Costimulation through OX40 is crucial for induction of an alloreactive human T-cell response
Summary
The alloreactive immune response is a series of events initiated by the interaction of T cells with allogeneic dendritic cells (DCs), involving alloantigen recognition and costimulatory signals. In this study, we investigated the role of OX40 in alloreactivity in vitro. We first demonstrated that anti-OX40 ligand (anti-OX40L) monoclonal antibody (mAb) could markedly suppress the mixed leucocyte reaction (MLR) of peripheral blood mononuclear cells (PBMC). To further define the contribution of the OX40/OX40L system to the MLR, we set up a co-culture system of CD4+ T cells and allogeneic monocyte-derived dendritic cells (DCs). After 2 days, OX40 expression was induced on CD4+ T cells and this induction was strongly inhibited by the addition of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)–Fc fusion protein, suggesting that the expression of OX40 during alloreaction is dependent on CD28 signalling. Next we examined the effects of anti-OX40L mAb, CTLA-4–Fc fusion protein and anti-human leucocyte antigen (HLA)-DR mAb on the proliferative response of CD4+ T cells to allogeneic DCs. The proliferation of T cells was almost completely suppressed by anti-OX40L mAb, which was comparable with that of CTLA-4–Fc. Measurement of interleukin-2 (IL-2) production in the culture supernatants showed that suppression of a proliferative response was at least in part ascribed to reduced IL-2 production. Furthermore, purified OX40L− allogeneic DCs could induce considerable proliferation of CD4+ T cells, which was suppressed by anti-OX40L mAb. These results suggest that the OX40/OX40L system is crucial for induction of the allogeneic T-cell response and the OX40/OX40L system is subsequent to and dependent on CD28 signalling, but is crucial for the end outcome of the human alloreactive T-cell response.
Abbreviations
-
- CTLA-4
-
- cytotoxic T lymphocyte-associated antigen-4
-
- DC
-
- dendritic cell
-
- GM-CSF
-
- granulocyte–macrophage colony-stimulating factor
-
- HLA
-
- human leucocyte antigen
-
- mAb
-
- monoclonal antibody
-
- MLR
-
- mixed leucocyte reaction
-
- OX40L
-
- OX40 ligand
-
- [3H]TdR
-
- tritiated thymidine.
Introduction
The allogeneic immune response, which is characterized by a high frequency of responding T cells and independence of prior sensitization, has become a major clinical issue, as the practice of organ transplantation, as well as that of haematopoietic stem cell transplantation, becomes generalized worldwide. Studies on the mixed leucocyte reaction (MLR), a common in vitro model of alloreactivity, have suggested the central role of allogeneic dendritic cells (DCs) as stimulators of T cells.1 It usually takes several days for the MLR to become apparent and, during this period, the multistep T cell–DC interaction appears to take place. Although triggering the T-cell receptor (TCR) complex by allogeneic major histocompatibility complex (MHC) initiates and elicits the subsequent series of events, the magnitude of the whole response is suggested to be largely influenced by species and quantity of available costimulatory signals.
It has been established that the CD28–CD80/CD86 interaction is the main pathway for DC-mediated T-cell costimulation.2 Nevertheless, other costimulatory molecules, such as OX40, 4-1BB and ICOS, have been shown to play important roles in their own way.3–5 Among these, OX40, a member of the tumour necrosis factor (TNF) receptor superfamily, is noted for its strong costimulatory activity for CD4+ T cells.3,6–11 Activated DCs express OX40 ligand (OX40L),10 a TNF superfamily molecule (alternatively designated as gp34), and accumulating evidence has suggested that the OX40/OX40L system has an essential function in the helper T cell–DC interaction. Studies with OX40L-knockout mice have shown that OX40L-deficient DCs have severely impaired activity to stimulate helper T cells.11,12 With regard to the role of OX40 in alloreactivity, a number of studies have suggested that the OX40/OX40L system is involved in both allograft rejection13 and graft-versus-host disease (GVHD).14,15 However, it is not fully understood how the OX40/OX40L system is integrated in the sequential process of the alloreactive T cell–DC interaction.
In the present study, we analysed the time course of OX40 expression and its contribution to the proliferative response of CD4+ T cells stimulated with allogeneic DCs. We performed relevant blocking experiments to define the distinctive significance of the OX40 pathway in alloreactivity compared with the CD28 pathway. Herein, we show that the OX40/OX40L system is subsequent to and dependent on CD28 signalling, but is crucial for the end outcome of the human alloreactive T-cell response.
Materials and methods
Culture medium
RPMI-1640 (Sigma, St Louis, MO), supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 292 µg/ml l-glutamine (penicillin–streptomycin–glutamine; Life Technologies, Rockville, MD) and 1% human plasma, was used as culture medium in all cultures except for the CTLL-2 bioassay.
Human plasma, primary cells and cell line
Heparinized peripheral blood from healthy volunteers was centrifuged and the plasma collected. Thereafter, human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll–Paque (Amersham Pharmacia Biotech, Bucks., UK) density-gradient centrifugation. Monocytes or CD4+ T cells were purified from PBMCs using CD14 microbeads or a CD4 T-cell isolation kit (Miltenyi Biotech, Bergish Gladbach, Germany), according to the manufacturer's instructions. The purity of the obtained cell populations was > 96%. CTLL-2 cells were maintained by culture in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS) (Life Technologies, Grand Island, NY), 5·5 × 10−5 m 2-mercaptoethanol (2-ME) (Life Technologies) and 50 U/ml recombinant human interleukin (IL)-2 (Shionogi Pharmaceutical, Osaka, Japan).
Cytokines and conditioned medium
Recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) was kindly provided by Novartis Pharma (Basel, Switzerland). Recombinant human IL-4 was purchased from Peprotech (London, UK). TNF-α, poly (I;C) and lipopolysaccharide (LPS) were from Sigma, and recombinant soluble CD40 ligand (CD40L) was from R & D Systems, Inc. (Minneapolis, MN). Monocyte-conditioned medium (MCM) was prepared in our laboratory. Briefly, PBMCs were suspended in RPMI-1640 containing 1% autologous plasma (5·1 × 106/ml) and plated onto human immunoglobulin (Welfide, Osaka, Japan)-coated 100-mm dishes (10 ml/dish). Cells were cultured for 20 hr and cell-free supernatants (MCM) were collected.
Monoclonal antibodies and fusion protein
Anti-OX40L (ik-1, mouse IgG1; ik-5, mouse IgG2a) and anti-OX40 (131, mouse IgG1) were established and purified from ascites fluid in our laboratory.16 ik-1 and 131 were biotinylated as described preveously.16 Fluorescein isothiocyanate (FITC)-conjugated anti-human leucocyte antigen (HLA)-DR (L243), anti-CD4 (SK3), phycoerythrin (PE)-labelled streptavidin and control monoclonal antibodies (mAbs) were purchased from Becton-Dickinson (Mountain View, CA). Purified IgG of anti-HLA-DR (G46-6) and isotype-matched control mAbs (UPC-10 and MOPC21) were purchased from Sigma; and anti-CD18 (TS1/18) was from Immunotech (Marseilles, France). Recombinant human cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)/Fc chimera was purchased from R & D Systems.
Generation of DC from peripheral blood monocytes
Freshly isolated monocytes were incubated in 100-mm dishes in RPMI-1640 without serum. After 12 hr, non-adherent cells were removed and the adherent cells were cultured for 7 days in RPMI-1640 supplemented with 50 ng/ml GM-CSF, 20 ng/ml IL-4 and 1% autologous plasma. On day 7, most cells became morphologically and phenotypically immature DCs (iDCs). To differentiate into mature DCs (mDCs), these cells were further cultured for 2 days with GM-CSF, IL-4, autologous plasma and one of the following reagents: MCM (20%), Poly (I;C) (12·5 µg/ml), TNF-α (15·0 ng/ml), or recombinant human soluble CD40L (1 µg/ml).
Primary MLR
Primary MLRs were set up in 96-well round-bottom plates (IWAKI, Tokyo, Japan), in 0·2 ml of RPMI-1640 supplemented with 5% human plasma (autologous to responder cells), in triplicate wells. The ratios of responder to stimulator were as follows: 1 : 1 for PBMC/allogeneic PBMC; 500 : 1 for CD4+ T cells/allogeneic mDCs; and 1000 : 1 for CD4+ T cells/allogeneic OX40L− mDCs; and the culture periods were 7, 5 and 7 days, respectively. Cultures were set up with medium alone or with one of the blocking reagents: anti-OX40L mAb (ik-5); anti-CD18 mAb (TS1/18); anti-HLA-DR mAb (G46-6); isotype-matched control mAbs (UPC-10 or MOPC21); or CTLA-4–Fc fusion protein. Cells were pulsed with [3H]thymidine ([3H]TdR) (0·5 µCi/well; MEN Life Science, Boston, MA) for the last 7 hr of culture. After harvesting cells, the incorporated radioactivity was measured in a liquid scintillation counter (Packard Instrument Company, Downers Grove, IL). To examine the time course of OX40 expression, purified CD4+ T cells were cultured with 40 Gy-irradiated allogeneic mDCs, at a ratio of 200 : 1, in the presence of control mAb (UPC-10), anti-HLA-DR (G46-6) or CTLA-4–Fc. Cells were collected on days 0, 2, 4, 6 and 8, and subjected to flow cytometric analysis.
Flow cytometric analysis
Immunofluorescence staining and subsequent flow cytometric analysis with a fluorescence-activated cell sorter (FACScan; Becton-Dickinson) were performed as described previously.16
CTLL-2 bioassay
CD4+ T cells were co-cultured with 40 Gy-irradiated allogeneic mDCs, at a ratio of 300 : 1, in a 96-well round-bottom plate (IWAKI) in RPMI-1640 supplemented with 10% heat-inactivated FCS (Life Technologies). Anti-OX40L (ik-5), anti-HLA-DR (G46-6), or isotype-matched control mAb (UPC-10) were added to the culture at a final concentration of 100 µg/ml. The cell-free supernatants were harvested after 72 hr. The IL-2 activity of each supernatant was measured by using the CTLL-2 bioassay, as described previously.17 The standard curve was drawn in order to measure the amount of IL-2 being produced.
Purification of OX40L− mDCs
mDCs were stained with biotinylated anti-OX40L (ik-1) and PE-labelled streptavidin. Then, OX40L− cells were purified using a cell sorter (EPICS ALTRA; Beckman Coulter Inc., Fullerton, CA). Both purity and viability (measured by Trypan Blue dye exclusion), immediately after sorting, were > 99%. Sorted OX40L− mDCs were co-cultured with allogeneic T cells to monitor induction of OX40L expression, or used as stimulator cells after irradiation (40 Gy) in the primary MLR, as described above.
Results
Anti-OX40L mAbs suppressed the two-way MLR
To determine whether the OX40/OX40L system is involved in the allogeneic response, we first set up a two-way MLR and examined the effects of anti-OX40L, anti-HLA-DR, CTLA-4–Fc, or anti-CD18. Alloreactive proliferation of PBMC was suppressed by anti-OX40L mAb, anti-HLA-DR mAb, or CTLA-4–Fc fusion protein when measured by [3H]TdR incorporation on day 7. (Two independent experiments gave similar results and the findings of one is shown in Fig. 1.) In particular, the suppressive effect of anti-OX40L mAb was conspicuous and comparable with that of anti-HLA-DR mAb, suggesting that the OX40/OX40L system plays a crucial role in alloreactivity.
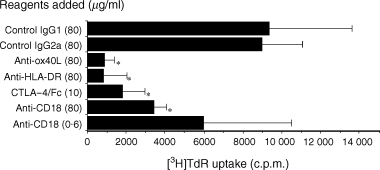
Effects of anti-OX40 ligand (OX40L), anti-human leucocyte antigen (HLA)-DR, anti-CD18 and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)-Fc fusion protein on the two-way mixed leucocyte reaction (MLR). Peripheral blood mononuclear cells (PBMC) from two different donors were mixed and co-cultured in a 96-well culture plate for 7 days in the presence of the indicated reagents. Cells were pulsed with [3H]thymidine ([3H]TdR) (0·5 µCi/well) for the last 7 hr of culture and the incorporated radioactivity was measured in a liquid scintillation counter. Results are expressed as mean counts ± standard deviation (SD) of triplicate samples. The asterisk indicates a P-value of < 0·05 compared with the suppressed reactivity.
OX40L is inducible on monocyte-derived DCs
Although the expression of OX40L in human tissue was shown to be relatively restricted, circulating DCs have been reported to express OX40L constitutively.9,11 As reported previously,9 monocyte-derived iDCs, obtained by culturing monocytes with GM-CSF and IL-4, did not express OX40L. To obtain OX40L+ DCs, we sought a culture condition that was suitable for the induction of OX40L expression. Therefore, iDCs were stimulated with soluble CD40L, poly (I;C), TNF-α, or MCM, and the expression of OX40L, in addition to the characteristic cell-surface markers of DCs, was analysed. Two days of culture with any of these reagents induced the maturation of iDCs, which was monitored by the enhanced expression of HLA-DR, CD1a, CD80, CD86 and CD83 (data not shown). In most DC preparations, these factors could also induce OX40L expression. The highest (and reproducible) induction of OX40L expression on DCs was observed when isolated monocytes were allowed to adhere overnight to plastic dishes before the addition of GM-CSF, and IL-4 (Fig. 2).
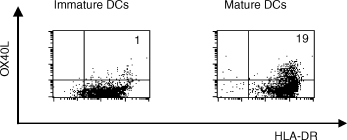
OX40 ligand (OX40L) expression on monocyte-derived dendritic cells (DC), in the presence or absence of stimulation with monocyte-conditioned medium (MCM). Purified monocytes were cultured in RPMI-1640 supplemented with granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4) and autologous plasma for 7 days to generate immature DCs. Then, cells were cultured for a further 2 days, with or without MCM. Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-human leucocyte antigen (HLA)-DR, biotinylated anti-OX40L (ik-1), and phycoerythrin (PE)-labelled streptavidin, and subsequent flow cytometric analysis, with a FACScan, was performed. The percentage of OX40L+ HLA-DR+ cells is indicated in the upper right corner of each dot-plot.
OX40 is inducible on T cells by allogeneic stimuli in a CD28-dependent manner
To examine the expression of OX40 on CD4+ T cells during the MLR, T cells were co-cultured with allogeneic mDCs treated with MCM, and OX40 expression was examined at different time-points. OX40 was detectable on CD4+ T cells after 2 days of culture, and this was sustained for 8 days. (Four independent experiments gave similar results and the data of a representative experiment is shown in Fig. 3.) The addition of anti-HLA-DR mAb (data not shown), or CTLA-4–Fc fusion protein, completely inhibited the induction of OX40 expression on CD4+ T cells, indicating that both TCR-mediated stimuli by alloantigens and CD28-mediated signals are required for the expression of OX40.
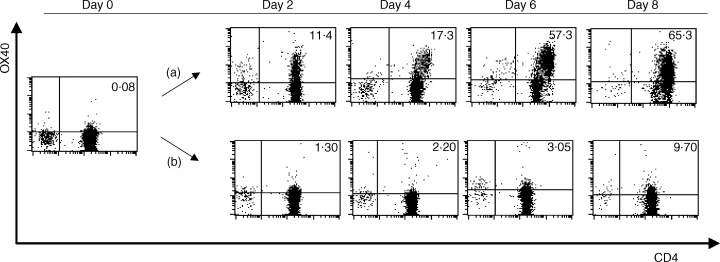
OX40 expression on CD4+ T cells stimulated by allogeneic dendritic cells (DCs) and the inhibitory effect of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)-Fc fusion protein against the expression of OX40. CD4+ T cells and allogeneic irradiated mature DCs (mDCs) were co-cultured in a 96-well culture plate at a ratio of 200 : 1. (a) Control monoclonal antibody (mAb) (MOPC21) or (b) CTLA-4–Fc fusion protein was added to the culture at a final concentration of 100 or 10 µg/ml, respectively. On days 0, 2, 4, 6 and 8, cells were collected, stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4, biotinylated anti-OX40L (ik-1) and phycoerythrin (PE)-labelled streptavidin, and subjected to flow cytometric analysis in a FACScan. The percentage of OX40+ CD4+ T cells is indicated in the upper right corner of each dot-plot.
The CD4+ T-cell response to allogeneic mDCs is strongly suppressed by blockade of the OX40/OX40L system
We next prepared a one-way MLR, comprising purified CD4+ T cells and monocyte-derived mDCs treated with MCM as stimulators. CD4+ T cells responded with vigorous cell growth as measured by [3H]TdR incorporation in addition to the increase in cell number. As shown in Fig. 4(a), the addition of anti-OX40L markedly suppressed the CD4+ T-cell response in a dose-dependent manner. Five independent experiments gave similar results. We observed strong inhibitory effects at various responder/stimulator ratios and after different culture periods (data not shown). It should be noted that the anti-OX40L mAb used was not toxic for DCs at 100 µg/ml (Fig. 4b). To further investigate the contribution of the OX40/OX40L system to the allogeneic CD4+ T-cell response, IL-2 bioassay of the culture supernatants was performed. As shown in Table 1, the addition of anti-OX40L mAb considerably suppressed IL-2 secretion, which was comparable with the inhibitory effects of anti-HLA-DR mAb. These findings suggest that the OX40/OX40L system constitutes a crucial costimulatory pathway in the alloreactive CD4+ T-cell response.
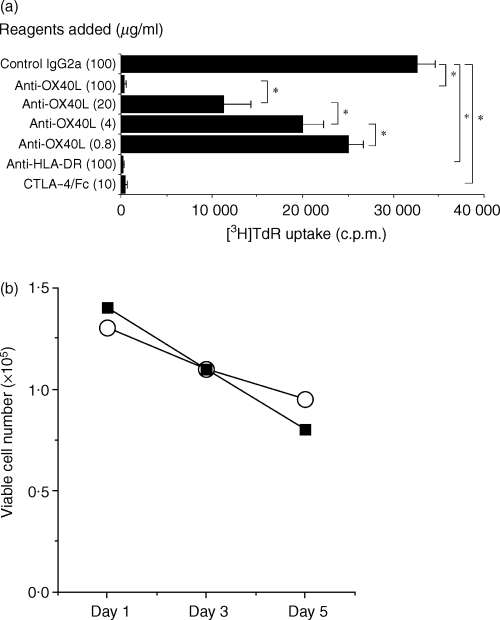
Effects of anti-OX40 ligand (OX40L) monoclonal antibody (mAb) on CD4+ T-cell proliferation stimulated by allogeneic dendritic cells (DCs). (a) CD4+ T cells and allogeneic irradiated mature DCs (mDCs) were mixed and co-cultured in a 96-well culture plate for 5 days in the presence of the indicated reagents. Cells were pulsed with [3H]thymidine ([3H]TdR) (0·5 µCi/well) for the last 7 hr of culture and the incorporated radioactivity was measured in a liquid scintillation counter. Results are expressed as mean counts ± standard deviation (SD) of triplicate samples. The asterisk indicates a P-value of < 0·05 compared with the suppressed reactivity. (b) Effects of anti-OX40L mAb on the viability of DCs. DCs were cultured in RPMI-1640 supplemented with 10% fetal calf serum (FCS) in the presence of 100 µg/ml control mouse IgG2a (UPC-10; open circle) or anti-OX40L mAb (ik-5; closed square) and the viable cell number was counted by using Trypan-Blue dye exclusion.
| Blocking reagent | [3H]TdR incorporation ± SD (c.p.m) | U/ml |
|---|---|---|
| Control mAb | 28925·3 ± 4666·0 | 53·50 |
| Anti-OX40L mAb | 9186·0 ± 402·3 | 0·22 |
| Anti-HLA-DR mAb | 8936·0 ± 652·7 | 0·19 |
- CD4+ T cells and irradiated allogeneic mature DCs (mDCs) were mixed and co-cultured in a 96-well culture plate at a ratio of 1 : 300, in triplicate. Control monoclonal antibodies (mAbs) (UPC-10), anti-OX40 ligand (anti-OX40L) mAb (ik-5), or anti-human leucocyte antigen (HLA)-DR mAb (G46-6) were added to the culture at a final concentration of 100 µg/ml. After 72 hr, cell-free supernatants were collected. Background proliferation for the CTLL-2 cells was 156·0 ± 28·3 [mean counts per minute (c.p.m.) ± standard deviation (SD)].
Purified OX40L− allogeneic DCs can stimulate CD4+ T cells via the OX40/OX40L system
Not all of the mDCs used expressed OX40L, and yet anti-OX40L mAb could almost completely inhibit allogeneic mDC-induced CD4+ T-cell proliferation. Therefore, we investigated whether the OX40/OX40L system is also involved in the alloreactive T-cell response induced by OX40L− mDCs. To address this, we purified OX40L− mDCs and set up the same coculture experiments of CD4+ T cells. As shown in Fig. 5(a), allogeneic OX40L− mDCs were able to elicit considerable levels of T-cell proliferation, and this was strongly inhibited by anti-OX40L mAb, suggesting that the OX40/OX40L system plays an important role, even in the MLR apparently induced by OX40L− mDCs. Two independent experiments gave similar results. Next, we examined whether OX40L expression was induced on OX40L− mDCs after coculture with allogeneic T cells. Contrary to our expectations, we could not detect expression of OX40L on sorted OX40L− mDC at any of the time-points tested (Fig. 5b).
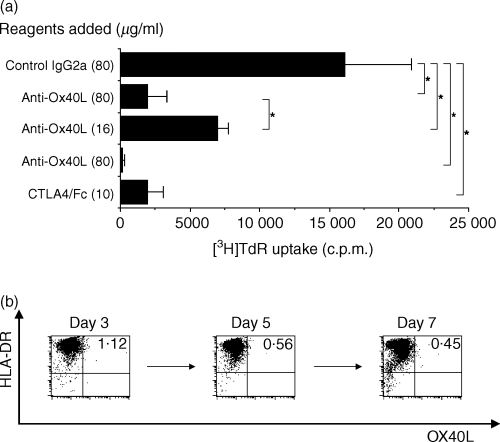
Effects of anti-OX40 ligand (OX40L) monoclonal antibody (mAb) on the proliferation of CD4+ T cells stimulated by purified OX40L− mature DCs (mDCs). (a) OX40L− mDCs were purified in a cell sorter and irradiated. CD4+ T cells and OX40L− mDCs were mixed at a ratio of 1000 : 1 and co-cultured in a 96-well culture plate for 7 days in the presence of the indicated reagents. Cells were pulsed with [3H]thymidine ([3H]TdR) (0·5 µCi/well) for the last 7 hr of culture and the incorporated radioactivity was measured in a liquid scintillation counter. Results are expressed as mean counts ± standard deviation (SD) of triplicate samples. The asterisk indicates a P-value of < 0·05 for comparison with the suppressed reactivity. (b) CD4+ T cells and allogeneic OX40L− mDCs were mixed at a ratio of 10 : 1 and co-cultured in a 96-well culture plate for 7 days. Cells were collected on days 3, 5 and 7, and the expression of OX40L was examined on mDCs.
Discussion
The benefit of organ transplantation, as well as haematopoietic stem cell transplantation, has been decreased by unfavourable alloreactivity manifesting as allograft rejection and GVHD. Better understanding of alloreactivity is needed to establish effective therapies for these disorders. Alloreactivity is characterized by a high frequency of responding T cells and independence of prior sensitization. Although allo-MHC recognition by the TCR was suggested to precede the subsequent series of events, the underlying molecular mechanism that culminates in T-cell proliferation and differentiation remains to be clarified. In the present study, we showed that anti-OX40L mAb could strongly inhibit both two-way MLR of PBMC and CD4+ T-cell proliferation induced by allogeneic monocyte-derived DCs. We confirmed that the anti-OX40L mAb we used had no toxic effect on DCs (Fig. 4b). These results suggest that costimulation through OX40 is crucial for induction of the alloreactive T-cell response. Although OX40 has a costimulatory function,3,6–11 blockade of the MLR by anti-OX40L mAb has not previously been reported. The present study, for the first time, substantiated that the alloreactive human T-cell response requires the OX40/OX40L system in vitro.
It is, however, not self-evident that blockade of the OX40/OX40L system resulted in almost complete suppression of T-cell proliferation in response to allogeneic mDCs because the DC samples used contained, at most, 20% OX40L+ cells. Based on our observation that OX40L expression was not induced on sorted OX40L− DCs during coculture with allogeneic T cells, the following two possibilities should be taken into consideration. First, OX40L− mDCs apparently express very low levels of OX40L that are difficult to detect but sufficient for T-cell costimulation. Second, OX40L+ mDCs exert inhibitory effects on T cells or other DCs when treated with anti-OX40L mAb by, for example, producing immunosuppressive cytokines, such as IL-10. We prefer the first possibility because purified OX40L− allogeneic mDCs were able to elicit considerable T-cell response levels, which were strongly inhibited by the addition of anti-OX40L mAb.
Considering the time course of OX40 expression during the T cell–DC interaction, and the requirement of CD28 signals for OX40 expression, a possible sequential process of CD4+ T cell–allogeneic DC interaction can be envisaged, as has been demonstrated in murine T cells.18,19 The TCR–allogeneic MHC interaction, together with CD28-CD80/86 binding, results in T-cell activation, leading to the expression of OX40 and CD40L. It is known that CD40 signalling induces expression of OX40L on DCs.9 Therefore, CD40L on T cells activates CD40+ DCs to induce expression or upregulation of OX40L, which in turn activates OX40+ T cells. This final step may be common to the majority of CD4+ T cells and accordingly crucial for appropriate cytokine production and a proliferative response of CD4+ T cells.
A number of studies using mouse or rat models have shown that the OX40/OX40L system is involved in alloreactivity in vivo, such as rejection of transplanted organs13 and GVHD after bone marrow transplantation.14–16 In humans, several studies have been published reporting that OX40+ T cells infiltrate the lesions of GVHD.20,21 Furthermore, we recently reported that OX40 expression is a predictive marker for the occurrence of chronic GVHD after allogeneic haematopoietic stem cell transplantation.22 Therefore, it is probable that the OX40/OX40L system is also critically involved in human alloreactivity in vivo. The present study provides insights into the basic understanding of the alloreactive human T-cell response and clues for the development of a new therapeutic approach to clinical disorders caused by alloreactivity.
Acknowledgments
This work was partly supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan. We thank Novartis Pharma for providing recombinant human GM-CSF and Kyoto Red Cross Blood Center for buffy coats from normal healthy volunteers.




