A review of the newer aromatase inhibitors in the management of metastatic breast cancer
Abstract
Background: Breast cancer remains a major cause of morbidity and mortality world-wide. While aminoglutethimide, a first-generation aromatase inhibitor, has equivalent efficacy to first-line tamoxifen in the palliative treatment of metastatic breast cancer, its toxicity profile has relegated this drug to a second- or third-line agent in this setting. Recently, several aromatase inhibitors have been released onto the market while others are in phase II and III clinical trials
Aim: To review the role of the new aromatase inhibitors in the management of metastatic breast cancer
Methods: Current literature, abstracts from meetings and information from pharmaceutical manufacturers have been summarized
Content: A review of the clinical pharmacology of the new aromatase inhibitors has been provided in addition to a synopsis of phase III clinical studies
Conclusion: The newer aromatase inhibitors have several advantages compared to aminoglutethimide and are a useful addition to the armamentarium of therapies employed in the palliative management of advanced breast cancer
INTRODUCTION
Breast cancer is a major cause of morbidity and mortality in women. The incidence varies throughout the world, with the highest rates reported in developed nations such as western Europe (99 per 100 000 women) and the United States (113 per 100 000 women). The occurrence of this disease is rising and by the year 2000 more than a million new cases will be diagnosed world-wide, with approximately 400 000 of these women expected to die from the disease ( 1)
Endocrine therapy, in particular tamoxifen, has an important role in the management of metastatic breast cancer as approximately one-third of tumours are believed to be hormone-dependent ( 1). In addition, tamoxifen's role in the adjuvant setting is well established, while its use in the prevention of breast cancer is currently under investigation ( 2, 3). Tamoxifen is the first line of therapy in advanced breast cancer in post-menopausal women. The response rate is approximately 30% in unselected patients but exceeds 50% in patients with oestrogen-positive malignancies ( 1). The first-generation non-steroidal aromatase inhibitor, aminoglutethimide, has been shown to be equally efficacious in this setting but has been relegated to a second- or third-line agent due to its toxicity profile ( 4, 5). Side-effects associated with the use of aminoglutethimide include sedation, rash and more importantly inhibition of cortisol production, necessitating hydrocortisone supplementation ( 6, 7). The increasing use of tamoxifen in the adjuvant setting has resulted in the need for second- and third-line therapies. Moreover, the long-term toxicity concerns of tamoxifen, including its thromboembolic and carcinogenic potential, has led to the continued search for alternative, non-toxic, hormonal treatment options ( 8)
Recently, several new aromatase inhibitors for the treatment of metastatic breast cancer in post-menopausal women have been marketed while others are currently in phase II and III clinical trials. Compared to aminoglutethimide, these newer agents appear to be associated with several advantages ( 8). The aim of this paper is to review the role of the newer aromatase inhibitors in the management of advanced breast cancer in post-menopausal women
PHARMACOLOGY
Classification
The aromatase inhibitors have been divided into two classes: non-steroidal and steroidal drugs ( 9). The non- steroidal, competitive aromatase inhibitors have been further classified into three generations: first, aminoglutethimide; second, the imidazole derivatives, e.g. fadrozole and rogletimide (also referred to as pyridoglutethimide); and third, the triazole derivatives, e.g. vorozole, letrozole and anastrozole ( 9, 10). The steroidal inhibitors are irreversible inhibitors of the aromatase enzyme and include the first-generation compound formestane and the second-generation drugs plomestane and exemestane. Data regarding plomestane are minimal, because technical problems in its development have been encountered ( Fig. 1) ( 9)
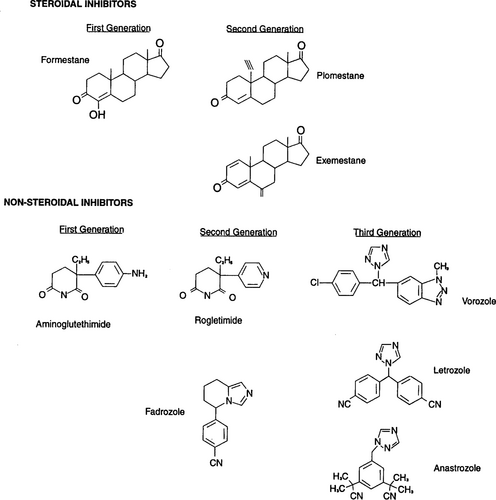
. Structures of aromatase inhibitors.
Mechanism of action
In post-menopausal women, the primary source of oestrogen arises from the peripheral conversion of adrenal androgens and androstenedione by the aromatase enzyme to oestrone. The latter is then metabolized to oestradiol by 17-β-hydroxysteroid dehydrogenase. Aromatase, a p450 enzyme, is found chiefly in fat cells, liver, muscle and breast tumour tissue with the 18-hydroxylase and 11-β-hydroxylase enzymes. The latter enzymes are responsible for the synthesis of aldosterone and cortisol, respectively ( 6, 8, 10, 11)
Because aminoglutethimide is a non-selective inhibitor of aromatase, the production of oestrogen as well as aldosterone and cortisol is reduced during its administration ( 12). In order to mimic endogenous cortisol secretion and prevent hypothalamic pituitary axis (HPA) suppression, corticosteroid replacement is typically required ( 6). In contrast, the newer agents have greater degrees of selectivity. However, at higher doses, 18-hydroxylase enzyme inhibition may occur with fadrozole. The triazole compounds and rogletimide have not been associated with adrenal suppression at clinically employed doses ( 9). For example, with letrozole, reductions in adrenal steroid synthesis have occurred at 6000–15 000 times the dose required to suppress oestrogen production ( 13). The newer aromatase inhibitors have equivalent or higher potency compared to aminoglutethimide ( 8, 9, 13, 14), although the potency of rogletimide appears to be lower ( 15)
The steroidal aromatase inhibitors bind covalently to aromatase and, despite rapid clearance from the plasma, they continue to inhibit the enzyme ( 9). Like the non-steroidal aromatase inhibitors, the non-com petitive inhibitors have equal or higher potency compared to aminoglutethimide. Both formestane and exemestane possess a higher degree of selectivity than aminoglutethimide and have not been associated with adrenal suppression and electrolyte changes ( 8, 14, 16).
Pharmacokinetics
The pharmacokinetics of the aromatase inhibitors have yet to be elucidated fully. The drugs appear to be well absorbed orally although the absorption of formestane is prolonged when administered via the intramuscular route ( 6, 17–20). The half-life (t) of aminoglutethimide varied from 12·6 to 19·5 h, but after a week of therapy the t typically falls to 8·6–14·3 h due to both auto?induction and a reduced volume of distribution (Vd). This short t has necessitated a four-times-a-day dosing regimen ( 6). In contrast, the t of the newer aromatase inhibitors is markedly longer, thus permitting twice-daily administration of fadrozole and daily dosing of vorozole, anastrozole (t=50 h) and letrozole (t=48 h) ( 13, 18, 21, 22). When administered orally, formestane is cleared rapidly from the body due to extensive liver metabolism (t=2–3 h). The intramuscular route of administration slows the absorption rate, which allows a fortnightly dosing regimen ( 19, 20). The pharmacokinetics of exemestane is largely unknown but it has been administered orally once daily in phase I and II studies ( 14, 23). The simplified dosing regimens of the newer aromatase inhibitors represent a further advantage over aminoglutethimide
Little is known about the distribution of the aromatase inhibitors, although both letrozole and anastrozole appear to be bound weakly to plasma proteins ( 17, 18). The Vd of aminoglutethimide is reported to vary from 66 to 70 litres, while the value for letrozole is 133 litres ( 6, 17)
The liver is the primary route of metabolism for the aromatase inhibitors. Letrozole is metabolized by the cytochrome p450 enzymes 3A4 and 2A6. Dose reductions for anastrozole and letrozole have not been recommended in patients with liver insufficiency although caution has been advised in patients with severe impairment ( 17, 18). The pharmacokinetics of formestane was unaltered in patients with liver metastases and abnormal liver function ( 20). The clearance of fadrozole appeared to decline with increasing weight in healthy post-menopausal women ( 24). The primary metabolites of letrozole and anastrozole are inactive and have been identified as 4–4 methanolbisbenzonitrile and a triazole derivative, respectively, while that of formestane is 4-OHA-G (17–20). Between 3% and 7% of aminoglutethimide is excreted as N-acetylaminoglutethimide in the urine ( 6)
Aminoglutethimide induces the mixed function oxidase enzymes, including cytochrome p450 3A, thus enhancing the metabolism of warfarin, theophylline, tamoxifen, dexamethasone and progesterone ( 6, 25). Letrozole inhibits the 2A6 and 2C19 cytochrome p450 subtype. Cimetidine did not alter the pharmacokinetics of letrozole nor did letrozole significantly modify the pharmacokinetics of warfarin ( 17). Anastrozole inhibited cytochrome p450 enzymes 1A2, 2C9 and 3A only at concentrations far exceeding that found in the therapeutic range. Based upon the latter observation, it is unlikely that anastrozole would result in clinically significant drug interactions with cytochrome p450 metabolized compounds ( 18, 26)
Effects on hormone levels
Fadrozole produced significant reductions in oestradiol, oestrone and oestrone sulphate after 2 weeks of drug therapy, with a dose-dependent effect noted for oestrone alone. After 12 weeks, however, a slight increase in hormone levels was noted although this was not confirmed by a subsequent study ( 27, 28). Rogletimide 800 mg twice a day produced less reduction in oestradiol levels (mean±SD 57·6±9·2%) than aminoglutethimide 250 mg q.i.d. (75·7±7·3%) although the difference was not statistically significant ( 15)
Lipton et al. found that 0·l mg daily of letrozole reduced oestrone and oestradiol levels within 24 hours by 55% and 62%, respectively ( 29). Maximum suppression was reported to have occurred within 2–3 days of therapy and appeared to be dose-related ( 17). By the end of a 2-week period of letrozole therapy, oestrone, oestradiol and oestrone sulphate concentrations fell by 95% with similar results reported by other authors ( 10, 13, 29, 30). Anastrozole reduced oestradiol levels by 70% within 24 hours and by 80% after a fortnight of therapy ( 18, 31). More than 90% inhibition of oestradiol occurred after 3 weeks of therapy with comparable results reported for oestrone ( 31). At doses ranging from 1 to 5 mg daily, vorozole lowered oestradiol levels by 89–91%, while oestrone and oestrone sulphate levels fell by more than 50% and 63%, respectively ( 22)
The steroidal aromatase inhibitors appear to be less effective than the non-steroidal compounds in reducing oestradiol concentrations, although the clinical significance of greater suppression is unproved ( 8, 23). Orally administered formestane lowered oestrogen levels significantly within 3 h of commencing therapy. By day 4 of treatment, oestradiol concentrations were less than 50% of baseline values with this suppression maintained until day 14. Both 250 mg and 500 mg of IM formestane reduced oestradiol levels to approximately 60% of baseline levels within 24 hours and remained less than 50% from days 7–14. A greater degree of variability in hormone suppression was noted with the lower dose and some recovery occurred towards the end of the 2-week dosage interval. Oestrone levels were also reduced to 40% of baseline values ( 20). Pickles et al. noted slightly less reduction in oestradiol and oestrone levels to 76% and 64% of baseline values, respectively, with a 2-weekly 250 mg intramuscular dose. Moreover, a rise to pre-treatment concentrations occurred after 8–16 weeks of therapy ( 32). Exemestane 25 mg daily reduced oestradiol levels to 28% of baseline values with comparable results reported by Zilembo et al. Doses exceeding 25 mg daily provided no further benefit ( 16, 23)
While aldosterone levels appear to have remained unchanged during fadrozole therapy, minor reductions in sodium and increases in potassium have been noted in some studies ( 27, 28, 33). Cortisol production and thyroid hormone levels have been unaffected by fadrozole ( 27, 33). A significant reduction in cortisol levels occurred after 2 weeks of 0·5 mg letrozole per day but the concentrations remained within normal limits. The results of two additional phase I studies reported consistent findings ( 10, 29). A small but statistically significant decline in cortisol concentrations occurred following the administration of vorozole 5 mg daily, although 2·5 mg daily did not alter steroid production after the ACTH (adreno?corticotrophin) stimulation test ( 21, 22). In contrast, anastrozole has not been associated with reductions in cortisol. Aldosterone and thyroid function tests have remained unaltered during therapy with letrozole, vorozole and anastrozole ( 17, 18, 21, 22, 29, 30)
Cortisol levels declined significantly with formestane although 11-deoxycortisol levels remained unchanged ( 20, 32). Exemestane did not appear to alter cortisol or aldosterone concentrations during therapy ( 16)
Sex hormone binding globulin (SHBG) levels were significantly suppressed (P< 0·05) with oral formestane and it was suggested that this was due to the high degree of first-pass metabolism of the drug through the liver. However, another study found a similar fall in SHBG levels following intramuscular administra- tion ( 32). Furthermore, SHBG concentrations declined during exemestane treatment ( 23). A comparable decline was noted with vorozole in two studies. The authors of the latter study suggested that the recent cessation of tamoxifen may have accounted for this finding ( 21, 22)
CLINICAL TRIALS
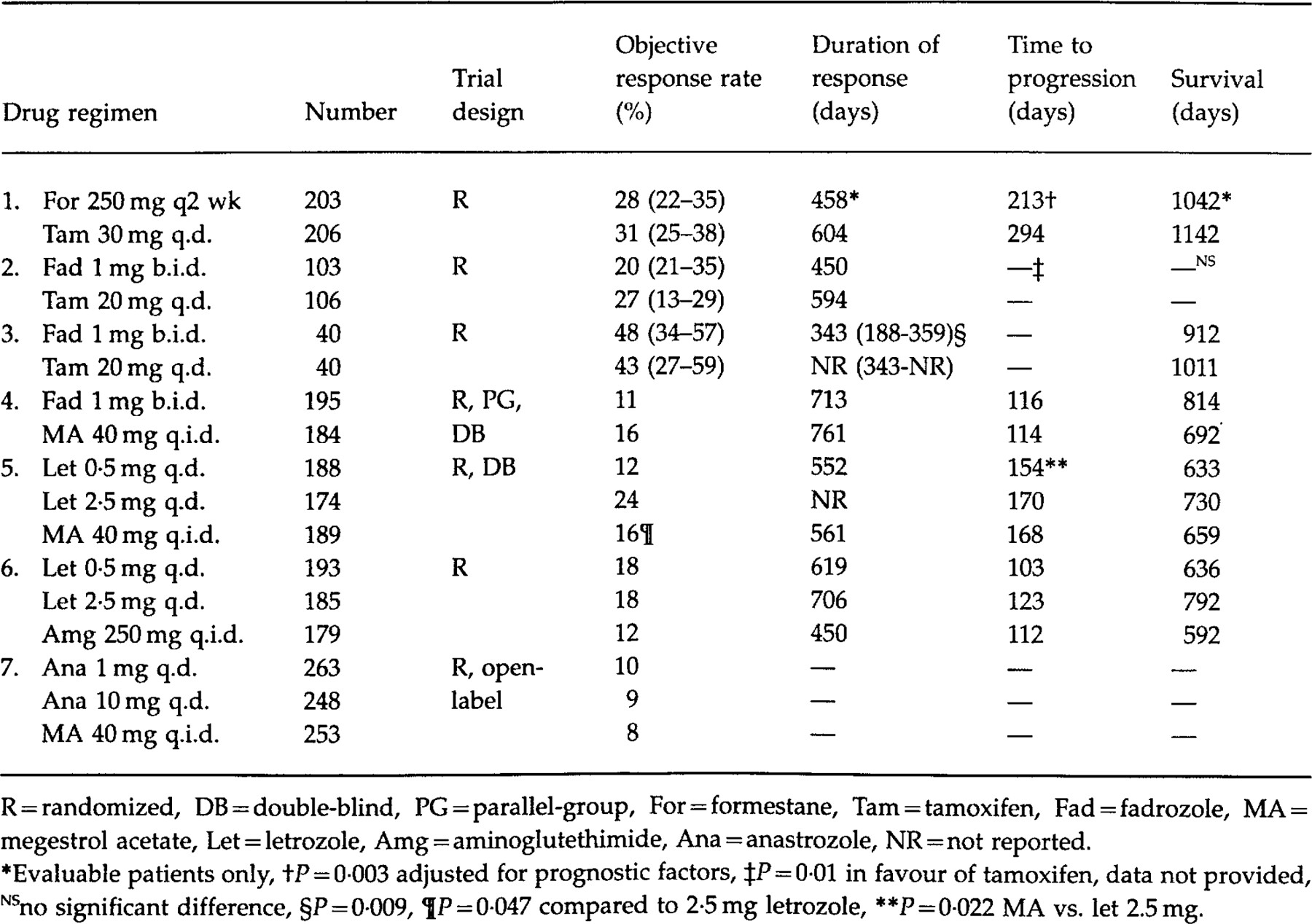
Phase I and II trials with the non-steroidal aromatase inhibitors revealed objective responses ranging from 4% to 36%, depending upon baseline prognostic characteristics ( 10, 13, 21, 22, 28–30, 33–35). The objective response rate includes both complete responses (defined as the complete disappearance of disease for at least 4 weeks) and partial responses (defined as at least a 50% reduction in measurable lesions and stabilization of unmeasurable tissue) ( 37, 42). Early studies with intramuscular formestane and oral exemestane demonstrated objective response rates from 23% to 35% ( 23, 32, 38). These promising results and low toxicity prompted the initiation of phase III studies ( Table 1)
Formestane
Carrion et al. compared the effects of formestane to tamoxifen as first-line hormonal therapy in a randomized, international trial. Baseline characteristics were similar in the two groups, although there was a higher proportion of patients with soft tissue metastases in the formestane arm. Two hundred and three patients were randomized to receive formestane 250 mg every 2 weeks intramuscularly while 206 subjects received tamoxifen 30 mg daily. The dose of tamoxifen employed in this trial was higher than has been typically recommended ( 2). The objective response rate (OR), as assessed by Union International Contre Le Cancer (UICC) criteria, was similar between the two groups according to intention-to-treat analysis [OR: formestane 28%, 95% confidence interval (CI) 22–35%, tamoxifen 31%, 95% CI 25–38%]. Patients with disease limited to soft tissue and those aged 70 years and over were more likely to respond to therapy. While survival among the evaluable patients was not significantly different between the groups, the time to progression (P=0·003) and time to treatment failure (P=0·001) were significantly longer in the tamoxifen group, despite adjustment for prognostic factors ( 14)
Fadrozole
Fadrozole 1 mg twice a day was compared to tamoxifen 20 mg a day as first-line therapy in the management of metastatic breast cancer. Unlike the previous study, women were eligible regardless of oestrogen-receptor status. Patients were stratified according to Eastern Cooperative Oncology Group (ECOG) status, oestrogen-receptor status and previous adjuvant therapy. Over a 6·5-year accrual period, 221 patients were allocated randomly to drug therapy with 12 patients excluded in the efficacy analysis. Nine of the excluded patients were ineligible according to the inclusion criteria, one received radiation therapy and two patients were lost to follow-up. The groups were well-balanced with respect to prognostic variables although the fadrozole patients were more likely to have had visceral metastases and disease in three or more sites; factors which are associated with a poor prognosis. Despite the difference between the cohorts, the objective response rate [OR: tamoxifen 27%, 95% CI 21–35%; fadrozole 20%, 95%CI 13–29%] as determined by the World Health Organisation (WHO) criteria, was not significantly different between the treatments (P=0·26). Sub-group analysis revealed that responses were more likely in patients with prior response to endocrine therapy, limited number of metastatic sites, oestrogen-positive or unknown status and those patients with soft tissue disease only. The duration of response and survival was comparable with both therapies. However, the time to treatment failure was longer with tamoxifen (P=0·05), although when adjustment for baseline prognostic factors was performed, the hazards ratio of 1·3 (95% CI 0·96–1·95) was non-significant (P=0·09). Crossover into the alternate treatment category was permitted with more patients who initially received fadrozole crossing over to the other drug (second-line therapy: tamoxifen 75%, fadrozole 60%). The secondary response rate appeared to be higher with tamoxifen at 29% compared to a 9% response rate with fadrozole, but the significance of this was not tested as the treatments were not randomized ( 39)
Similarly, in another trial fadrozole (n=40) was compared to tamoxifen (n=40) as first-line therapy in advanced breast cancer. However, only women with oestrogen receptor-positive or unknown status were eligible. Intention-to-treat analysis revealed no significant difference in the response rate between tamoxifen (OR 47·5%, 95% CI 33·0%-56·5%) and fadrozole (OR 42·5%, 95% CI 27·0–59·1%), although the study may have lacked power to detect a difference. The overall response rate in this trial appeared to be higher than in the previous studies. This may have been due partly to the inclusion of a large proportion of patients with soft tissue metastases in the study, in which the majority of responses were seen. The median duration of response was significantly shorter (P=0·009) in the fadrozole group at 343 days (95% CI: 188–359 days) while this parameter had yet to be attained in the tamoxifen arm (343–NR). However, both the time to treatment failure (tamoxifen 5·4 months, fadrozole 5·1 months) and survival (tamoxifen 33·7 months, fadrozole 30·4 months) were similar. The author's explanation for the disparate results was the 10-year age difference between the two groups. However, in a formestane study older patients were found to obtain a higher response rate than their younger counterparts ( 14, 40)
Buzdar et al. reviewed the results of two phase III trials, which assessed the efficacy of second-line fadrozole and megestrol acetate in over 650 women. The studies were randomized, parallel group but, unlike the prior trials, were double-blinded. Individuals with oestrogen receptor-negative status were excluded from the studies. Subjects were randomized to receive fadrozole l mg twice a day or megestrol acetate 40 mg four times a day, both with matching placebo. In the first study, the objective response rate to fadrozole was 11·3% vs. 16·3% with megestrol acetate while the respective figures in the second trial were 13·4% and 11·5%, which were not significantly different. The median duration of response, time to progression and survival were similar with both drug therapies in each trial. Patients with a disease-free interval equal to or greater than 2 years and patients with soft tissue disease alone were more likely to benefit from therapy ( 37)
Letrozole
The results of a trial comparing letrozole to megestrol acetate as second-line therapy have been published in abstract from. Five hundred and fifty-one patients with oestrogen receptor-positive or unknown tumours were randomized to receive letrozole 0·5 mg daily (n=188), letrozole 2·5 mg daily (n=174) or megestrol acetate 160 mg daily (n=189) in a double-blind manner. UICC criteria were employed to assess response to therapy, which was subsequently confirmed by a blinded external committee. A significantly higher response rate (P=0·047) was observed in patients receiving letrozole 2·5 mg daily (OR: 23·6%) compared to patients taking the progestin (OR: 16·4%). Furthermore, the time to progression (P=0·022) and treatment failure (P=0·002) was significantly longer in patients receiving the higher dose of the aromatase inhibitor. A dose–response effect was also reported ( 17, 41)
In a similar trial, aminoglutethimide 500 mg four times a day with steroid supplementation was compared to letrozole, employing the same doses as the previous study. A team of independent external reviewers verified the results. The response rate was not significantly different between the three groups, although the relative risk of progression was lower with letrozole 2·5 mg daily ( 17)
Anastrozole
The results of two trials comparing double-blind anastrozole at two dosage levels to open-label megestrol acetate were combined, because the study designs were identical. Patients were eligible for inclusion if they had oestrogen receptors present in the breast tissue or had demonstrated a previous response to hormonal therapy. UICC criteria were employed in assessing the response to therapy and a computer program was employed to determine efficacy in patients with measurable disease (> 75% of patients in the trial) in an attempt to limit investigator bias. Two hundred and sixty-three patients were assigned to receive anastrozole 1 mg daily, 248 patients to anastrozole 10 mg daily while 253 subjects received megestrol acetate 40 mg four times daily. The objective response rate was not significantly different between the three groups (anastrozole l mg 10·3%, anastrozole 10 mg 8·9%, megestrol acetate 7·9%) with responses found primarily in subjects with soft tissue disease alone. The overall response rate in this trial was noticeably low but may have been due to the stringent definition of a response. Responses were noted regardless of tamoxifen usage in the adjuvant or metastatic setting. The time to treatment failure and progression as well as survival were similar between the three groups ( 42).
ADVERSE EFFECTS
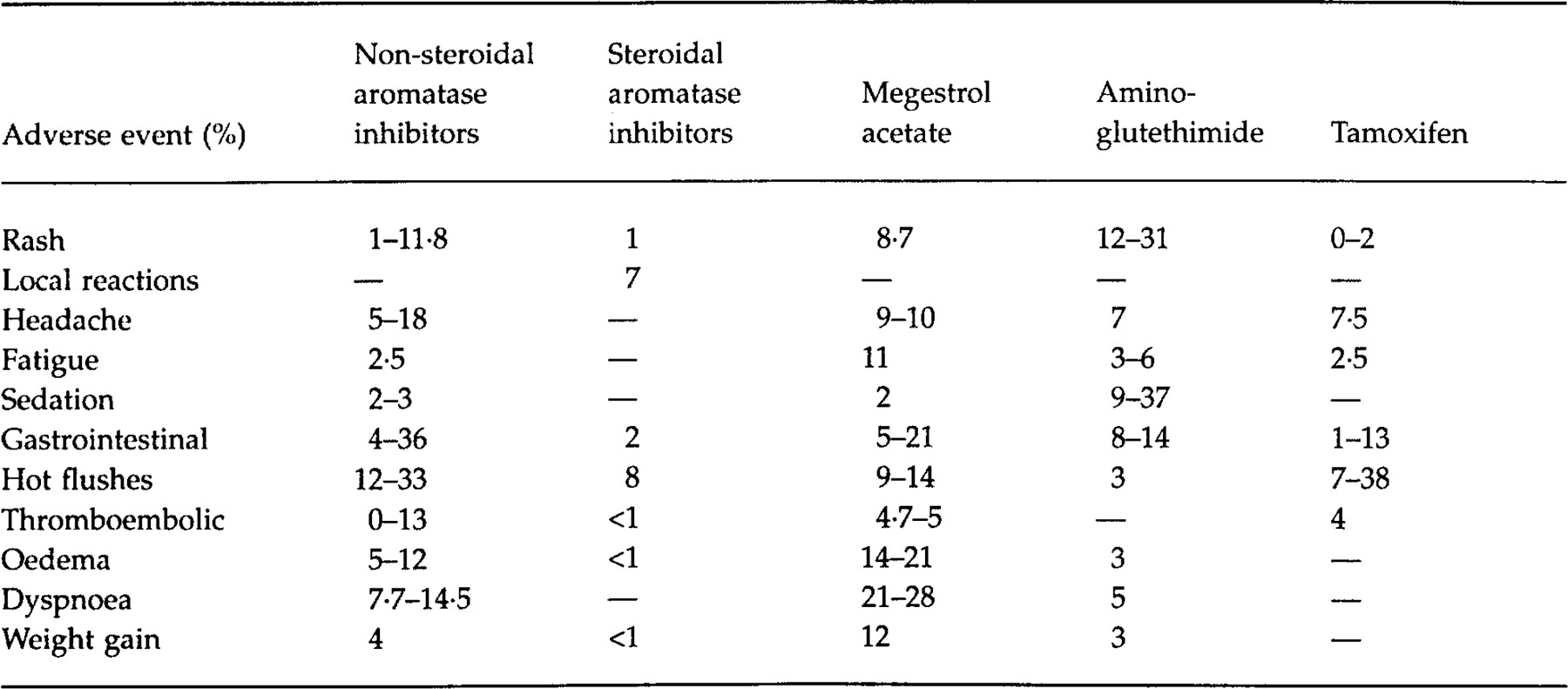
The discontinuation rates with the newer aromatase inhibitors trials have varied from l% to 8% in phase III studies with comparable withdrawal rates in the other treatment arms ( 14, 17, 37, 39, 42). However, in one study lower withdrawal rates of 2–3% with letrozole were noted compared to 8% with megestrol. Interestingly, discontinuations due to reasons other than progression were reported by a similar percentage of patients receiving either aminoglutethimide or letrozole ( 17)
The adverse effects reported in phase III clinical trials have been summarized in Table 2. Aminoglutethimide is related to the sedative glutethimide, thus lethargy may be experienced in one-third of patients. Somnolence was reported by 9% of patients taking aminoglutethimide whereas only 2–3% of letrozole patients complained of this side-effect in a direct comparative trial ( 17). While fatigue and lethargy were common adverse effects reported by patients assigned to aminoglutethimide, there appeared to be no difference in the incidence between the newer aromatase inhibitors and either tamoxifen or megestrol ( 14, 37, 40, 42). A transient rash has been reported in 12–30% of patients during the first week of therapy with aminoglutethimide. This is not an indication for drug discontinuation and may be managed by doubling the dose of the steroid. In a phase III comparative trial, the incidence of rash was lower with letrozole (2–3%) ( 5, 7, 29). Following the administration of intramuscular formestane, between 8% and 25% of patients experienced local reactions, ranging from inflammation to a mass at the site. In a phase II study the incidence of sterile abscesses was 6·4% with formestane ( 14, 38)
The incidence of gastrointestinal side-effects was higher in patients assigned to non-steroidal aromatase inhibitors compared to those patients receiving megestrol acetate in four trials ( 17, 18, 42). In the only study comparing a steroidal aromatase inhibitor to tamoxifen, the incidence of nausea and vomiting was similar ( 14)
Hot flushes are a frequent side-effect of antioestrogen therapy. The incidence has varied from 2% to 33% with the newer aromatase inhibitors, with similar rates in patients receiving tamoxifen or megestrol acetate ( 14, 39, 40, 42)
A concern with tamoxifen has been the propensity to induce thromboembolic events because it may reduce levels of antithrombin III ( 8). In a study by Thürlimann and colleagues, a 4% incidence in thromboembolic events was noted with three deaths occurring in the tamoxifen group, while no events were reported in the fadrozole arm ( 39). A higher incidence of thromboembolic adverse effects was noted in patients receiving megestrol compared to those taking either letrozole or anastrozole, although this difference was not significant in the latter trial ( 17, 18, 41, 42).
More patients receiving megestrol acetate reported oedema (14–21%) compared to those taking either fadrozole (11–13%) or anastrozole (7–11%) ( 37, 42). This may have explained the higher incidence of dyspnoea with the progestin (23–28%) compared to fadrozole (7·7–14·5%) ( 37). Similarly, oedema may have accounted for the higher proportion of subjects experiencing a weight gain of more than 10% (≈2·7–4·5 kg) with megestrol acetate ( 41, 42)
CONCLUSION
amoxifen remains the first-line therapy in post-menopausal women with advanced breast cancer, because the efficacy of the newer aromatase inhibitors is largely unknown in this setting ( 14, 39, 40). However, these agents may be recommended if progression on tamoxifen occurs, because these compounds appear to be equally efficacious to aminoglutethimide and megestrol acetate, but have been associated with a lower degree of toxicity. In particular, less somnolence and rash is experienced compared to aminoglutethimide and a lower incidence of oedema and weight gain is observed when compared to megestrol. Moreover, convenient dosing, fewer drug interactions and the lack of need for corticosteroid supplementation further increases the appeal of these newer agents. Data from clinical trials suggest that women with oestrogen or progesterone receptor-positive, a long disease-free interval and non-visceral disease will benefit the most from therapy. Furthermore, these drugs may be an alternative to tamoxifen if side-effects to this first-line agent occur. The role of aromatase inhibitors in the adjuvant and preventive setting is currently unknown ( 8). However, their lack of oestrogenic effects on the uterus and thromboembolic potential may provide an advantage over tamoxifen, although this has yet to be proved