Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1-positive carcinoma cells
Summary
The tumour-associated antigen mucin 1 (MUC1) is a multifunctional protein involved in protection of mucous membranes, signal transduction, and modulation of the immune system. More than 70% of cancers overexpress MUC1, making MUC1 a potential target for immunotherapy. In the present study, MUC1 transgenic mice were crossed with syngeneic strains that express the polyomavirus middle-T oncogene (PyMT) driven by the mouse mammary tumour virus promoter long-terminal repeat (MMTV-LTR). The resultant breed (MMT mice) developed spontaneous MUC1-expressing mammary carcinomas with 100% penetrance at 8–15 weeks of age. As found in human breast cancer, the mammary carcinoma in MMT mice arose in multiple stages. Immunization with fusions of dendritic cells and MUC1-positive tumour cells (FC/MUC1) induced MUC1-specific immune responses that blocked or delayed the development of spontaneous breast carcinomas. In contrast, there was no delay of tumour development in MMT mice immunized with irradiated MC38/MUC1 tumour cells. The efficacy of fusion cells was closely correlated with the timing of initial immunization. Immunization with FC/MUC1 initiated in MMT mice at < 1, 1–2 and 2–3 months of age rendered 33, 5 and 0% of mice free of tumour, respectively, up to 6 months. Whereas mice immunized in the later stage of tumour development succumbed to their disease, immunization resulted in control of tumour progression and prolongation of life. These results indicate that immunization with FC/MUC1 can generate an anti-MUC1 response that is sufficient to delay the development of spontaneous mammary carcinomas and control tumour progression in MMT mice.
Introduction
Active specific immunotherapy is an approach used to elicit and boost immune effector mechanisms to achieve an augmented antitumour response.1–3 T-cell-mediated immunity directed against tumour antigens has been documented in animal models and patients with breast carcinomas.4–7 These responses, however, are often ineffective in eradicating the tumour. The activation and boosting of the host's immune surveillance against antigens selective for or overexpressed in breast carcinomas represent a potentially useful antitumour strategy. One such tumour antigen is mucin 1 (MUC1). MUC1 is a high-molecular-weight glycoprotein that is overexpressed in human breast cancers.8 Aberrant glycosylation of MUC1 in breast carcinoma cells results in the generation of distinct epitopes not found in normal tissues.9,10 Studies have demonstrated that these cryptic epitopes are recognized by cytotoxic T lymphocytes (CTLs) in patients with breast carcinomas5,11 and in animal models.12–15 These findings suggest that the MUC1 antigen may represent a target for immunotherapy of breast cancer.
Effective antigen presentation is the key to an efficacious tumour vaccine. Dendritic cells (DCs) have been identified as the most potent antigen-presenting cells.16–18 Fusions of DCs with carcinoma cells have proved to be effective in the induction of antitumour immunity. Vaccination with fusions of murine tumour cells and syngeneic DCs has been shown to eliminate established tumour metastases in wild-type and MUC1 transgenic (MUC1.Tg) mice. These studies were conducted in murine models of transplanted tumour cells. The injected tumours usually grow quickly and the host immune system, while potentially competent, does not have sufficient time to generate an effective antitumour response. Furthermore, these models are not appropriate for cancer prevention studies because the tumours lack the premalignant or early lesions that are the critical stage at which the host must mount an effective immune response. We have produced a transgenic murine model (MMT) that expresses the polyomavirus middle-T (PyMT) oncogene under control of the mouse mammary tumour virus promoter long-terminal repeat (MMTV-LTR)19 and develops spontaneous mammary carcinomas. The spontaneous mammary tumours also express the MUC1 tumour-associated antigen.15 One advantage of this model is that tumours develop from normal cells in their natural tissue microenvironment with a viable immune system and progress through multiple stages found in human cancer.19–21
In the present study, MMT mice were immunized with fusions of DCs and MUC1-positive tumour cells (FC/MUC1) to determine the effectiveness of cancer prevention and treatment in a murine model relevant to human cancers. We demonstrate that immunization with FC/MUC1 induces specific anti-MUC1 immunity that delays the development of spontaneous breast carcinomas and controls the progression of tumours in MMT mice. These results indicate that MUC1 is a potent immunogenic antigen capable of inducing an immune response and tumour rejection.
Materials and methods
Mice
Female C57BL/6 mice, 6 to 8 weeks old, were purchased from Taconic (Germantown, NY). The transgenic mice included: (i) MT mice expressing the polyomavirus middle-T oncogene driven by the mouse mammary tumour virus (MMTV) long-terminal repeat (LTR) that develop spontaneous mammary carcinomas,19 (ii) MUC1 transgenic mice (MUC1.Tg) expressing the human MUC1 antigen in a tissue-specific fashion similar to that in humans,22 and (iii) MMT mice that express the PyMT and the MUC1 antigen and develop spontaneous mammary carcinomas.15 The MT mice were generated by breeding the female wild-type C57BL/6 strain with male MT mice. The MMT mice were generated by crossing the female C57BL/6 strain of MUC1.Tg mice with male MT mice. All mice were congenic on the C57BL/6 background at N > 10. The mice were selected for the expression of the PyMT oncogene and/or MUC1 using the polymerase chain reaction (PCR).22,23 Only female mice positive for either MT (MT mice) or MT/MUC1 double transgenes (MMT mice) were used for the experiments. The mice were maintained in microisolator cages under specific pathogen-free conditions.
Cell culture and fusion
Murine MC38 adenocarcinoma cells (C57BL/6) were stably transfected with a MUC1 cDNA (MC38/MUC1).24 Cells were maintained in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mml-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. DCs were obtained from bone marrow cultures of C57BL/6 mice.25 The methods of DC generation and fusion with MC38 or MC38/MUC1 tumour cells in the presence of 50% polyethylene glycol have been described.26 Briefly, DC and MC38/MUC1 cells were collected, washed twice in serum-free medium, and counted. DCs were mixed with MC38/MUC1 cells in a 10 : 1 ratio. The fusion process was carried out with 50% polyethylene glycol (PEG) in prewarmed Dulbecco's phosphate-buffered saline (PBS) without Ca2+ or Mg2+ at pH 7·4. After washing twice, the fused cells were plated in 24-well culture plates for 5 days. Then the cells were plated in six-well culture plates in complete RPMI 1640 medium supplemented with 20 ng/ml recombinant murine GM-CSF (Sigma Chemical Co., St. Louis, MO). By day 5 of culture, the unfused tumour cells had become firmly attached to the tissue culture flask, while the fused cells could be dislodged by gentle pipetting. The latter were then collected and analysed by flow cytometry for antigen expression.
PCR
The mice were examined for MUC1 and MT genes by PCR analysis. Ten-µg aliquots of tail and mammary tumour tissue were digested with proteinase K. DNA was extracted using the Dneasy™ Tissue Kit (QIAGEN, Valencia, CA). PCR was carried out in a total volume of 50 µl in Perkin-Elmer Gene Amp tubes (Perkin-Elmer, Norwalk, CT) with the following reagents: 5 µl 10 × PCR buffer including 15 mm MgCl2; 0·02% formamide; 0·2 mm dNTPs; 100 nm 5′-CTTGCCAGCCATAGCACCAAG-3′ (bp 745–765) forward primer, and 100 nm 5′-CTCCACGTCGTGGACATTGATG-3′ (bp 1086–1065) reverse primer for the MUC1 gene; 100 nm 5′-AGTCACTGCTACTGCACCCAG-3′ (bp 282–302), and 100 nm 5′-CTCTCCTCAGTTCCTCGCTCC-3′ (bp 817–837) primers for the MT gene; 1·25 units of Taq polymerase; 2 µl of tail DNA (approximately 500 ng) and reagent quality H2O. The amplification programme consisted of one cycle for 10 min at 94° and 40 cycles of 30 s each at 94°, 61° and 72°. The PCR product of each reaction was analysed by size fractionation in a 1% agarose gel. Amplification of MUC1-positive DNA resulted in a 500-bp fragment22 and that of MT-positive DNA in a 491-bp fragment.23
Immunoblotting
Mammary carcinomas from MT or MMT mice were harvested, rapidly frozen in liquid nitrogen and stored at −80° until use. The samples were lysed in a solution containing 0·05 m sodium chloride, 0·02 m Tris, pH 7·4, 100 µg/ml leupeptin, 50 µg/ml aprotinin and 1% NP-40. Equivalent amounts of protein were separated in 5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were stained with Ponceau S, photographed, blocked in 5% non-fat dry milk in PBS + 0·05% Tween 20 and probed with anti-MUC1 mAb (DF-3) for 1 hr at 25°. Membranes were incubated with horseradish proxidase (HRP)-conjugated rabbit anti-mouse antibody (Amersham, Pharmacia Biotech, Piscataway, NJ) for 1 hr at 25°. The antigen–antibody complexes were visualized by Electro Chemiluminescence (ECL, Amersham).
Histological and immunohistochemical staining
Groups of MT or MMT mice were killed at various ages (from 3 weeks to 6 months). Mammary tissue or tumour was harvested and fixed in 2% paraformaldehyde. Sections (5 µm) were cut and stained with hematoxylin and eosin (H&E). For detecting MUC1 expression, sections were stained with anti-MUC1 mAb (DF3) for 30 min at room temperature and then subjected to indirect immunoperoxidase staining using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Flow cytometry
DC, FC/MUC1 and MC38/MUC1 carcinoma cells were double stained with fluorescein-isothiocyanate (FITC)-conjugated-mAb HMPV (anti-MUC1, BD Pharmingen, San Diego, CA) for 30 min on ice. After washing twice with PBS, the phycoerythrin (PE)-conjugated mAb M5/114 (anti-MHC class II, BD Pharmingen) was added for another 30 min on ice. Splenocytes were purified by passage through nylon wool and stained with the following antibodies: anti-CD4 (H129·19), anti-CD8 (53-6·7), anti-NK1·1 (PK136), anti-αβ TCR (H57-597) and anti-γδ TCR (GL3) (BD Pharmingen) for 30 min on ice. The cells were washed with PBS and incubated with FITC-conjugated anti-rat, mouse and hamster immunoglobulin G (IgG) for an additional 30 min on ice. All the cells were washed, fixed, and analysed by FACScan (Becton Dickinson, Bedford, MA) with CellQuest analysis software (Becton Dickinson).
Vaccination
Groups of 4-week-old MT or MMT mice were vaccinated subcutaneously with 5 × 105 FC/MUC1 cells, FC/MC38 cells or MC38/MUC1 tumour cells that had been exposed to 60-Gy ionizing radiation (Gammacell 1000 Atomic Energy of Canada, Ottawa, Canada). In another experiment, MMT mice were immunized at varying ages. The immunization was repeated four additional times at monthly intervals. The mice were followed for up to 6 months. From 6 weeks of age, the mice were palpated every other day to assess the presence of mammary tumours. A progressively growing mass was regarded as tumour and was measured by calipers in two perpendicular diameters. Tumour incidence was determined and recorded. Mice with tumours ≥ 2 cm were killed. The mice were cared for according to the Institutional Animal Care and Use Committee Guidelines.
Humoral immune responses
Microtitre plates were precoated overnight at 4° with 100 µl/well of MUC1 antigen (5 units/ml in PBS, pH 7·4). MUC1 antigen was purified from supernatant of ZR-75 human breast cancer cells using the method previously described.27–30 Briefly, the supernatant obtained by culturing ZR-75 cells for 3 or 4 days in the serum-free medium was concentrated in a stirred ultrafiltration cell (Millipore, Billerica, MA) on a YM30 filter. The concentrate was centrifuged and applied to an agarose-wheat germ lectin column (Pharmacia, Peapack, NJ). The DF3/MUC1 antigen containing fractions was eluted with 1 m N-acetylgulcosamine. The elute was dialysed, concentrated, and then applied to Sephacryl S-300 (Pharmacia). The DF3/MUC1 antigen-containing fractions were monitored by immunoassay, concentrated and then incubated with mAb DF3-Sepharose 4B. The MUC1 antigen was eluted with 3 m MgCl2. The MgCl2 fraction was dialysed against water and then concentrated. Anti-mouse IgG at 100 µl/well (1 µg/ml in PBS, pH 7·4) was used to precoat the well as a control. Each well was washed three times with PBS/Tween (0·05% v/v Tween 20) and blocked with 120 µl/well 5% horse serum in PBS for 1 hr at room temperature. After washing, 4-fold dilutions of mouse sera were added to each well for 2 hr. The plates were washed and incubated with sheep anti-mouse IgG conjugated to horseradish peroxidase (Amersham, Piscataway, NJ). Antibody complexes were detected by development with o-phenylenediamine (Sigma) and measured in an enzyme-linked immunosorbent assay (ELISA) microplate autoreader EL310 (Bio-Rad, Hercules, CA)at an OD of 490 nm.
51Cr cytotoxicity assay
Splenocytes were isolated from MMT mice by Ficoll separation. The target cells include mammary carcinoma cells isolated from MMT or MT mice, MC38/MUC1, MC38 (the parent cells of MC38/MUC1), and YAC-1 cells. These target cells were prelabelled with 51Cr for 1 hr at 37° and added to wells of 96-well v-bottom plates with T cells (effector cells) for 5 hr at 37°. The supernatants were assayed for 51Cr release in a gamma counter and CTL activity was determined at the indicated effector:target (E:T) ratios. Percentage of specific 51Cr release was determined by the following equation:
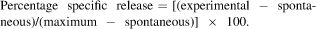
Statistical analysis
Statistical significance was determined using Student's t-test.
Results
Characterization of mammary carcinomas in MT and MMT mice
MMT mice have been generated by breeding MT mice with MUC1.Tg mice. Bitransgene-positive mice were selected using PCR. Mammary tumours first appeared in the female MMT mice at about 8 weeks of age. All mice developed mammary tumours by 15 weeks of age (range from 8 to 15 weeks) with a median of 12 weeks. The mammary tumours were multiple in nature with synchronous kinetics. The tumours progressed rapidly, and the mice became moribund at 16–18 weeks of age (Fig. 1a) when they were killed.

Mammary carcinoma development in MMT and MT mice. (a) Growth rate of spontaneous mammary carcinomas in MMT (●) and MT (○) mice. Three mice were used at each time-point. The error bars represent SE of the mean. (b) Histological examination with H&E staining of spontaneous mammary tumours in MT and MMT mice at different ages (×16).
To establish the pattern of tumourigenesis, groups of MMT and MT mice were killed at varying time-points and their mammary tissue was subjected to histological examination. At least three stages of tumour development were observed. Mammary glands appeared normal until the 3rd week of age. Focal hyperplasia began to appear in the 4th week, which evolved into dysplasia and carcinoma in situ. Invasive tumours emerged at 8 to 9 weeks (Fig. 1b). A similar trend was observed in MT mice. These findings indicate that expression of PyMT resulted in transformation of the mammary epithelia and rapid production of mammary carcinomas in MT and MMT mice. Expression of MUC1 shortened the latency period to some extent, although no significant difference was found between the two groups. More importantly, multiple stages of tumour development, similar to those found in human cancers, were observed.
Expression of MUC1 on mammary carcinoma cells in MMT mice
To assess expression of MUC1 in the mammary carcinomas of MMT mice, western blotting, PCR and immunohistochemical staining techniques were used. Western blot showed the expression of MUC1 in spontaneous mammary carcinoma cells from MMT, but not from MT, mice at a level comparable to that found in MCF-7 human breast cancer cells (Fig. 2a). At the DNA level, the presence of MUC1 and PyMT was detected in tumour cells from MMT mice by PCR analysis. In contrast, only PyMT was detected in tumour cells from MT mice (Fig. 2b). Furthermore, we detected MUC1 expression on mammary tumour cells from MMT, but not MT, mice with immunohistochemical staining (Figs 2c and d). Whereas MUC1 expression was confined to the apical or lumenal surface of normal mammary epithelia, MUC1 was detectable over the entire surface of spontaneous mammary tumour cells. Taken together, these results indicate that MUC1 is overexpressed on mammary carcinoma cells in MMT mice.
MUC1 expression on mammary carcinomas. (a) Lysates from MMT carcinoma (lane 1), MT carcinoma (lane 2) and human MCF-7 tumour cells (lane 3) were analysed by western immunoblotting with anti-MUC1 mAb DF3. (b) PCR was used to detect MT and MUC1 genes in mammary carcinoma from MMT and MT mice. (c, d) Immunohistochemical staining with mAb DF3 to detect the expression of MUC1 on mammary carcinomas from MT and MMT mice (×40).
Induction of anti-MUC1 immunity in MMT mice by immunization with FC/MUC1
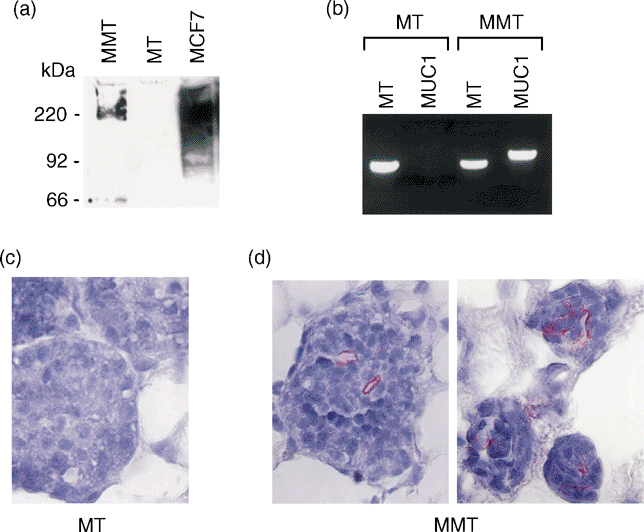
MC38/MUC1 carcinoma cells were successfully fused with syngeneic DCs. The fused cells (FC/MUC1) expressed tumour-derived MUC1 and DC-derived MHC class II molecules (Table 1a). In contrast, fusion of DC with MC38 tumour cells (FC/MC38) resulted in the expression of MHC class II molecules but not MUC1 (Table 1a). The fusion efficiency was 21·16%, as determined by dual expression of MUC1 and MHC class II. To assess induction of anti-MUC1 humoral immune response, MT and MMT mice were immunized subcutaneously with FC/MUC1. The immunization was repeated four additional times at 4-week intervals. Irradiated MC38/MUC1 tumour cells, fusions of DCs and MC38 tumour cells (FC/MC38) or PBS injection were used as controls. The sera from immunized and control mice were collected 7 days after each immunization and analysed for the presence of anti-MUC1 antibody by ELISA assay. An anti-MUC1 humoral response was induced in MMT mice immunized with FC/MUC1, but not with irradiated MC38/MUC1, FC/MC38 or PBS injection (Fig. 3a). The anti-MUC1 antibody titre increased after the second immunization, peaked after the third and fourth immunizations, then decreased and was maintained at lower levels at 6 months of age (Fig. 3b). In contrast, there was no anti-MUC1 antibody detected in MMT mice injected with PBS (Fig. 3b). These results indicate that immunization with FC/MUC1 is associated with production of anti-MUC1 antibodies in MMT mice.
|
(a) |
||||
|---|---|---|---|---|
| % positive cells | ||||
| DC | MC38/MUC1 | FC/MUC1 | FC/MC38 | |
| Anti-MUC1 | 0·10 | 98·01 | 68·14 | 0·24 |
| Anti-MHC II | 82·08 | 1·36 | 27·98 | 25·88 |
| Anti-MUC1/MHC II* | 0·22 | 0·13 | 21·16 | 4·02 |
|
(b) |
|||
|---|---|---|---|
| Antibodies | % positive splenocytes† | ||
| PBS | MC38/MUC1 | FC/MUC1 | |
| Anti-CD4 | 27·22 | 26·03 | 29·52 |
| Anti-CD8 | 36·02 | 34·56 | 39·92 |
| Anti-NK | 8·92 | 7·98 | 8·04 |
| Anti-αβ TCR | 41·14 | 42·31 | 43·16 |
| Anti-γδ TCR | 2·22 | 2·08 | 1·96 |
- * Percentage of double-positive cells.
- † Splenocytes were collected from MMT mice immunized five times with FC/MUC1 or irradiated MC38/MUC1 tumour cells, or treated with PBS.
- NK, natural killer; TCR, T-cell receptor.
Anti-MUC1 humoral response induced in MMT mice by FC/MUC1 immunization. (a) The measurement of a single serum sample from one female MMT mouse or her littermates. The mice at 4 weeks of age were immunized with 5 × 105 FC/MUC1 cells (●), irradiated MC38/MUC1 cells (▪) or FC/MC38 cells (□), or treated with PBS (○). The immunization was repeated at 8 and 12 weeks. Mice were killed 1 week after the last immunization. Serum from the mice was collected and anti-MUC1 antibodies were detected by ELISA. The results are expressed as the mean ± SD of three replicates. (b) Serum was collected from MMT mice of various ages (three or four mice per group) that had been immunized at different times with 5 × 105 FC/MUC1 cells (●) or treated with PBS (○) and assayed for the presence of anti-MUC1 antibody. Each dot represents the level of anti-MUC1 antibody (1 : 5 dilution) at the indicated age and number of immunizations. (c) MMT mice were immunized with 5 × 105 FC/MUC1 cells (●) at 4 weeks of age. The immunization was repeated at 8 and 12 weeks. Their littermates were immunized with FC/MC38 cells (□), irradiated MC38/MUC1 cells (▪) or PBS (○). One week after the last immunization, the mice were killed and splenocytes were purified through nylon wool to remove antigen-presenting cells. T cells were incubated with mammary carcinoma cells from MMT mice at the indicated effector:target ratios. CTL activity was determined by the 51Cr release assay. The results are expressed as the mean ± SD of three replicates. (d) Splenocytes were isolated from MMT mice of various ages (three or four mice per group) that had been immunized at different times with 5 × 105 FC/MUC1 cells (●) or PBS (○). CTL activity against mammary carcinoma cells from MMT mice at an effector:target ratio of 100 : 1 was determined by 51Cr release. Each dot represents the CTL activity at the indicated age and number of immunizations. (e) Splenocytes were isolated from MMT mice immunized five times with 5 × 105 FC/MUC1 (right panel) or irradiated MC38/MUC1 tumour cells (middle panel) or treated with PBS (left panel). CTL activity against MC38 (○) and MC38/MUC1 (●), MT (□) and MMT (▪) mammary carcinoma cells, and YAC-1 (▵) cells at the indicated effector:target ratio was determined by 51Cr release.
To assess the induction of CTL against MUC1-positive tumour cells, splenocytes from MMT mice immunized with FC/MUC1 were collected at multiple time-points. Table 1b shows the phenotypes of the splenocytes. The standard 51Cr release assay was used to determine the CTL activity against spontaneous mammary tumour cells from MMT mice (MMT tumour cells, MUC1-positive). CTL activity against MMT tumour cells (37%) was induced in splenocytes from MMT mice immunized with FC/MUC1 (Fig. 3c). In contrast, there was no apparent CTL activity induced in their littermates immunized with irradiated MC38/MUC1, FC/MC38 or PBS (Fig. 3c). The CTL activity against MUC1-positive targets was detectable at 20–38% lysis throughout the experiment (Fig. 3d). To assess the specificity of CTL activity, multiple targets were used. CTL activity against MC38/MUC1, MMT tumour and, to a lesser extent, MC38 cells was induced by FC/MUC1 immunization (Fig. 3e, right panel). This result is consistent with our previous findings that polyclonal CTLs are induced by the FC/MUC1 vaccination against MUC1 antigen as well as unknown tumour antigens expressed by MC38 and MC38/MUC1. In contrast, there was little, if any, CTL activity against MT and YAC-1 cells (Fig. 3e, right panel). As expected, no CTLs were induced in MMT mice immunized with irradiated MC38/MUC1 tumour cells (Fig. 3e, middle panel) or treated with PBS (Fig. 3e, left panel). Taken together, the results indicate that immunization with FC/MUC1 induces polyclonal and antigen-specific CTLs.
Inhibition of spontaneous mammary carcinomas in MMT mice by fusion cell immunization
To assess the efficacy of immunization with fusion cells in vivo, MMT mice were immunized with FC/MUC1 cells, FC/MC38 cells or irradiated MC38/MUC1 cells, or injected with PBS. Immunization was begun at 4 weeks of age. The mice received four additional immunizations at 4-week intervals (Fig. 4a). All MT mice treated with PBS, irradiated MC38/MUC1 cells or FC/MUC1 developed mammary tumours at 8–15 weeks and became moribund 4 weeks after tumour appearance (Fig. 4b, left panel). In contrast, immunization of MMT mice with FC/MUC1 significantly delayed the development of spontaneous mammary tumours (Fig. 4b, right panel). The specificity of these responses against MUC1 is supported by the finding that the immunization of MMT mice with FC/MC38 (MUC1-negative) or immunization of MT mice with FC/MUC1 gave no protection against the development of mammary carcinomas (Fig. 4b, left panel). These results indicate that the anti-MUC1 immune responses induced by the immunization with FC/MUC1 can block or delay the development of mammary tumours in a predisposed model.
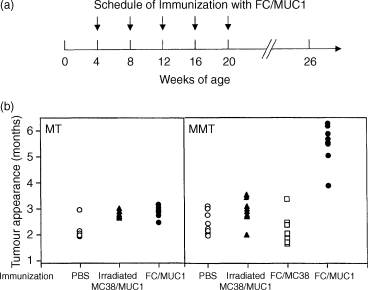
Inhibition of spontaneous mammary carcinoma in vivo. (a) Schedule of immunization with FC/MUC1. Female MMT and MT mice were vaccinated subcutaneously with 5 × 105 FC/MUC1 cells, FC/MC38 cells or irradiated MC38/MUC1 cells at the base of tail at < 1 month of age. Immunization was repeated for four additional times at 4-week intervals. (b) Tumour incidence in MT (left panel) and MMT (right panel) mice immunized with FC/MUC1 cells (●), FC/MC38 cells (□), irradiated MC38/MUC1 cells (▴) or PBS (○). Each dot represents the appearance of tumour for individual mice at the indicated age.
Control of tumour progression with fusion cell immunization
One of the characteristics of MMT and MT mice is that they develop mammary tumours in multiple stages. We have shown that immunization of MMT mice in the early stage of tumourigenesis can block tumour development. Therefore, it would be of interest to determine whether immunization of MMT mice in the later stage of tumourigenesis can control the progression of spontaneous mammary tumour. To address this issue, MMT mice were divided into three groups according to age at initial immunization. The immunization was begun in the first group of mice at < 1 month of age, in the second group of mice at 1–2 months and in the third group of mice at > 2 months. The immunization was repeated four additional times at 4-week intervals. Immunization of MMT mice with FC/MUC1 at < 1, 1–2 and > 2 months of age rendered 33, 5 and 0% of mice free of tumours, respectively, up to 6 months (Fig. 5a). As expected, all the PBS-treated MMT mice developed mammary tumours and became moribund 4 weeks after tumour occurrence (Fig. 5b, left). However, immunization of MMT mice with FC/MUC1 in the early stages of tumour development rendered 33% mice free of the disease for at least 6 months (Fig. 5b, right), and delayed development of tumour in those in which mammary tumour developed. The percentage of tumour-free mice was statistically significant compared with 1–2 month, >2 months, and non-vaccine groups respectively (P < 0·05, P < 0·0001 and P < 0·0001). Invasive mammary carcinomas were found in samples from PBS-treated mice (Fig. 5c, upper and lower panels). In contrast, there was no tumour formation in MMT mice immunized with FC/MUC1 (Fig. 5d, upper and lower panels). These results indicate that the efficacy of immunization is dependent on the age at which the mice were immunized.
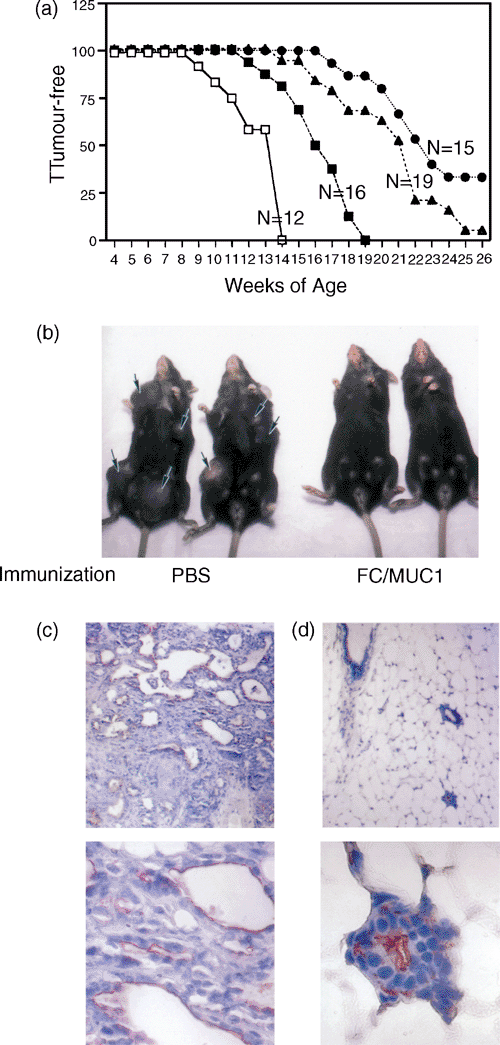
Immunoprevention and immunotherapy with FC/MUC1 immunization. (a) Tumour incidence in MMT mice in which immunization with FC/MUC1 cells was begun at <1 month (●), 1–2 months (▴), or over 2 months (▪). Non-vaccinated age-matched MMT mice were used as the control (□). (b) Photograph of female littermates of MMT mice at age 14 weeks treated with PBS (left panel) or at age 26 weeks treated with FC/MUC1 fusion (right panel). The mice were < 1 month old when treatment with PBS or FC/MUC1 immunization was commenced. (c, d) Photomicrograph of mammary carcinoma from a non-vaccinated MMT mouse at the age of 14 weeks (left panels) and of mammary tissue from a vaccinated MMT mouse at the age of 26 weeks (right panels). The mice were < 1 month old when treatment with PBS or immunization was commenced. The upper panels have magnifications of ×10 and the lower panels ×40.
Discussion
Cancers expressing MUC1 antigen occur in 72% of new cases.31 MUC1 has been recognized as a multifunctional protein that is involved in protection and lubrication of mucous membrane, signal transduction and modulation of the immune system.32 Although MUC1 is both tumour-associated and expressed in normal tissues, there is a striking difference in expression between normal tissues and cancer cells. MUC1 is normally expressed on the apical surface of secretory epithelia lining the lumen of ducts. Carcinoma cells, in contrast, exhibit loss of polarization and express MUC1 at high levels over the entire cell surface.8 Moreover, sialyated O-linked glycans attached to the MUC1 tandem repeats differ structurally in normal and transformed epithelia.33 Incomplete glycosylation of MUC1 in carcinoma cells could expose the tandem repeat epitopes that are normally cryptic.9,34 Taken together, these features make MUC1 an attractive target for immunotherapy.
The aim of the present study was to determine whether immunization of MMT mice with FC/MUC1 can induce anti-MUC1 immunity that is sufficient to prevent or delay the development of spontaneous mammary tumour in vivo. The results demonstrate that immunization with FC/MUC1 induces anti-MUC1 immune responses. Such immune responses resulted in delay of mammary tumour development driven by a potent oncogene in a genetically modified mouse. Early immunization rendered 33% of mice free of disease for up to 6 months. These results are consistent with our previous findings that DC/tumour fusion cells induce potent antitumour immunity.26,35
Fusion cells express DC-derived costimulatory ligands, MHC class I and II molecules and tumour cell-derived antigens, including MUC1. The fusion cells process and present MUC1 in the context of MHC and costimulatory molecules and induce both arms of cellular-mediated immunity in vivo and in vitro.35,36 In the present study, we showed that immunization with FC/MUC1 was associated with the induction of an anti-MUC1 immune response, a delay of mammary tumour development and control of tumour progression. These results further strengthen the notion that MUC1 is an immunogenic antigen capable of eliciting immune responses to reject MUC1-positive tumours.
The finding that the efficacy of the vaccine is correlated with the timing of initial vaccination is of interest but not unexpected. The MMT mice are born with a potent activated oncogene, PyMT. The PyMT antigen is associated with a number of signal transduction pathways37–41 that promote cell growth and/or survival. This oncogene affects the entire mammary tree and results in widespread transformation of the mammary epithelia.19,20 In this model, reversal of early tumour development or elimination of established tumour is needed to alter the progression of disease. In this context, immunization with FC/MUC1 significantly prolonged the latent period of tumour development, especially when the immunization was initiated in the early stage of tumour development. These results indicate that an effective immune response can be induced to counter the tumorigenesis driven by a potent oncogene. Furthermore, MUC1 was targeted in the present study. A possibility is that the efficacy of vaccine can be improved if multiple antigens are targeted. One of the advantages in using DC/tumour fusion cells is that the fusion cells are capable of processing and presenting multiple tumour antigens, including those unidentified, and inducing polyclonal CTLs. Collectively, the findings in the present study raise hope that a tumour vaccine can be developed for preventive and therapeutic use.
Acknowledgments
This work was supported by National Cancer Institute Grant R01 CA87057; by the US Department of Defense Breast Cancer Research programs, Grant 990344; and by the Susan G. Komen Breast Cancer Foundation, Grant 9825.




