
An experimental study of the effects of nutrient supply and Chaoborus predation on zooplankton communities of a shallow tropical reservoir (Lake Brobo, Côte d'Ivoire)
Summary
1. Based on two mesocosm experiments and 10 in vitro predation experiments, this work aimed to evaluate the impact of nutrient supply and Chaoborus predation on the structure of the zooplankton community in a small reservoir in Côte d'Ivoire.
2. During the first mesocosm experiment (M1), P enrichment had no effect on phytoplankton biomass (chlorophyll a) but significantly increased the biomass of some herbivorous zooplankton species (Filinia sp, Ceriodaphnia affinis). During the second experiment (M2), N and P enrichment greatly increased phytoplankton biomass, rotifers and cladocerans (C. affinis, C. cornuta, Moina micrura and Diaphanosoma excisum). In both experiments, nutrient addition had a negative impact on cyclopoid copepods.
3. Larger zooplankton, such as cladocerans or copepodites and adults of Thermocyclops sp., were significantly reduced in enclosures with Chaoborus in both mesocosm experiments, whereas there was no significant reduction of rotifers and copepod nauplii. This selective predation by Chaoborus shaped the zooplankton community and modified its size structure. In addition, a significant Chaoborus effect on chlorophyll a was shown in both experiments.
4. The preference of Chaoborus for larger prey was confirmed in the predation experiments. Cladocerans D. excisum and M. micrura were the most selected prey. Rotifer abundance was not significantly reduced in any of the 10 experiments performed.
5. In conclusion, both bottom-up and top-down factors may exert a structuring control on the zooplankton community. Nutrients favoured more strictly herbivorous taxa and disadvantaged the cyclopoid copepods. Chaoborus predation had a strong direct negative impact on larger crustaceans, favoured small herbivores (rotifer, nauplii) and seemed to cascade down to phytoplankton.
Introduction
Food resources and predation are among the main factors structuring zooplankton communities. Nutrient availability influences primary production and phytoplankton composition (Kilham & Kilham, 1984) and indirectly affects food availability for herbivorous zooplankton. Selective predation regulates the zooplankton biomass and composition and thus modifies the grazing pressure by particle-feeding zooplankton which, in turn, modifies the abundance and composition of food available for grazers. The importance of such cascading trophic interactions (Carpenter, Kitchell & Hodgson, 1985) has been demonstrated in many ecosystems. Moreover, it has been shown in temperate areas that the combination of such top-down and bottom-up factors regulates plankton populations and drives seasonal succession (Threlkeld, 1987; Hu & Teissier, 1995). However, if the role of nutrient supply and predation on plankton community in temperate freshwater ecosystems is well documented (Benndorf et al., 2002; Gliwicz, 2002), studies examining their combined effects on tropical ecosystems are very scarce. Moreover, the trophic cascade models for temperate environments, based on linear food chain structure, may not apply in tropical waters, where complex food webs predominate due to preponderance of omnivory (Lazzaro, 1997).
Since the 1960s, predation by planktivorous fish has attracted the most attention (Lazzaro, 1987). There is now an increasing interest in predation by invertebrates (Pinel-Alloul, 1995) which occasionally has a greater effect than predation by fish (Lane, 1979; MacKay et al., 1990). In contrast to zooplanktivorous fish, which visually select large prey, invertebrates generally feed on smaller zooplankton species such as rotifers, nauplii and Bosmina (Dodson, 1974; Taylor, 1980; Zaret, 1980), although examples of invertebrate predation on large zooplankton have also been reported (Elser et al., 1987; MacKay et al., 1990; Wissel & Benndorf, 1998). One of the most important invertebrate predators in freshwater lakes is the larva of the Dipteran Chaoborus. These larvae occur in many freshwater habitats, from temporary ponds to large lakes, all over the world. They are particularly common throughout the tropics and are often a major component of the limnetic food web in African lakes such as Lake George in Uganda (McGowan, 1974), Lake Malawi (Irvine, 1997), Lake Chad (Saint-Jean, 1983) or small Opi Lake in Nigeria (Hare & Carter, 1987). Many studies have documented their feeding behaviour and their predation impact on zooplankton, but most concern temperate lakes (Pastorok, 1980; Elser et al., 1987; Riessen et al., 1988; Mumm, 1997; Wissel & Benndorf, 1998). Studies in tropical or subtropical water bodies mainly concern deep lakes such as Lake Malawi (Irvine, 1997), but knowledge of shallow tropical lakes or reservoirs is scarce (Saint-Jean, 1983; Hare & Carter, 1987; Roche et al., 1993). However, in such shallow tropical water bodies, Chaoborus larvae could have an important control because zooplanktivorous fish are rare or absent (Fernando, Tudorancea & Mengestou, 1990; Fernando, 1994) and because visual predators, if present, are disadvantaged by the high turbidity linked to wind resupension of sediments.
Several hundred small reservoirs have been constructed in the sahelian areas of West Africa (about 500 in Côte d'Ivoire) since the dry period of the 1970s, mainly to supply water for agriculture and ranching. Understanding their ecological functioning is important in order to improve water quality and to optimise fish resources that are now increasingly exploited (Vallet, 1993). These shallow reservoirs often have high primary production, but their fish yields remain relatively low, because of ineffective gear or low fishing intensity, and/or low efficiency in primary production transfer to higher trophic levels. One reason could be associated with the irregularity in nutrient supply which appears very stochastic in such shallow ecosystems, and is linked to high intensity winds and storms that induce strong vertical movement of the water column and resuspend sediments. These episodic nutrient pulses alternate with N-limited or P-limited periods (Arfi et al., 2001). Another reason could be a mismatch between the phytoplankton community dominated by large and elongated cyanobacteria (Anabaenopsis) or chlorophytes (Monoraphidium) (R. Arfi, personal communication) and grazer assemblages mainly composed of small species such as rotifers and the cladocerans Ceriodaphnia cornuta and Moina micrura (Aka et al., 2000). The third reason could be strong predation pressure by Chaoborus larvae which are present in 80% of the lakes and sometimes have biomasses substantially exceeding that of the zooplankton (Aka et al., 2000).
Based on mesocosm and microcosm studies in a small reservoir in Côte d'Ivoire, this work aimed to evaluate the impact of nutrient supply and Chaoborus predation on the structure of the zooplankton community through analysis of trophic relationships between compartments (phytoplankton, herbivorous zooplankton, carnivorous zooplankton, Chaoborus). To our knowledge, this is the first simultaneous study of nutrient and Chaoborus predation effects on the plankton communities of a shallow tropical freshwater ecosystem.
Material and methods
Study site
Lake Brobo, located 30 km east of Bouaké (7°40’N, 4°49’W) in the centre of Côte d'Ivoire, is a reservoir built in 1986 on a gravelly lateritic soil which bars the Soungourou River. It is a water body of 85 ha with an average depth of 3 m and a catchment of about 33 km2. Phytoplankton and microphytobenthos are the main contributors to primary production, whereas periphyton on macrophytes or on dead flooded trees are negligible (Thomas et al., 2000). As in many other African reservoirs, fish populations are composed of both indigenous and introduced species (Da Costa, Traoré & Tito de Morais, 1998). Generalist feeders (mostly Cichlids and Silurids, but also Heterotis niloticus, Schilbe sp., and some Chrysichtys sp.) represent the most important fraction of the catches, whereas zooplanktivorous or insectivorous fishes (such as Hemisynodontis membranaceus or Brachynosynodontis batensoda) are rare or absent V. Benech, personal communication).
Mesocosm experiments
Experimental design
Two mesocosm experiments were run at the end of the dry season: from 16 March to 4 April, 1998 (experiment M1) and from 27 March to 15 April, 2000 (experiment M2). We used cylindrical enclosures (0.8 m diameter, 1.7 m height, 0.8 m3 water volume) with polyethylene walls and a bottom of 60 μm mesh-sized fabric. At the beginning of each experiment the top circle of each enclosure was fixed onto a floating square structure built with polyvinyl chloride (PVC) pipe (20 cm in diameter) and the ballasted lower circle was lowered quickly. The enclosure self-filled through the 60 μm fabric bottom, filtering out zooplankton and Chaoborus. These enclosures were installed at a 2-m deep station, close to the shore. An identical quantity of zooplankton was then poured in each enclosure. This zooplankton was previously collected with a 64 μm mesh plankton net (40 cm diameter aperture) during day time to avoid Chaoborus larvae (mostly concentrated near the bottom in daylight conditions). The volumetric fraction added to each enclosure was calculated for the density of the zooplankton in the enclosure to be roughly equivalent to the in situ density.
In the first experiment (M1), three treatments were applied in triplicate: no nutrient addition and no Chaoborus (C = Control); enrichment with phosphorus nutrients (P); no nutrient addition but addition of Chaoborus larvae (CH). In the second experiment (M2) we used a 2 × 2 factorial design with duplicate enclosures for each of the four treatments: no nutrients enrichment and no Chaoborus (C = Control); enrichment with nitrogen and phosphorus (NP); no nutrient addition but addition of Chaoborus (CH); enrichment with nutrients and Chaoborus (NP + CH).
Nitrogen and phosphorus sources were ammonium sulfate [(NH4)2SO4] and potassium di-hydrogen phosphate (KH2PO4), respectively. For experiment M1, PO4-P was added every day in the three nutrient-supplied enclosures to maintain, with only small fluctuations, the phosphorus concentration at around 2 μg PO4-P L−1. During experiment M2, the same scenario was chosen for phosphorus supply, whereas the objective was to simultaneously maintain N concentrations close to 15 μg NH4-N L−1 through daily additions. Nine days were necessary to progressively enhance N concentrations in nutrient-supplied enclosures close to the target value. However, high N concentrations were not registered for more than 3 days, after which they again became as low as in control enclosures (around 2 μg NH4-N L−1). During the same time, pH changed in nutrient-supplied enclosures from 7 at the beginning of the experiment to 8.5 after several days and for the remainder of the experiment. It is possible that denitrification led to some N loss from enclosures.
The Chaoborus larvae, mostly 3rd and 4th instars (collected at night with a 350 μm mesh WP2 net) were added in equivalent quantity to each enclosure, on the first day at a density close to the natural density. The Chaoborus density was checked every day by quickly counting the larvae in the whole zooplankton sample (see below). According to these estimations, subsequent additions were carried out during the experiment (on days 4 and 8 during M1; on days 5, 10 and 15 during M2) to maintain the density close to the target value (2–3 individuals L−1) and thus compensate for the losses because of mortality or emergences of adults. Average Chaoborus densities in the Chaoborus enclosures (CH and NP + CH) were 2.4 ± 1.3 and 3.0 ± 1.1 individuals L−1 during M1 and M2 respectively, whereas the respective in situ densities at the same periods were 2.2 ± 0.6 and 3.2 ± 1.5 individuals L−1.
Sampling and analysis
Sampling was carried out every morning between 7 and 8 a.m. and every evening between 6 and 7 p.m. for physico-chemical factors and chlorophyll a, and every evening between 7 and 8 p.m. for zooplankton. Temperature and dissolved oxygen were measured on vertical profiles with a multiparametric probe (YSI 57). Conductivity, pH, nutrients (nitrates, ammonia and soluble reactive phosphorus), were measured on water samples taken using a PVC tube (4 cm in diameter and 1.8 m length). This tube was vertically plunged inside each enclosure up to 1.5 m in order to integrate the entire water column. Measurements of pH and conductivity were immediately performed with a conductimeter (Taccusel CRDV 62) and a pH-meter (Orion SA 720) (IM Lab, Lille, France). Ammonia was measured spectrophotometrically using the method of Koroleff (1970, in Strickland & Parsons, 1972). Nitrites, nitrates and phosphates were measured with an autoanalyser (Technicon, Tarrytown, NY, USA) according to the methods detailed in Strickland & Parsons (1972).
Zooplankton were sampled using a PVC tube of 1.25 m length and 10 cm diameter that ended with a closing device consisting of a funnel equipped with a 60 μm mesh window and a rubber tube closed with a Mohr grip. Samples were fixed in a formalin solution at 5% final concentration. Organisms were enumerated from subsamples taken using wide bore piston pipettes (0.5–5 mL). At least 100 individuals of the main taxa were counted from subsamples and the rarest taxa were estimated on the whole sample.
Individual weights of most taxa were estimated from their size measured under a dissecting microscope (objective 5×, ocular 20×). For rotifers, size was converted into volume using formulae established by Ruttner-Kolisko (1977 in Bottrell et al., 1976) and volume into dry weight using a 3.9% ratio for Asplanchna and a 10% ratio for other taxa (Doohan, 1973, in Bottrell et al., 1976). For crustacean species, we used length (L)–dry weight (DW) relationships from Bottrell et al. (1976) for Ceriodaphnia spp., from Saint-Jean & Bonou (1994) for Moina micrura, and from Pagano and Saint-Jean (unpublished data) for Diaphanosoma excisum (DW = 4.42 L2.55), Mesocyclops sp. (DW = 5.7 L3.1) and Thermocyclops sp. (DW = 4.8 L2.63). For all taxa we assumed a carbon/dry weight ratio of 0.45 (Pagano & Saint-Jean, 1993). For cyclopoid nauplii we used a mean and constant individual weight of 0.08 μg C based on unpublished data.
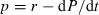
where r is estimated as the slope of the time-numbers (or time-biomass) regression line in the control treatments during the zooplankton growing phase.
Predation experiments
Ten predation experiments were performed to determine the feeding selectivity of Chaoborus larvae on natural zooplankton assemblages. Zooplankton and Chaoborus larvae were collected in the same ways as for enclosure experiments. After the zooplankton collection, performed in the late afternoon (5–6 p.m.), identical sets of zooplankton (made volumetrically) were gently poured in eight 2-L transparent polycarbonate bottles that had been previously filled with surface lake water that had been passed through a 60 μm mesh sieve. Ten to twenty Chaoborus larvae (3rd and 4th instars) were added 2–3 h after the zooplankton (the Chaoborus collection being carried at 7–8 p.m.) to four of the eight bottles. The bottles were then capped and suspended at 0.5 m depth in the lake. After a 22–24 h incubation (8 p.m. to 6–8 p.m.), the bottles were retrieved and the contents of each was filtered through a 64 μm mesh to retain zooplankton and Chaoborus larvae which were then added to a 5% buffered formalin solution for enumeration, which was performed as described above for zooplankton samples. Predation rates (prey Chaoborus−1 h−1) on the various prey types were calculated by subtracting the mean final density of each prey in the experimental bottles (with Chaoborus) from its mean density in the control bottles (without Chaoborus). Ingestion was considered as null if there was no significant difference between the two means (t-test, P > 0.05).
Selectivity was calculated using the normalised selectivity coefficient (Wi) defined by Vanderploeg & Scavia (1979).
Statistical analysis
In the mesocosm experiments, one-way anovas were performed to test whether differences between enclosures may have occurred before the addition of nutrients and/or Chaoborus (day 0). The effects of treatments on the environmental data (chlorophyll a, pH and oxygen) and on the abundance and biomass percentages of the main zooplankton taxa were assessed using one-way (for experiment M1) or two-way (for experiment M2) analysis of variance with repeated measures (anovar) using STATISTICA software (StatSoft France, 1995). The analysis compares the means of different treatments using several dates, assuming that in each experimental unit sampling dates are not independent (Winer, 1971). The between-treatment effects were nutrients, Chaoborus and their interactions. The within-treatment effects were time and the interactions between time and the between-treatment effects. All sampling dates were considered in the analyses except the day 0. To supplement this analysis, post hoc Scheffé tests were performed to compare the responses between treatments. For normality correction, environmental data and zooplankton abundance were log-transformed [log(x + 1)], whereas percentages of biomass were arcsine-transformed.
Results
Mesocosm experiments
Environmental parameters
In both experiments on day 0 (before the addition of nutrients or Chaoborus), the different environmental parameters were not different between treatments (one-way anova, P > 0.05). Average water column temperature ranged between 28.8 and 30.4 °C during M1 and between 28.3 and 30.7 °C during M2. In both experiments, there was no temperature difference between enclosures, or between enclosures and the lake. Oxygen concentration in enclosures without enrichment usually remained around 8 mg L−1, and pH around 7–8. Higher values up to 12 mg O2 L−1 and pH 8.5 occurred in enriched enclosures during M2.
Chlorophyll a ranged between 10 and 20 μg L−1 and displayed almost the same pattern in the different treatments during M1 from days 1 to 7 (Fig. 1a). Afterwards, a slight increase was observed in the CH treatment but not in the C and P treatments. The anovar analyses showed a significant Chaoborus effect on chlorophyll a (P = 0.042), but no significant nutrient effect (P > 0.05). During M2, chlorophyll a concentrations showed low variations around 20 μg L−1 in the lake and enclosures without nutrient addition (C and CH), but sharply increased from days 2 to 8 in enclosures where nutrients were added (NP and NP + CH) (Fig. 2b). Furthermore, the chlorophyll a increase was more important with the NP + CH treatments (up to 110 μg L−1) than with the NP treatment (up to 98 μg L−1). The anovar showed that nutrients and Chaoborus effects on chlorophyll a were significant (P < 0.001 in both cases), and Scheffé test confirmed the difference between NP + CH and NP treatments (P = 0.011).
Time variations of the mean chlorophyll a concentration in the lake (L) and in the enclosures for the different treatments (C: control; P: phosphorus; NP: nitrogen + phosphorus; CH: Chaoborus, NP + CH: nitrogen, phosphorus and Chaoborus) during the mesocosm experiments M1 (a) and M2 (b).
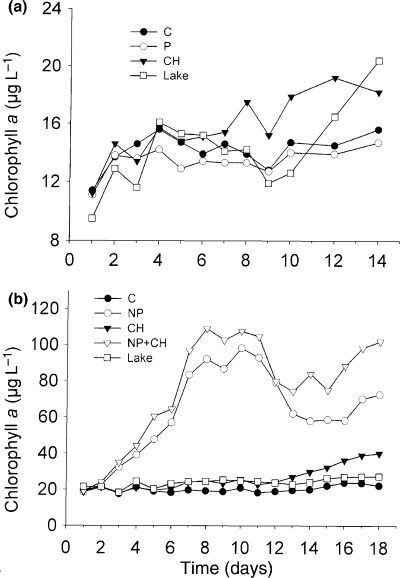
Time variations of the mean biomass of total zooplankton (a), total rotifers (b), Thermocyclops sp. (c, d) and cladocerans (e–h) in the enclosures for the different treatments (C: control; P: phophorus; CH: Chaoborus) during the first mesocosm experiment (M1).
Zooplankton
In both experiments on day 0 (before the addition of nutrients or Chaoborus), biomass of total zooplankton or of the main taxa were not different between treatments (one-way anova, P > 0.05), except in M1 for the cladoceran D. excisum, which was significantly (P = 0.048) more abundant in P-enclosures than in CH-enclosures. During both experiments the lake zooplankton was dominated by one cyclopoid (Thermocyclops sp.), four rotifers (Brachionus caudatus, B. angularis, Filinia sp. and Asplanchna brighwelli) and four cladocerans (D. excisum, M. micrura, C. cornuta and C. excisum) (Tables 1 and 2).
| Control | PO4 | Chaoborus | Lake | |
|---|---|---|---|---|
| Total zooplankton | 548.5 ± 146.6 | 566.3 ± 140.4 | 553.4 ± 120.7 | 449.9 ± 119.5 |
| Rotifers | 182.8 ± 122.5 | 196.4 ± 111.3 | 257.6 ± 126.8 | 208.4 ± 135.6 |
| Hexarthra sp. | 0.1 ± 0.1 | 0.4 ± 0.3 | 1.0 ± 0.7 | 1.1 ± 0.8 |
| Lecane sp. | 0.0 ± 0.0 | 0.5 ± 0.3 | 0.1 ± 0.2 | 0.1 ± 0.2 |
| Brachionus calyciflorus | 2.6 ± 5.2 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.0 ± 0.0 |
| B. falcatus | 0.2 ± 0.4 | 0.2 ± 0.2 | 2.5 ± 3.3 | 4.3 ± 6.4 |
| B. angularis | 11.7 ± 8.2 | 22.9 ± 17.9 | 68.5 ± 61.0 | 31.5 ± 27.9 |
| B. caudatus | 143.3 ± 117.8 | 149.1 ± 115.5 | 155.3 ± 133.7 | 155.6 ± 146.2 |
| B. plicatilis | 0.1 ± 0.2 | 0.8 ± 0.8 | 0.8 ± 1.3 | 0.0 ± 0.1 |
| Platyias platulus | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| Polyarthra sp. | 0.1 ± 0.2 | 0.1 ± 0.1 | 3.5 ± 6.9 | 0.0 ± 0.0 |
| Keratella sp. | 0.0 ± 0.1 | 0.0 ± 0.0 | 0.3 ± 0.4 | 0.1 ± 0.2 |
| Filinia sp. | 2.6 ± 2.4 | 4.0 ± 2.4 | 5.6 ± 3.6 | 7.1 ± 3.4 |
| Trichocerca sp. | 8.2 ± 7.9 | 1.2 ± 0.4 | 5.6 ± 1.5 | 1.1 ± 0.7 |
| Asplanchna brightwellii | 13.6 ± 8.0 | 16.4 ± 8.8 | 13.2 ± 8.6 | 7.3 ± 5.6 |
| Conochiloides sp. | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.3 ± 0.6 | 0.0 ± 0.0 |
| Philodina sp. | 0.1 ± 0.2 | 0.0 ± 0.1 | 0.2 ± 0.3 | 0.0 ± 0.0 |
| Cephalodella sp. | 0.1 ± 0.1 | 0.3 ± 0.3 | 0.1 ± 0.2 | 0.2 ± 0.2 |
| Undetermined sp. | 0.0 ± 0.0 | 0.6 ± 0.9 | 0.3 ± 0.4 | 0.0 ± 0.0 |
| Thermocyclops sp. | ||||
| Nauplii | 84.7 ± 22.7 | 86.9 ± 18.5 | 120.1 ± 33.0 | 90.6 ± 23.5 |
| Copepodites | 19.6 ± 9.6 | 20.1 ± 8.4 | 22.1 ± 5.2 | 28.9 ± 4.6 |
| Adults | 22.0 ± 4.1 | 20.3 ± 4.4 | 13.7 ± 2.6 | 17.2 ± 3.6 |
| Cladocerans | 119.6 ± 25.4 | 121.3 ± 21.1 | 70.0 ± 20.8 | 52.4 ± 6.9 |
| Diaphanosoma excisum | 50.8 ± 11.1 | 62.6 ± 12.7 | 21.8 ± 9.2 | 21.4 ± 6.8 |
| Ceriodaphnia cornuta | 40.6 ± 17.7 | 38.8 ± 19.2 | 32.0 ± 13.1 | 20.9 ± 5.0 |
| Ceriodaphnia affinis | 20.8 ± 6.5 | 13.2 ± 3.7 | 7.4 ± 2.7 | 4.9 ± 1.7 |
| Moina micrura | 7.5 ± 3.1 | 6.8 ± 2.8 | 8.8 ± 3.2 | 5.2 ± 0.9 |
| Control | NP | Chaoborus | NP + Chaoborus | Lake | |
|---|---|---|---|---|---|
| Total zooplankton | 474.4 ± 37.5 | 484.4 ± 90.2 | 297.1 ± 58.1 | 525.3 ± 97.1 | 332.3 ± 53.3 |
| Rotifers | 68.8 ± 16.2 | 323.8 ± 105.3 | 172.7 ± 52.3 | 434.1 ± 102.8 | 112.0 ± 27.5 |
| Hexarthra sp. | 0.4 ± 0.4 | 1.3 ± 1.5 | 2.9 ± 3.2 | 3.2 ± 2.3 | 0.5 ± 0.5 |
| Lecane sp. | 3.4 ± 2.5 | 31.9 ± 30.1 | 2.9 ± 2.3 | 65.0 ± 60.1 | 1.7 ± 1.1 |
| B. falcatus | 1.8 ± 0.6 | 2.6 ± 1.8 | 4.0 ± 1.2 | 2.7 ± 1.5 | 2.3 ± 0.6 |
| B. angularis | 4.2 ± 4.4 | 42.8 ± 33.9 | 9.6 ± 8.3 | 33.4 ± 21.9 | 3.2 ± 2.6 |
| B. caudatus | 41.0 ± 10.4 | 192.2 ± 88.3 | 127.4 ± 42.6 | 239.0 ± 97.8 | 89.0 ± 29.4 |
| B. plicatilis | 0.2 ± 0.3 | 0.8 ± 0.5 | 0.3 ± 0.3 | 0.9 ± 0.8 | 0.3 ± 0.3 |
| Platyias platulus | 2.2 ± 1.7 | 7.2 ± 4.5 | 4.3 ± 3.1 | 19.7 ± 16.8 | 1.2 ± 0.9 |
| Keratella sp. | 0.7 ± 0.6 | 0.7 ± 0.8 | 0.7 ± 0.6 | 1.0 ± 0.6 | 1.1 ± 0.7 |
| Filinia sp. | 2.3 ± 1.2 | 4.0 ± 3.5 | 2.4 ± 1.6 | 5.6 ± 2.7 | 6.0 ± 3.8 |
| Trichocerca sp. | 4.5 ± 1.7 | 7.5 ± 3.4 | 7.5 ± 3.4 | 11.0 ± 4.0 | 1.4 ± 0.6 |
| Asplanchna brightwellii | 4.4 ± 2.9 | 11.7 ± 7.3 | 3.5 ± 2.5 | 7.3 ± 4.4 | 1.8 ± 1.3 |
| Conochiloides sp. | 2.1 ± 2.2 | 6.6 ± 4.2 | 4.7 ± 4.9 | 29.2 ± 38.6 | 1.6 ± 1.4 |
| Philodina sp. | 0.4 ± 0.5 | 4.9 ± 4.5 | 1.1 ± 1.5 | 7.3 ± 6.5 | 0.4 ± 0.5 |
| Cephalodella sp. | 0.4 ± 0.4 | 0.6 ± 0.5 | 0.3 ± 0.3 | 1.3 ± 0.7 | 0.3 ± 0.4 |
| Scaridium sp. | 0.0 ± 0.1 | 1.0 ± 0.8 | 0.1 ± 0.3 | 0.7 ± 0.7 | 0.2 ± 0.3 |
| Mytilina sp. | 0.3 ± 0.3 | 3.1 ± 3.2 | 0.2 ± 0.3 | 1.6 ± 2.4 | 0.5 ± 0.5 |
| Encentrum velox | 0.7 ± 0.5 | 3.0 ± 2.4 | 0.8 ± 0.9 | 3.7 ± 2.5 | 0.5 ± 0.6 |
| Undetermined sp. | 0.1 ± 0.1 | 2.0 ± 1.7 | 0.2 ± 0.3 | 1.5 ± 1.5 | 0.2 ± 0.3 |
| Thermocyclops sp. | 172.6 ± 28.7 | 98.1 ± 46.7 | 111.0 ± 20.5 | 65.0 ± 26.0 | 203.5 ± 47.0 |
| Nauplii | 130.6 ± 25.9 | 62.3 ± 37.2 | 83.4 ± 16.7 | 46.4 ± 20.9 | 161.5 ± 46.3 |
| Copepodites | 21.6 ± 7.7 | 18.7 ± 10.5 | 17.0 ± 4.5 | 10.8 ± 4.8 | 19.1 ± 3.0 |
| Adults | 20.4 ± 6.0 | 17.1 ± 6.4 | 10.6 ± 2.3 | 7.7 ± 1.9 | 22.9 ± 3.8 |
| Cladocerans | 30.2 ± 11.1 | 62.5 ± 13.9 | 13.5 ± 3.2 | 26.2 ± 5.4 | 16.7 ± 3.2 |
| Diaphanosoma excisum | 5.5 ± 1.4 | 20.9 ± 7.0 | 1.8 ± 0.5 | 4.0 ± 2.8 | 6.4 ± 1.8 |
| Ceriodaphnia cornuta | 2.3 ± 0.8 | 1.7 ± 0.9 | 1.5 ± 0.5 | 1.1 ± 0.4 | 1.5 ± 0.4 |
| Ceriodaphnia affinis | 3.0 ± 1.0 | 2.4 ± 0.8 | 3.5 ± 0.9 | 2.0 ± 0.5 | 1.3 ± 0.5 |
| Moina micrura | 19.5 ± 9.1 | 37.6 ± 14.3 | 6.6 ± 2.2 | 19.1 ± 5.3 | 7.5 ± 1.9 |
In M1, total zooplankton biomass was similar in the C and P enclosures with peaks on days 4 and 5 respectively, and lower biomass afterwards (Fig. 2a). Zooplankton biomass was lower in the CH enclosures after the second day of experiment and the anovar confirmed a significant effect of Chaoborus on total zooplankton biomass (Table 3). Rotifer biomass (Fig. 2b) showed similar patterns in the different treatments but peaked sooner (day 2) in the P enclosures than in the C and CH enclosures (day 5). There was no significant effect of the two treatments on total rotifer biomass, but phosphorus had a significant effect on Filinia sp, and Chaoborus had significant effects on B. angularis, B. caudatus and Filinia sp. (Table 3). Thermocyclops nauplii displayed similar biomass and variation pattern in the different treatment enclosures during the first week of experiment, but afterwards increased in the CH enclosures and decreased in the P enclosures (Fig. 2c). Copepodites and adults had similar variation patterns in the different treatments, but lower biomasses in the CH enclosures between days 3 and 5 (Fig. 2d). The anovar showed Chaoborus effects on the different stages of Thermocyclops but no phosphorus effect (Table 3). The four cladoceran species increased initially in all enclosures (Fig. 2e–h). D. excisum and C. affinis reached lower abundances in the CH enclosures than in C and P enclosures, but the differences between treatments were less clear for the two other species. Chaoborus effects were significant only for C. affinis, D. excisum and M. micrura, and a phosporus effect was also detected for C. affinis (Table 3).
| Phosphorus and time | Chaoborus and time | |||||
|---|---|---|---|---|---|---|
| P | T | P × T | CH | T | CH × T | |
| d.f. | 1 | 10 | 10 | 1 | 10 | 10 |
| Total zooplankton (μg C L−1) | ns | *** | ns | * | *** | ns |
| Rotifers (μg C L−1) | ns | *** | ** | ns | *** | ns |
| Brachionus angularis | ns | *** | *** | ** | *** | ** |
| B. caudatus | ns | *** | *** | * | *** | *** |
| Filinia sp. | * | *** | *** | * | *** | *** |
| Asplanchna sp. | ns | *** | *** | ns | *** | *** |
| Thermocyclops sp. (μg C L−1) | ns | *** | * | ns | *** | ** |
| Nauplii | ns | *** | ** | * | *** | ** |
| Copepodites | ns | *** | *** | *** | *** | *** |
| Adults | ns | *** | ns | ** | * | ns |
| Cladocerans (μg C L−1) | ns | ** | ns | *** | *** | ** |
| Ceriodaphnia affinis | ** | *** | *** | *** | *** | *** |
| C. cornuta | ns | *** | *** | ns | *** | *** |
| Diaphanosoma excisum | ns | *** | * | *** | *** | *** |
| Moina micrura | ns | *** | *** | * | *** | *** |
| Biomass percentages | ||||||
| Rotifers (%) | ns | *** | * | * | *** | ** |
| Thermocyclops (%) | *** | ** | * | *** | *** | *** |
| Cladocerans (%) | ** | *** | ns | *** | *** | *** |
| Individual length (μm) | ns | *** | * | *** | *** | *** |
- Statistical significances are *P < 0.05; **P < 0.01; ***P < 0.001; ns: not significant (P > 0.05); d.f.: degree of freedom.
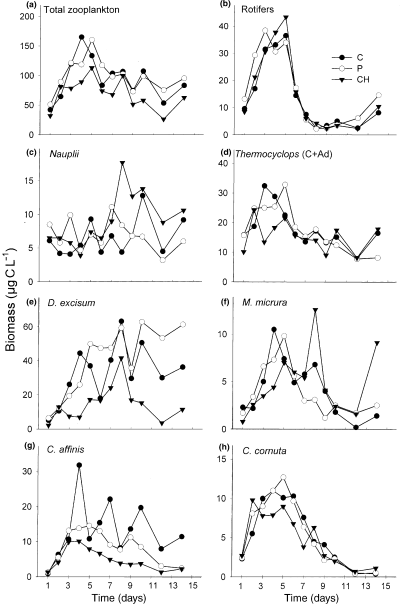
During M2, zooplankton biomass (Fig. 3a) peaked sooner and reached higher values in the NP enclosures than in the C enclosures and showed very low values and no peak in the enclosures with Chaoborus (CH and NP + CH). The anovar confirmed that both treatments had significant effects on zooplankton biomass (Fig. 4a, Table 4). Total rotifer biomass displayed higher values in the NP and NP + CH enclosures than in the C and CH enclosures (Fig. 3b). It followed roughly the same variation in the C and CH enclosures until day 11, but afterwards became higher in the CH enclosures. The anovar showed significant nutrient effects on total rotifer biomass and on the more abundant rotifer species (B. angularis, B. caudatus and Lecane sp.), whereas Chaoborus had significant effects on total rotifer biomass and Lecane sp. (Fig. 4b, Table 4). Nauplii of Thermocyclops (Fig. 3c) decreased in all the enclosures during the first week and reached lower values in the enclosures where nutrients were added (NP and NP + CH) than in the other enclosures (C and CH). Copepodites and adults also decreased at the beginning of the experiment but increased again and peaked on day 6 in the enclosures without Chaoborus (C and NP) whereas they continued to decrease in the enclosures with Chaoborus (Fig. 3d). The anovar showed significant nutrient and Chaoborus effects on total Thermocyclops biomass, nutrient effects on nauplii and copepodites and Chaoborus effects on adults (Fig. 4c, Table 4). Both treatments had significant effects on the cumulated. biomass of copepodites and adults (Fig. 4d). D. excisum and M. micrura reached highest densities in the enclosures with nutrient alone (NP) and lowest densities in the enclosures with Chaoborus (Fig. 3e and f), both treatments having significant effects on these two species (Fig. 4d and f, Table 4). In contrast, the two less abundant cladoceran species, C. affinis and C. cornuta showed neither a clear trend (Fig. 3g and h) nor a significant difference between treatments (Fig. 4g and h, Table 4).
Time variations of the mean biomass of total zooplankton (a), total rotifers (b), Thermocyclops sp. (c, d) and cladocerans (e–h) in the enclosures for the different treatments (C: control; NP: nitrogen + phosphorus; CH: Chaoborus; NP + CH: nitrogen, phosphorus and Chaoborus) during the second mesocosm experiment (M2).
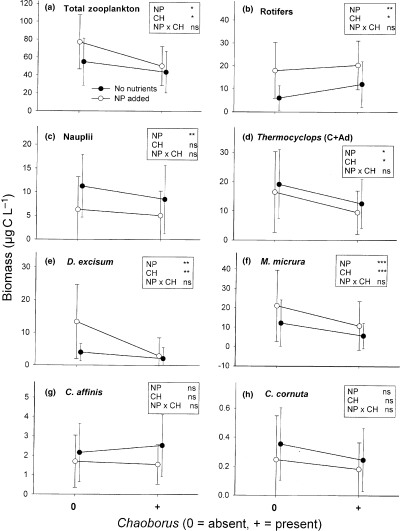
Mean biomass of total zooplankton (a), total rotifers (b) Thermocyclops sp. (c, d) and cladocerans (e–h), computed from days 1 to 18 during the M2 experiment (n = 36) for the four treatment levels: with (+) or without (0) Chaoborus, with (open circles) or without (black circles) nutrients. The significance levels of treatments in the anovar are indicated within boxes: *P < 0.05; **P < 0.01; ***P < 0.001; ns = not significant (P > 0.05).
| NP | CH | NP × CH | T | NP × T | CH × T | NP × CH × T | |
|---|---|---|---|---|---|---|---|
| d.f. | 1 | 1 | 1 | 16 | 16 | 16 | 16 |
| Total zooplankton | * | * | ns | *** | ns | * | ns |
| Rotifers | ** | * | ns | *** | *** | ns | ns |
| B. angularis | * | ns | ns | *** | * | ns | ns |
| B. caudatus | * | ns | ns | *** | *** | ns | ns |
| Filinia sp. | ns | ns | ns | *** | ** | ns | ns |
| Lecane sp. | *** | * | ** | *** | *** | ns | ns |
| Asplanchna sp. | ns | ns | ns | *** | ns | ns | ns |
| Thermocyclops sp. | * | * | ns | *** | *** | * | ns |
| Nauplii | ** | ns | ns | *** | *** | ns | ns |
| Copepodites | * | ns | ns | *** | *** | ns | ns |
| Adults | ns | ** | ns | *** | ns | *** | ns |
| Cladocerans | * | * | ns | *** | * | * | ns |
| C. affinis | ns | ns | ns | *** | ns | * | ns |
| C. cornuta | ns | ns | ns | *** | ns | ns | ns |
| D. excisum | ** | ** | ns | ns | ns | * | ns |
| M. micrura | *** | *** | ns | *** | ns | * | ns |
| Biomass percentages | |||||||
| Rotifers (%) | * | * | ns | *** | ** | ns | * |
| Thermocyclops (%) | *** | ns | * | *** | *** | * | ns |
| Cladocerans (%) | ns | * | ns | *** | *** | ns | ns |
| Individual length (μm) | ns | ** | ns | *** | ** | ** | ns |
- Statistical significances are *P < 0.05; **P < 0.01; ***P < 0.001; ns: not significant (P > 0.05); d.f.: degree of freedom.
In both experiments, the average percentage of cladocerans was lower in the enclosures with Chaoborus and no nutrient addition than in the other enclosures and this lower percentage of cladocerans was compensated by copepods during M1 and by rotifers during M2 (Fig. 5). The anovar analyses confirmed the significant effect of Chaoborus on cladoceran (M1 and M2), cyclopoid (M1) and rotifer (M2) percentages (Tables 3 and 4). During M1 and M2, the percentage of copepods decreased progressively and reached very low values at the end of the experiments in the nutrient-enriched enclosures, whereas it varied little in the other enclosures (Fig. 5). The negative effect of nutrient addition on copepod percentage in both experiments was confirmed by the anovar analyses (Tables 3 and 4). The anovar showed a negative effect of Chaoborus on the mean individual length of zooplankton (as of ratio biomass/numbers) in both experiments. During M2, the nutrient addition seemed to have also a negative effect on the mean zooplankton length, mainly because of the increase in the percentage of rotifers, but the anovar did not fully confirm this trend (Table 4), despite the P-value (P = 0.07) was close to the significance level (0.05).
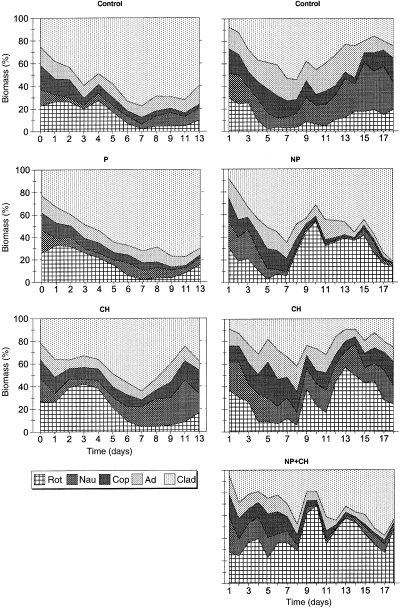
Time variations of the biomass percentages of rotifers (rot), nauplii (nau), copepodites (cop) and adults (ad) of Thermocyclops sp. and cladocerans (clad) for the different treatments (C: control; P: phosphorus; NP: nitrogen + phosphorus; CH: Chaoborus; NP + CH: nitrogen, phosphorus and Chaoborus) during the mesocosm experiments M1 (left) and M2 (right).
Predation rates of Chaoborus in the CH enclosures were computed daily. They ranged from 5 to 58 (mean = 29 ± 5) prey Chaoborus−1 day−1 during M1 and from 11 to 29 (mean = 20 ± 1) prey Chaoborus−1 day−1, during M2, which represented daily rations ranging from 13 to 126% (mean = 58 ± 10%) and from 21 to 55% (mean = 38 ± 2%) of body carbon for M1 and M2 respectively.
Predation experiments
In the 10 predation experiments, the most frequently ingested prey were the copepodites and adults of Thermocyclops and the four cladocerans (t-test, P < 0.05). Nauplii of Thermocyclops were significantly removed only when they were very abundant (>1000 L−1). There was no significant reduction in rotifer prey in the predation bottles compared with the control bottles. The daily ingestion rate ranged between 5 and 76 prey Chaoborus−1 (mean = 16.1 ± 19.1 prey Chaoborus−1), according to the type of prey, and represented 5 to 70% (mean = 25.5 ± 19.7%) of body carbon.
The average selectivity values for each prey type (i.e. crustaceans) are shown in Fig. 6. The highest Wi were found for M. micrura and D. excisum and the smallest values for nauplii of Thermocyclops. Paired t-tests showed that the mean Wi of M. micrura and D. excisum were not significantly different from each other but that both were significantly higher than the Wi of all other prey (P < 0.05).
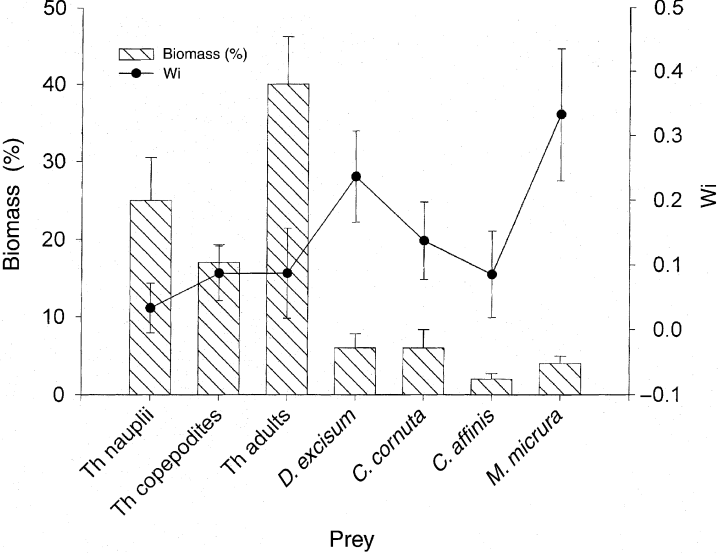
Mean selectivity of Chaoborus for crustacean prey (Wi, line) and mean biomass percentage of the same prey in control flasks (bars) over the 10 predation experiments. Vertical bars represent standard error.
Discussion
Nutrient effects
Nutrient enrichment with N and P greatly increased the phytoplankton biomass during the second mesocosm experiment (M2). The enhanced algal production probably favoured the increase of herbivorous zooplankton taxa such as rotifers and cladocerans. During the M1 experiment, where P was added alone, there was no nutrient effect on chlorophyll a. However, significant P effects on the biomass of some herbivorous species (Filinia sp., C. affinis) and on the biomass percentages of cladocerans and copepods suggested that phosphorus addition could have very slightly enhanced the primary production and/or modified the phytoplankton community structure and thus the food composition for zooplankton. It has been well documented that fertilisation increases phytoplankton abundance and/or changes community structure (Dillon & Rigler, 1974; Schindler, 1978; Qin & Culver, 1995). As in other studies (Lancaster & Drenner, 1990; Qin & Culver, 1995), we found that the degree of algal enhancement was greater in the treatment combining predation and nutrients than in the treatment with nutrients alone. This could be due to the lowered grazing pressure (in the presence of predation), which increases the algal response to fertilisation (Lancaster & Drenner, 1990). The less evident effect of enrichment on phytoplankton when phosphorus was added alone is consistent with accounts of a less important role of phosphorus for phytoplankton in tropical areas as compared with temperate areas (Talling & Talling, 1965). Previous results from 49 reservoirs from northern Côte d'Ivoire have also suggested that P limitation is not important in such environments (Arfi et al., 2001).
Zooplankton community structure changed in response to nutrient addition, whether of both N and P or of P alone. Strictly herbivorous zooplankton (rotifers and cladocerans) were favoured to the detriment of populations with mixed trophic behaviour (cyclopoid copepods). Paul et al. (1995) observed a similar positive effect of nutrient addition on rotifers because of increased standing stock (two to fourfold) of suitable algae. However, in contrast to the present study, they also observed a strong increase in cyclopoid nauplii (threefold) because of increase of female clutch size and survival of nauplii. In the present study, nutrient addition had no positive effect on nauplii, had a negative effect on nauplii during M2, and seemed generally unfavourable to cyclopoids, whose biomass percentage was particularly low at the end of each experiment in enclosures where nutrients have been added (see Fig. 5). This negative effect of nutrients on cyclopoids might be because of poor algal quality (size and nature) for nauplii and/or adults. It could also be due to a strong competition with parthenogenic herbivores (rotifers and cladocerans), which possess higher intrinsic growth capacities and thus seem better adapted to exploit rapidly the stock of suitable algae. However, the lack of information about phytoplankton composition and the food-selectivity of organisms limit discussion of this point.
Chaoborus effects
In both mesocosm experiments, the presence of Chaoborus had clear effects on zooplankton community structure. The predation impact was particularly oriented towards larger organisms such as cladocerans or copepodites and adults of Thermocyclops sp., which were significantly reduced in enclosures with Chaoborus, whereas there was no significant reduction of rotifers or copepod nauplii. The preference of Chaoborus for larger prey was confirmed in the predation experiments. The cladocerans D. excisum and M. micrura were always the most selected prey, and rotifer prey were never significantly reduced in any of the 10 predation experiments. This selective predation by Chaoborus shaped the zooplankton community and modified its size structure, as evidenced by the significant decrease in the average individual length of zooplankton in enclosures with Chaoborus. This trend is opposite to the most common pattern observed in temperate waters, where invertebrate predation generally induces a shift of the zooplankton size-spectrum toward larger sizes (Pinel Alloul, 1995). It is also consistent with the results of Irvine (1997) who found that larvae of Chaoborus edulis in Lake Malawi avoided the smallest prey and selected mainly cladocerans. In the present study this effect is probably maximised because the experiments used mainly third and fourth instars, which are known to feed on larger prey than the younger larvae (Irvine, 1997). It could also be related to the small proportion of rotifer biomass in the zooplankton assemblages (<40% during M1 and <20% during M2, see Fig. 5) and the rarity of soft-bodied species among the rotifer community (see Tables 1 and 2). Several studies (Moore & Gilbert, 1987; Havens, 1990) have shown that soft-bodied rotifers such as Synchaeta are more vulnerable to Chaoborus predation than are loricate species.
Direct effects of invertebrate predation on zooplankton communities have been demonstrated in many studies. Chaoboridae can eliminate 2–90% of the population of their prey per day (Pastorok, 1980; Von Ende & Dempsey, 1981). The mean predation rates estimated during the mesocosm experiments (29 and 20 prey Chaoborus−1 day−1 representing daily rations of 76 and 36% of body carbon for M1 and M2 respectively) were consistent with the mean values found during our predation experiments (16 prey Chaoborus−1; 26% of body carbon) and with values from the literature for several Chaoborus species (Pastorok, 1980; Pinel-Alloul, 1995). When comparing these predation rates to the Chaoborus and zooplankton densities, we estimated that 14–240% (mean = 130 ± 22%) of the prey population (15–280% in terms of biomass, mean = 120 ± 15%) would have been removed daily by Chaoborus predation in the enclosures. These high rates, even higher than the maximal values reported by Pastorok (1980) reinforce the idea of a very strong direct predation impact.
Indirect effects of predation by Chaoborus were also evident in our mesocosm experiments. Indeed, the smaller organisms appeared to be indirectly favoured as revealed by the density increase of nauplii (M1) and rotifers (M2) after several days in enclosures with Chaoborus as compared with control enclosures (see 2, 3). This increase of small organisms could have resulted from the decrease of their main predators (adults and last copepodites of Thermocyclops) and/or of their main herbivore competitors (cladocerans, young copepodite stages). It is well known that the last copepodite stages and the adults of cyclopids are at least partially carnivorous (Williamson & Gilbert, 1980) and that their predation impact on rotifer community structure can be very intense (Stemberger & Evans, 1984). Their diminution after several days in our mesocosm experiments, linked to Chaoborus predation, should have consistently reduced their predation impact on rotifers and nauplii and favoured the increase of these small organisms. The impact of predation by invertebrates on interspecific competition within the zooplankton community is also well documented. Neill (1984) demonstrated that the presence of Chaoborus trivittatus enhanced rotifers by decreasing their competition with cladocerans. The same situation probably applied in our experiments where the sharp decrease of cladocerans, linked to Chaoborus predation, should have resulted in decreased competition with other herbivores. The potential indirect effects of Chaoborus predation (decrease of cyclopid predation or decrease of competition) could have contributed separately or together to favour small organisms after several days in the mesocosm experiments.
The heavy predation by Chaoborus on zooplankton seemed to cascade down to phytoplankton, because in both experiments a significant Chaoborus effect on chlorophyll a was shown (see Tables 3 and 4). Many studies have shown that zooplanktivory can affect phytoplankton communities through cascading effects (Carpenter et al., 1985; McQueen, Post & Mills, 1986; Perin et al., 1996). It could have positive effects by reducing herbivory (Carpenter & Kitchell, 1988) or by stimulating algal production through excretion by predators, regenerating nutrients (Braband, Faafeng & Nielssen, 1990). It could also have negative effects by reducing zooplankton nutrient recycling (Ramcharan, France & McQueen, 1996).
In conclusion, our study showed that both bottom-up and top-down factors may exert a structuring control on the zooplankton community in Lake Brobo. Nutrients favoured more strictly herbivorous taxa and disadvantaged copepods. In the field, a sudden nutrient enrichment linked, for example, to a wind-driven event could translate into an increase in rotifers and cladocerans and a decline in copepods. On the other hand, Chaoborus predation had a strong direct negative impact on large crustaceans and favoured small herbivores (rotifers, nauplii) presumably by controlling their main food competitor. This strong impact, shown here experimentally, could also operate directly in situ, given the high Chaoborus densities observed in Lake Brobo of 2–4 individuals L−1, similar to the densities in the mesocosm experiments, and much higher than those previously recorded in the literature (Havens, 1990; Irvine, 1997).
Acknowledgments
This research is a part of the research program “Petits Barrages” granted by IRD. We thank F. Laloé, X. Lazzaro and two anonymous referee for helpful comments on the manuscript.




