOEG |
| XI | Sea bass anti-Phdp 003 cCPS prepared in YOEG |
| XII | Sea bass anti-Phdp 003 cCPS prepared in YO |
- YO: yeast extract peptone and salts; YOEG: yeast extract peptone, salts, EDDA, glucose; YFO: yeast extract fish peptone and salts; YFOEG: yeast extract fish peptone, salts, EDDA, glucose.
1. Sea bass serum against live Phdp cells. Thirty-five sea bass were i.p. injected with low numbers of live Phdp 003 cells (1 mL of 104 cells mL−1) grown in BHIB and suspended in PBS. Another 10 fish were injected with an equal volume of PBS and served as controls. This serum is designated probe I.
2. Sea bass serum against inactivated bacterial cells. Cells of isolate 003 (grown in YO and YOEG) were HI for 1 h at 56 °C and injected i.p. into sea bass on days 0 and 25. These sera are designated probes II and III, respectively.
3. Sea bass serum against Phdp ECPs produced in vivo. Sea bass were surgically implanted i.p. with sterilized dialysis tubing with 2, 12 and 50 kD molecular weight cut-off pore sizes containing 1–2 mL of a Phdp cell suspension (102 cells mL−1). The experiment was terminated after 1 week. All dialysis bags were collected and the suspension was centrifuged to collect the bacterial cells. Portions of the supernatants from each molecular weight cut-off membrane were pooled, filter sterilized, and their protein content measured using the Bradford dye assay. A portion of the ECPs, after adjustment of the protein content with PBS to similar levels (approximately 3 mg mL−1), was resuspended 1:1 in ESB and stored at −70 °C for electrophoresis and WB analysis. The same mixture was adjusted to 500 μg mL−1 of protein with PBS. Twenty sea bass were injected i.p. with 0.5 mL of these ECPs prepared in vivo. Controls received a similar volume of sterile PBS. Serum samples isolated from immunized fish were designated as probe IV.
4. Sea bass serum from a natural pasteurellosis outbreak. After a pasteurellosis outbreak in a commercial fish farm, blood was collected from surviving fish. The sera isolated were tested by enzyme-linked immunosorbent assay (ELISA) against Phdp bacterial cells (Bakopoulos, Adams & Richards 1997d; Bakopoulos, Volpatti, Papapanagiotou, Richards, Galeotti & Adams 1997e) and samples with reactions >0.2 (OD450 nm) from control sera, were pooled and stored. This serum was designated probe V.
5. Sea bass serum against Phdp 003 formalin-inactivated (FI) cells grown in YOEG (probe VI). Bacterial cells were inactivated using 0.5% formalin overnight at 4 °C. They were then washed twice in PBS and used in immunizations.
6. Sea bass serum against ECPs of Phdp 003 grown in 2× YFO (probe VII).
7. Sea bass serum against HI Phdp 003 ECPs prepared in YOEG (probe VIII). The ECPs were prepared in the latter medium and were HI for 1 h at 56 °C.
8. Sea bass serum against ECPs of Phdp 003 grown in YOEG (probe IX).
9. Sea bass serum against phenylmethyl sulfonyl fluoride (PMSF) treated ECPs of Phdp 003 grown in YOEG (probe X). The ECPs were treated with 0.75 μg of PMSF protein ECP μg−1 for 1 h at 4 °C before fish immunization.
10. Sea bass serum against Phdp 003 cCPS produced in YOEG (probe XI) and in YO (probe XII). Similar amounts of cCPS prepared as described previously were i.p. injected into sea bass.
As the scope of this study was to determine the specificity of the various sera against the antigen mixtures used for immunization, none of the sera were titrated by ELISA (except for the sea bass serum collected after a natural pasteurellosis outbreak). Instead, all the sea bass sera were used at the same dilution in WB analysis, as described subsequently.
Assessment of the phenotype and antigenicity of Phdp cells, ECPs and cCPS produced in various laboratory media
The Phdp strain 003 was grown in various media formulations and materials. Cells, ECPs and CPS were produced. These samples were analysed phenotypically using electrophoresis in 10% (cCPS), 12% (cells) and 15% (neat ECPs) polyacrylamide gels as described by Laemmli (1970). Gels were stained with Coomassie blue (cells, cCPS) and silver stain (BioRad silver stain kit) (ECPs, cCPS). The samples were analysed antigenically by WB analysis as described elsewhere (Wiens, Turaga & Kaatari 1990). The protocol was as follows: the nitrocellulose paper onto which the antigens were transblotted was incubated overnight with sea bass sera (1:40 dilution). Then, neat anti-sea bass IgM Mab M6 (Bakopoulos et al. 1997c), was added for 90min at room temperature (RT). An anti-mouse IgG-biotin conjugate (1:1000 dilution) was then added for 30 min at RT, followed by the addition of streptavidin-HRP (1:500 dilution) for 30 min before the colour development of the reactions.
Preliminary WB analyses were performed in order to determine the presence of any reactions of non-immune or control sera towards the antigen mixtures that were used for the immunization of experimental animals.
Results
Electrophoresis of Phdp cells grown in eukaryotic cell media
The use of tissue culture media and fish eukaryotic cells with or without the addition of sera (FCS, HI fish serum) for growth of Phdp did not result in the synthesis of new products. A representative electropherogram of the analyses of the cells prepared in eukaryotic cell culture media and cells is shown in Fig. 1. In fact, reduction in synthesis of products between 31 and 14.5 kDa was noted when bacterial cells were grown in bacteriological media (lane 2, Fig. 1) and near-in vivo conditions were compared (lanes 3–14, Fig. 1). Low molecular weight material below 14.5 kDa was expressed in higher quantities in the eukaryotic cell culture media (lanes 8–10, 12–13, Fig. 1).
SDS-PAGE (12% acrylamide) of Photobacterium damselae ssp. piscicida isolate 003 whole cells grown in in vivo resembling conditions. Lanes: 1, MW standards BioRad broad range; 2, cells grown in BHIB + 2% NaCl used for inoculations; 3, cells grown in L15; 4, cells grown in L15 + FCS; 5, cells grown in DMEM + FCS; 6, cells grown in L15 + HI fish serum; 7, control RBCs + L15 + FCS + HI fish serum; 8, cells grown in RBCs + L15; 9, cells grown in RBCs + L15 + FCS; 10, cells grown in RBCs + L15 + HI fish serum; 11, control HKCs + L15 + FCS + HI fish serum; 12, cells grown in HKCs + L15; 13, cells grown in HKCs + L15 + FCS; cells grown in HKCs + L15 + HI fish serum. Coomassie staining.
Electrophoresis of Phdp 003 whole cells grown in novel bacteriological media
Figure 2 presents the electrophoretic analysis of Phdp isolate 003 cells grown in novel bacteriological media. All the growth media appeared to induce the synthesis of similar products by the pathogen. The media BHIB and M1 (lanes 2 and 7) did not induce the synthesis of a 21.3 kDa protein. This was produced in high quantities in the YO, YOEG and 2× YO media (lanes 3–5). Interestingly, the synthesis of this 21.3 kDa protein was accompanied by the proportional synthesis of another protein at 14 kDa and by the lack of synthesis of another protein molecule.
SDS-PAGE (12% acrylamide) of Photobacterium damselae ssp. piscicida isolate 003 whole cells grown in various media. Lanes: 1, prestained MW standards BioRad broad range; 2, cells grown in BHIB + 2% NaCl; 3, cells grown in YO; 4, cells grown in YOEG; 5, cells grown in 2× YO; 6, cells grown in 2× YOEG; 7, cells grown in Medium 1 + 2% NaCl; 8, cells grown in Medium 2 + 2% NaCl; 9, cells grown in YFO; 10, cells grown in YFOEG; 11, cells grown in 2× YFO; 12, cells grown in 2× YFOEG. Coomassie staining.
Electrophoresis of Phdp ECPs
A range of ECPs prepared in the novel bacteriological media were electrophoresed in 15% acrylamide gels as shown in Fig. 3. This analysis revealed that the 2× YFO, 2× YFOEG and M2 media (lanes 6, 7 and 5, respectively) induced high amounts of ECPs below 21.3 kDa and at approximately 7.6 kDa. These were either produced in low quantities or not produced at all by bacteria cultured in YO, YOEG and M1 media (lanes 2–4, respectively).

SDS-PAGE (15% acrylamide) of Photobacterium damselae ssp. piscicida isolate 003 neat ECPs prepared in various media. Lanes: 1, prestained MW standards BioRad broad range; 2, ECPs prepared in YO; 3, ECPs prepared in YOEG; 4, ECPs prepared in Medium 1 + 2% NaCl; 5, ECPs prepared in Medium 2 + 2% NaCl; 6, ECPs prepared in 2× YFO; 7, ECPs prepared in 2× YFOEG. Silver staining.
Electrophoresis of Phdp cCPS extracts
Crude CPS was prepared from Phdp 003 cells grown in all the novel bacteriological media. These were analysed by electrophoresis in 10% acrylamide gels (data not shown). Polysaccharide material at very high MW was observed in the stacking gel. The polysaccharide nature of this molecule, as well as the protein nature of the products observed from 48.3 to 34.7 kDa (a doublet and a single band), was confirmed by Coomassie staining of a similar gel (data not shown). The synthesis of these proteins was induced by all the growth media, while some additional proteins were seen around 79 kDa.
WB analysis of Phdp cells
Sea bass probe V
Sea bass sera isolated from a natural pasteurellosis outbreak were pooled and tested in WB analysis of Phdp 003 cells grown in the novel media as shown in Fig. 4. The probe reacted in a similar fashion with all the cell samples from high MW down to >21.3 kDa. Quantitative differences were noted with an antigen at 48.3 kDa for cells grown in BHIB, 2× YO, 2× YFO and 2× YFOEG (lanes 2, 5, 11 and 12, respectively); for these media the reaction was much stronger in comparison with the other samples. Marked differences between samples were noted against three products at 21.3, 14 and 11 kDa. The most complete reaction was noted for cells grown in the YO, YOEG, 2× YO, YFO and YFOEG media (lanes 3–5, 9 and 10, respectively). Fainter reactions were noted for the rest of the media for the Ags concerned, except for cells grown in BHIB, 2× YFO and 2× YFOEG (lanes 2 and 11–12), where these reactions were very difficult to visualize.
WB analysis of Photobacterium damselae ssp. piscicida 003 cells grown in various media with sea bass serum isolated after a natural pasteurellosis outbreak (probe V). Lanes 1–12 as for Fig. 2.
The reactions of probe V and probes I, II, III and VI are summarized in Table 3. A selection of reactive cell products with these probes is presented, as well as the reaction seen with each probe. A more detailed report on the reaction of each probe is presented below.
| Products kDa probe | 206 to >48.3 | 48.3 | 45 | 42 | 37, 34.7, 32 | 29.3 | 24 | 23 | 21.3, 14, 11 |
|---|---|---|---|---|---|---|---|---|---|
| V | +a | + | − | + | −, +, +fa | − | + | − | +, +, + |
| I | −a | − | + | + | +f, +v, + | − | + | − | +, −, − |
| II | + | − | − | +v | +, +, + | − | + | + | −, −, − |
| III | + | +va | − | − | +sa, +f, +f | + | +s | +s | −, +f, +f |
| VI | + | +v | − | +v | +s, +, +f | − | +f | +s | +, +f, +f |
- a +: Positive; −: negative; v: variable; f: faint; s: strong.
Sea bass probe I
Reactions were similar for most of the cell samples with evident quantitative differences. The strongest reactions were noted for the YO, YOEG, 2× YO and 2× YOEG media. Qualitative differences were noted especially for cells grown in BHIB, M1 and M2, while cells grown in the YFO, YFOEG, 2× YFO and 2× YFOEG media reacted faintly with the probe, especially below 21.3 kDa. In comparison with the reactions of probe V, reactions from high MW down to 48.3 kDa with probe I were absent. Some novel Ags reacted below 48.3 and 34.7 kDa (Table 3).
WB analysis of Phdp 003 cells with probe II
Similar reactions with all the bacterial samples were noted. Some quantitative differences were seen with an Ag at 42 kDa. Comparison of the reaction of this probe and probe V reveals that probe II retained a faint reaction at 79 kDa, reacted with material between 79 and 48.3 kDa, lacked the reaction at 48.3 kDa and reacted with a higher range of products from below 48.3 to 21.3 kDa (Table 3). Below the latter MW, very faint reactions were seen, while very strong reactions were evident towards the dye front in contrast to probe V.
WB analysis of Phdp 003 cells with probe III
Similar reactions were evident between cell samples prepared in the different media with the exception of the YFO, YFOEG and 2× YFO media. No reaction was seen with an Ag at 29.3 kDa for the YFO, YFOEG, 2× YFO and 2× YFOEG media. Variation was noted in the recognition of a product at 48.3 kDa. In comparison with probe II, probe III reacted more clearly with Ags from below 79 to 48.3 kDa. A strong reaction was noted for the top Ag of the triplet at 34.7 kDa. Reactions were mainly directed to one of the Ags at 29.3 kDa, while the reactions at 24 and 23 kDa were very strong. Below 21.3 kDa, faint but more discrete reactions were achieved, especially for cells grown in BHIB, YO, YOEG, 2× YO and 2× YOEG. When the reaction of this probe was compared with that of probe V, the former reacted with a higher range of Ags from high MW to >21.3 kDa and at just above 21.3 kDa. Faint reactions were noted for the Ags below 21.3 kDa, except for the Ags at the dye front where reactions were much stronger (Table 3).
WB analysis of Phdp 003 cells with probe VI
Similar reactions were obtained between the different cell samples but with quantitative variations. Comparison of the reactions of probe V with probe VI showed similar reactions from high MW to 21.3 kDa. Reactions were fainter between the latter serum and the Ags from 21.3 kDa to the dye front, in contrast to probe V. The best reactions, in respect to the Ag range recognized, were seen with cells grown in the YO and the 2× YO media.
WB analysis of ECPs
Western blot analysis of neat ECPs was performed with probes IV, VII, VIII, IX, X. Only faint reactions were noted against materials between 79 and 48.3 kDa. Figure 5 presents the reaction of probe VIII against the neat ECPs of Phdp. This reaction is representative of the reactions achieved with the other anti-neat ECP probes.
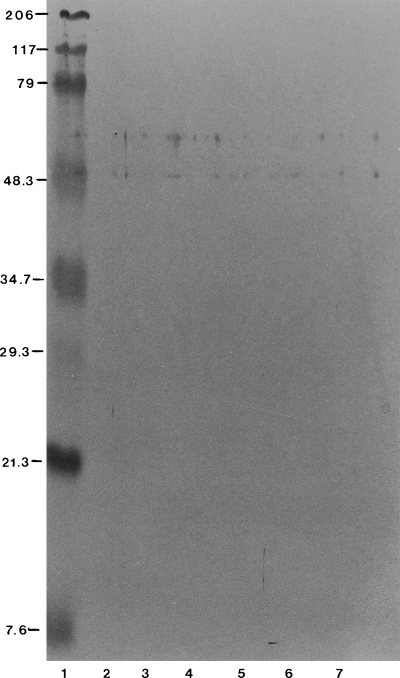
WB analysis of Photobacterium damselae ssp. piscicida 003 neat ECPs prepared in various media with sea bass serum raised against P. damselae 003 HI neat ECPs prepared in YOEG (probe VIII). Lanes 1–7 as for Fig. 3.
WB analysis of cCPS
(a) WB analysis with probe V. Reactions were noted with two protein Ags between 48.3 and 34.7 kDa, especially for the YO, YOEG, 2× YO, YFO, YFOEG, 2× YFO and 2× YFOEG media (lanes 3–5 and 9–12, respectively, Fig. 6).
WB analysis of Photobacterium damselae ssp. piscicida 003 cCPS prepared in various media with sea bass serum isolated after a natural pasteurellosis outbreak (probe V). Lanes: 1, prestained MW standards BioRad broad range; 2, cCPS prepared in BHIB + 2% NaCl; 3, cCPS prepared in YO; 4, cCPS prepared in YOEG; 5, cCPS prepared in 2× YO; 6, cCPS prepared in 2× YOEG; 7, cCPS prepared in Medium 1 + 2% NaCl; 8, cCPS prepared in Medium 2 + 2% NaCl; 9, cCPS prepared in YFO, 10, cCPS prepared in YFOEG; 11, cCPS prepared in 2× YFO; 12, cCPS prepared in 2× YFOEG. Silver staining.
(b) WB analysis with probe XI. Similar reactions (data not shown) were observed in comparison with probe V in respect to the Ags recognized. This probe reacted with these Ags in all the cCPS samples except for cCPS prepared by cells grown in BHIB.
(c) WB analysis with the probe XII. Similar Ags were recognized in comparison with the previous analyses except for cCPS prepared from cells grown in BHIB and 2× YOEG. This probe recognized an Ag at 34.7 kDa only for the YO medium (data not shown).
None of the pre-immune or control sera reacted with any of the Ags with the immune sera prepared in this study.
Discussion
A number of cell and ECP samples of Phdp prepared after growth in various novel bacteriological media were assessed in respect to conservation and/or induction of the synthesis of new products. These were compared with samples generated under near-in vivo growth conditions.
Initially attempts were made to identify products expressed by the pathogen when it was cultured in environments that can sustain the growth of eukaryotic cells. These environments were enriched with the presence or absence of serum (HI FCS or sea bass serum) and/or eukaryotic cells of a host animal (sea bass red blood, spleen and kidney cells). Thus, the pathogen was provided with some of the constituents that may be present during its development inside the fish host.
Interestingly, these ‘growth media’ did not promote the synthesis of any novel products and the most complete range of cell components was evident for cells grown in BHIB. It can be speculated that the expression of some molecules may be switched off when the pathogen is grown under conditions resembling those of the host (e.g. limited nutrient supply, low growth, anti-pathogen host defences) as demonstrated for Pasteurella multocida by Choi-Kim, Maheswaran, Felice & Molitor (1991). The fact that the pathogen expresses the most complete range of cell components when grown in BHIB (in comparison with in vivo growth) was used in subsequent steps of the study. The bacterial cell protein profile induced after growth in BHIB was used as a matrix for comparisons with the protein profiles that were induced after growth in novel bacteriological media.
Novel media were prepared which induced the synthesis of the most complete range of cell components and some novel products when compared with Phdp cells grown in BHIB. The analysis of cell products in different media showed that most of the media induced the synthesis of similar products by Phdp, from high MW to 31kDa. Below this MW, only the yeast peptone, yeast fish-peptone (supplemented with the salt formulation) and Medium 2 formulations induced the synthesis of novel products. These media also induced the synthesis of the most complete range of ECPs.
An extract of the cCPS synthesized by Phdp, first described by Bonet et al. (1994), was also investigated. The analyses concerned mainly the protein material that the CPS preparation method releases from bacterial cells. This might be either related to the CPS and/or situated at the bacterial cell outer surface, making it easily accessible to the fish immune system. The protein material was present in all the cell samples tested irrespective of the presence of carbohydrates in the growth medium.
Sea bass sera were prepared against Phdp cells grown in novel media. These cells were used untreated or after inactivation for immunization. Sera from fish after a natural pasteurellosis outbreak were also analysed. Novel products synthesized below 21.3 kDa for Phdp cells prepared in the yeast peptone and yeast fish-peptone media and Medium 2 formulations were recognized by all sera. These data suggest that the media induce the expression of novel antigens which are also naturally synthesized and which are also immunogenic for sea bass.
During immunological analyses, differences were noted in the recognition of cell components between sera produced against Phdp cells grown in supplemented media or media containing low levels of carbohydrates. Sea bass serum raised against Phdp cells grown in media devoid of carbohydrates (probe II), recognized a lower range of products in comparison with those recognized by the sea bass serum raised against bacterial cells grown in glucose-rich media (probe III). In addition, a stronger reaction was observed for certain products by the latter sera. Growth of Phdp cells, in glucose-rich media, induces the synthesis of a polysaccharide capsule (Bonet et al. 1994). Perhaps this cell component influences antigen processing and presentation by sea bass antigen-presenting cells. On the other hand, the antigens with which the sera reacted strongly are bacterial cell surface related and could be more easily accessible for processing by sea bass leucocytes.
Western blot analyses of Phdp ECPs, produced in novel bacteriological media, using a number of probes prepared against Phdp ECPs synthesized in vivo and in vitro showed that the probes recognized only three to four ECPs between 48.3 and 34.7 kDa irrespective of the treatment of and/or the antigens used for the immunization of sea bass. Interestingly, probe I also reacted with these products suggesting that these ECPs are apparently cell components that are released into the culture medium by the pathogen. The other ECPs visualized do not appear to be immunogenic in their present form. This has been also shown to be the case for the Aeromonas salmonicida ECPs (Hastings & Ellis 1988). The ECPs, being soluble antigens, may not induce an immune response and their particularization or/and the addition of adjuvants might prove beneficial in this respect (Andrianov & Payne 1998). Studies in our laboratory (data not presented) have shown that both in vivo and in vitro prepared ECPs are extremely toxic for susceptible fish and they seem to be solely responsible for the peracute deaths associated with pasteurellosis outbreaks. Thus, achieving an immune response towards these antigens may be an important way ahead in future vaccine development.
The sea bass serum collected after a natural pasteurellosis outbreak (probe V) confirmed which growth media for Phdp lead to synthesis and expression of products recognized by the host. This serum recognized the novel products that are induced by these media, in comparison with the BIHB growth medium, especially for the YO formulations and to a lesser degree for the other media. The other WB analyses performed showed that the serum prepared against FI Phdp cells prepared in YOEG (probe VI) reacted in a similar manner to probe V. Although the reaction was somewhat fainter for the products below 21.3 kDa, it was still stronger than probes I, II and III. Probe V also reacted with the protein material of cCPS prepared in the final media, especially for the YO and YFO formulations, suggesting that this material is conserved both in vivo and in vitro.
In conclusion, the culture media that induced expression of components recognized by sea bass sera (especially probe V) were identified as those based on yeast extract – peptone supplemented with the salts and Medium 2. Bacterial cells grown in YOEG promoted the synthesis of products which after formalin-inactivation and immunization of sea bass raised Abs of similar specificity to Abs in sera isolated after natural outbreaks of the disease.
An important group of determinants in pathogenesis, the ECPs, are not good immunogens in their present form despite their inactivation. Thus, particularization of the antigens and/or the use of adjuvants is required if a response to a wider range of antigens is required. Interestingly, the inclusion of ECPs in experimental vaccine mixtures consisting of inactivated whole bacterial cells administered either via immersion or i.p. injection resulted in better protection of challenged fish in comparison with vaccine mixtures without the inclusion of ECPs (Magarinos, Noya, Romalde, Perez & Toranzo 1994; Mazzolini, Fabris, Vismara, Passera, Geschia & Giorgetti 1998).
During these studies it was demonstrated that supplementation of carbohydrates in the laboratory media affects the range of Abs produced. This in turn affects the antigens recognized, suggesting the synthesis of capsular material, which may interfere with the recognition of important protective antigens against pasteurellosis (Bonet et al. 1994).
Acknowledgements
This study was supported by the European Commission FAIR programme, contract CT97-3449. We wish to thank Mr K. Poulos for the assistance provided during fish handling.
References
Received: 20 December 2001 Accepted: 30 May 2002