Effect of salinity on growth, survival and oxygen consumption of juvenile brown shrimp, Farfantepenaeus californiensis (Holmes)
Abstract
The brown shrimp, Farfantepenaeus californiensis (Holmes), is a species native to north-west Mexico, where its culture potential is presently being addressed. Because of the climatic conditions prevailing in the region, salinities over 40 g L−1 is a commonly encountered problem. In the present study, the effect of salinity on the growth and mortality of juvenile F. californiensis is described. The change in short-term routine metabolism at different salinities was also evaluated in order to define the adaptive capacity of the shrimp and to provide insight into the changes in the pathways of energy distribution. Groups of shrimp were exposed to increasing salinity (25, 35, 45 and 55 g L−1), and growth and survival rates after 75 days were determined in duplicate 1.8-m3 tanks for each salinity level. Significant differences were found in final weight, growth rate and mortality of shrimp as a result of salinity level. Final mean shrimp weights at increasing salinity levels were 10.0, 9.4, 8.6 and 7.8 g. Corresponding mortality was 24.4%, 15.1%, 33.6% and 55.7%. Oxygen consumption was found to depend significantly on salinity and was equivalent to 0.0027, 0.0037, 0.0043 and 0.0053 mg g−1 min−1 respectively for the increasing salinities. The increased rate of oxygen consumption at high salinities reflects the response of the organism to osmoregulatory and ionic imbalances. Increased energy requirements to fulfil basic metabolic function as salinity increased resulted in a reduction in the energy that could be diverted to growth. Consequently, the culture of the brown shrimp at salinities over 35 g L−1 would probably result in reduced yields.
Introduction
The brown shrimp, Farfantepenaeus californiensis (Holmes) (Perez-Farfante & Kensley 1997), is native to the north-west region of Mexico, with a distribution ranging from California to Peru (de la Cruz 1981), and has considerable commercial importance.
The potential for commercial culture of the species has been addressed by Figueroa (1996). However, the lack of fresh water and the high summer temperatures in the region result in pond salinities consistently over 35 g L−1 (Hernández-Llamas, Magallón-Barajas, Lechuga-Devéze, Bustillos-Guzmán & López-Cortés 1995). Moreover, low water exchange rates (i.e. 5% or lower) usually result in salinities ranging from 41 g L−1 to 49 g L−1 (Porchas 1999). Martinez-Cordova, Villarreal-Colmenares & Porchas (1995), working in the arid region of north-west Mexico with Litopenaeus vannamei (Boone), found that a water exchange rate of 5% negatively affected survival and yield of shrimp. On the other hand, Martínez-Cordova, Villarreal-Colmenares, Porchas-Cornejo, Naranjo-Paramo & Aragón-Noriega (1997) showed that 6 h per day aeration in low water exchange ponds resulted in yields of L. vannamei similar to those obtained when a 15% exchange rate was used. Aeration is generally acknowledged to benefit pond water quality, particularly by preventing extreme oxygen depletion. At low dissolved oxygen (DO) levels, usually at or below 2 mg L−1 (Broom 1970), shrimp are stressed and frequently reduce their feeding rate, resulting in depressed growth rates and poor food conversion ratios (Villarreal 1984). In addition, the oxygen consumption rate of shrimp is influenced by external factors. Yagi & Ceccaldi (1984) found that salinity and temperature were the main factors influencing oxygen consumption by the common prawn, Palaemon serratus (Pennat). This metabolic response is frequently observed in other crustaceans (Dall 1986; Dalla Via 1978a). These antecedents clearly indicate that in-depth knowledge of the biological response of F. californiensis to salinity is essential to determine their culture potential.
In the present study, the effect of different salinities on growth and survival of juvenile F. californiensis reared in fibreglass tanks is described. The change in short-term routine metabolism, defined by the instantaneous oxygen consumption rate, at different salinities, was also evaluated in order to define the adaptive capacity of the shrimp. From these results, a preliminary insight into the changes in the pathways of energy distribution by the brown shrimp is presented.
Materials and methods
Effect of salinity on growth and survival
Laboratory-produced juvenile brown shrimp (F. californiensis) were acclimated to the experimental units (1.8-m3 fibreglass tanks, 2 m × 1.5 m × 0.6 m) for 2 weeks prior to the commencement of the trial. The shrimp were fed to excess with a diet consisting of Artemia nauplii, fresh-frozen squid and a 36% crude protein commercial pellet, at a rate of 15% of the total biomass per day. During this time, the salinity of each unit was gradually adjusted by adding fresh water or brine-rock salt (Coelho 1984) until four duplicate treatments were obtained: 25, 35, 45 and 55 g L−1. Brine-rock salt was obtained from evaporation of sea water in salterns as described by Lopez (1994). Animals were exposed to a maximum rate of salinity change of 5 g L−1 per day, and were held at their final experimental concentration for a minimum of 7 days before the commencement of the trial. All enclosures were aerated with air diffusers and received a daily water exchange of 20% with salinity-adjusted, filtered (10 µm) sea water. Dissolved oxygen, salinity and temperature of water were recorded daily. Ionized ammonia and pH levels were recorded weekly.
A group of juveniles were reselected according to size, to standardize the initial mean weight to 5.86 ± 0.14 g. Shrimp were then randomly stocked in each tank at a density of 33.3 juveniles m−2 (100 juveniles per tank). Feed, which consisted of equivalent dry-weight amounts of a commercial shrimp ration (36% crude protein) and frozen squid, was distributed once a day at a rate of 10% of the total biomass per day. Rearing time was 75 days and individual weight increases were recorded to the nearest 0.01 g at 15-day intervals. Analysis of variance and regression analysis procedures available in statistica (StatSoft, Tulsa, OK, USA) were used to test the response of final weights, mortality, and growth rates to salinity level. Means for duplicate tanks for each level were used to conduct the statistical analyses. Estimates of percentage mortality were calculated from initial and surviving shrimp populations contained in each tank replicate.
Effects of salinity on oxygen consumption
Juveniles were randomly selected from one of the experimental tanks after approximately 60 days of rearing, placed in a 0.112-m3 plastic tank (0.7 m × 0.4 m × 0.4 m) containing 60 L of filtered (5 µm) and UV-sterilized sea water at the experimental salinity, and maintained at 27 °C for 24 h before each run. No food was supplied during this time. For each salinity level, the routine rate of oxygen consumption by individual F. californiensis was measured in 28 2-L containers (respirometers) immersed in a constant-temperature water bath at 27 ± 1 °C. One shrimp was placed in each of 28 respirometers for each salinity. The respirometers were covered with a blue epoxic paint to reduce the effect of light intensity. Following the procedures of Liao & Murai (1986), the shrimp were acclimated to the respirometers for 2 h, each respirometer containing 1.85 L of water saturated with oxygen by an air diffuser. This minimized the effect of handling stress. After acclimation, four shrimp, selected at random, were removed from respirometers before sealing all the experimental enclosures with plastic caps. The containers from which the shrimp were removed served as blanks to estimate oxygen consumption by micro-organisms and the oxygen electrode.
The time course of oxygen depletion by juvenile F. californiensis was determined for 120 min. Gas transfer between the air pocket, left in the respirometer to allow for water displacement by the oxygen probe, and the water was assumed to be negligible. Recordings of oxygen saturation using a YSI-57 oxygen meter (YSI Inc., Box 279, Yellow Springs, OH, USA) were done every 10 min. After the trial, all shrimp were weighed to the nearest 0.01 g.
The rate of oxygen consumption was determined from the slope of the linear regression of dissolved oxygen on time, after adjusting for shrimp weight, water volume and the mean consumption in the blanks. Differences in shrimp oxygen consumption between experimental treatments were defined by analysis of variance. Both linear regression and analysis of variance were conducted using procedures available in statistica (StatSoft, Tulsa, OK, USA).
Effect of salinity on the energy distribution of F. californiensis
Following the method of Capuzzo (1986), the generalized energy budget for crustaceans can be described by the equation:
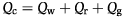 (1)
(1)where Qc is energy consumed, Qw is energy unassimilated and lost as nitrogenous waste products, Qr is energy used for metabolism and Qg is energy diverted to growth.
On the other hand, the assimilated energy (Qa) is the energy consumed minus the energy unassimilated or wasted (Qc–Qw). By substitution in equation 1, this results in:
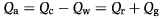 (2)
(2)This equation indicates that, in sexually immature organisms, all the assimilated energy not used as metabolic fuel would be diverted to growth. To obtain an insight as to how the energy balance changes as salinity increases, energy equivalents for oxygen [3.376 cal mg−1 (Brody 1945)] and for shrimp [3656 cal g−1 dry tissue (New 1987)] were used to define assimilated energy as defined by equation 2.
Results
There were no significant differences in temperature, dissolved oxygen, pH and unionized ammonia (NH3) between the experimental units enclosures for the duration of the trial (Table 1). In general, those water quality parameters were within the acceptable range for penaeid shrimp (Lawrence 1985).
| Parameter | n | Mean value |
|---|---|---|
| Temperature (°C) | 75 | 27.23 ± 0.69 |
| Oxygen (mg L−1) | 75 | 5.85 ± 0.21 |
| pH | 10 | 7.85 ± 0.55 |
| NH3 (mg L−1) | 10 | 0.18 ± 0.05 |
Significant differences were detected between treatments in final weight, growth rate and mortality (Table 2). Mortality was significantly higher at higher salinities (45 and 55 g L−1); however, similar mortalities were obtained for the 25 and 35 g L−1 treatments (Table 2). Regression analysis indicated that mortality did not depend linearly on salinity (P > 0.05), but a quadratic (i.e. second-order polynomial) provided an adequate fit (Fig. 1).
| Salinity (g L−1) | Weight (g) | Growth rate (g d−1) | Mortality (%) | |
|---|---|---|---|---|
| Initial | Final | |||
| 25 | 5.95 ± 0.11a | 10.02 ± 0.21a | 0.054 ± 0.0005a | 24.49 ± 1.7a |
| 35 | 5.95 ± 0.09a | 9.39 ± 0.19ab | 0.046 ± 0.0008ab | 15.16 ± 1.56a |
| 45 | 5.66 ± 0.11a | 8.57 ± 0.23bc | 0.039 ± 0.0009bc | 33.63 ± 1.1b |
| 55 | 5.85 ± 0.13a | 7.75 ± 0.23c | 0.025 ± 0.0007c | 55.79 ± 1.25c |
- * Values that do not share a common letter for each column are significantly different (P < 0.05).
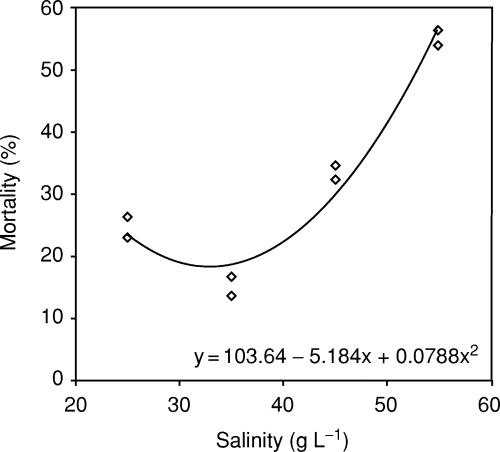
Mortality of F. californiensis juveniles at different experimental salinity levels. Regression coefficients of a quadratic equation to predict response were significant (P < 0.005, r2 = 0.97).
The time course of oxygen depletion by the brown shrimp at different salinities is shown in Fig. 2. Linear regression provided an adequate fit for these relationships, in which the oxygen consumption was essentially constant and thus independent of DO level, down to approximately 1.5 mg L−1 at 27 °C and 55 g L−1. The duration of the trials was insufficient to estimate the lower limit of oxygen independence.
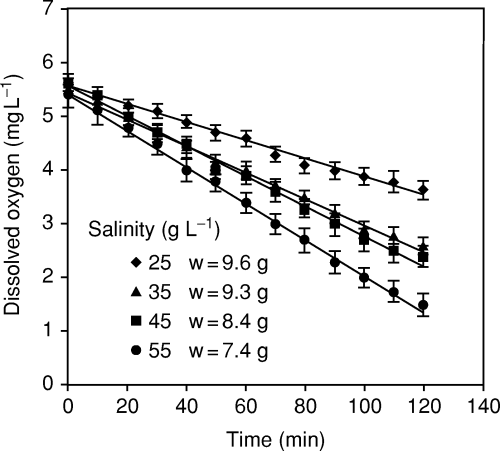
Time course of oxygen depletion by F. californiensis in closed respirometers at 27 °C. Vertical lines represent the standard error of mean dissolved oxygen values. Linear regressions were significant for all salinity levels (P < 0.001). Average weights (w) are from Table 3.
There were significant differences in oxygen consumption (mg g−1 min−1) between salinities (Table 3). On the other hand, a linear regression showed that oxygen consumption increase was dependent on salinity (Fig. 3). Thus, at 55 g L−1 oxygen consumption was 25.7% higher than at 45 g L−1, 41.0% higher than at 35 g L−1 and 99.9% higher than at 25 g L−1. After transforming the growth rate and the oxygen consumption rate for each treatment into energy equivalents (Table 4), an inverse relationship between salinity and assimilated energy was obtained (Fig. 3).
| Salinity (g L−1) | n | Mean weight (g) | Oxygen consumption (mg g−1 min−1) |
|---|---|---|---|
| 25 | 24 | 9.62 ± 0.15 | 0.0026 ± 0.00008a |
| 35 | 24 | 9.32 ± 0.19 | 0.0037 ± 0.00013b |
| 45 | 24 | 8.41 ± 0.20 | 0.0042 ± 0.00016b |
| 55 | 24 | 7.46 ± 0.21 | 0.0053 ± 0.00018c |
- * Values with different letter for each column are significantly different (P < 0.05).
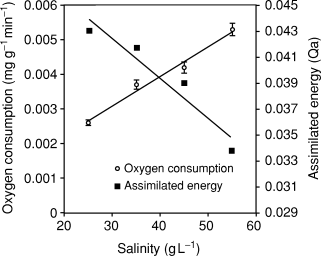
Oxygen consumption and assimilated energy in response to experimental salinity levels for F. californiensis juveniles. Vertical lines represent the standard error of mean oxygen consumption values. Linear regressions to predict response were significant (P = 0.009 and P = 0.039 respectively).
| Salinity (g L−1) | Growth rate | Oxygen consumption | Assimilated energy (Qa)* | ||
|---|---|---|---|---|---|
| (g d−1) | (cal g−1 min−1) (Qg) | (mg g−1 min−1) | (cal g−1 min−1) (Qr) | ||
| 25 | 0.054 | 0.0343 | 0.0026 | 0.0087 | 0.0430 |
| 35 | 0.046 | 0.0292 | 0.0037 | 0.0125 | 0.0417 |
| 45 | 0.039 | 0.0248 | 0.0042 | 0.0142 | 0.0390 |
| 55 | 0.025 | 0.0159 | 0.0159 | 0.0179 | 0.0338 |
- *Assimilated energy was defined as Q a = Qr + Qg.
Discussion
Farfantepenaeus californiensis is predominately an oceanic species (de la Cruz 1981), and thus adapted to an environment in which conditions tend to remain stable. The results of this study indicate that an increase in salinity from oceanic levels (35 g L−1) negatively affects both growth and survival.
Reduced growth rates associated with increased salinity may be related to reduced feed consumption. Although no direct data were obtained in our study with respect to food intake, it was evident from visual observations that shrimp reared at the highest salinity were lethargic in general and consumed less feed than shrimp reared at lower salinities. Assimilated energy (Qa) was inversely proportional to salinity, with the rate of energy diverted to growth compared with energy diverted to metabolic functions ranging from 4:1 at 25 g L−1 to roughly 1:1 at 55 g L−1. It appears that an increase in the energy requirements to fulfil routine actions reduces the amount available for tissue growth. At a commercial level, the effect of reduced growth rates would be reduced profitability. In addition, the mortality rate at salinities over 35 g L−1 significantly increased, perhaps reflecting the loss in capacity to adapt to changes in osmotic, sodium or chloride concentrations in the surrounding environment (Castille & Lawrence 1980). In our work, brine-rock salt was used as a practical way to obtain salinity levels of seawater above 35 g L−1. The response of the brown shrimp to changes in salt composition must still be determined.
The accuracy of oxygen consumption recording and the potential consumption of oxygen by organisms other than experimental animals have been major concerns in the design of systems that monitor the oxygen consumption of aquatic animals. The use of empty chambers (blanks), in either parallel or serial recordings, has been widely accepted as a way to overcome these concerns. Using serial blanks, Sutcliffe, Carrick & Moore (1975) reported that micro-organisms introduced to the respirometer by the experimental organism may constitute an important source of oxygen depletion, which should be taken into account. However, Villarreal (1990) indicated that consumption by the blank on a parallel system for marron, Cherax tenuimanus (Smith), was approximately 6% of the consumption of the experimental organisms. The oxygen depletion in the blanks during the salinity trials in this study was always less than 10% of the mean oxygen consumption by the shrimp. Blank adjusted recordings provided a better estimate of the actual consumption of shrimp.
In closed systems, a statistical description of the time course of the reduction in DO, due to the respiration by an organism, offers a considerable improvement in the methodology (Morrissy, Caputi & House 1984). This is preferable to estimates of respiration on oxygen measurements at the start and finish of a trial. The slope of a linear regression of DO with respect to time provided the best estimate of the respiration rate of F. californiensis for all the experimental salinities. This indicates that the rate of oxygen consumption was constant and independent of DO. Table 5 presents a comparison between the routine metabolic rate of F. californiensis at 35 g L−1 and other penaeid shrimp. The brown shrimp showed an oxygen consumption similar to Kuruma prawn, Marsupenaeus japonicus (Bate) (Perez-Farfante & Kensley 1997), another closed thelycum, temperate-water species, and was generally within the range of reported values for penaeid shrimp.
| Species | Temperature (°C) | Weight (g) | Oxygen consumption (mg g−1 min−1) | Source |
|---|---|---|---|---|
| Penaeus esculentus (Haswell) | 25.0 | 12.0 | 0.0028 | Dall (1986) |
| Marsupenaeus japonicus (Bate) | 26.5 | 6.0 | 0.0035 | Sacayanan & Hirata (1986), cited as Penaeus |
| Penaeus monodon (Fabricius) | 25.0 | 9.2–12.0 | 0.0050 | Liao & Murai (1986) |
| Farfantepenaeus brasiliensis (Latreille) | 25.0 | 10.0 | 0.0048 | Cited as Penaeus (Scelzo & Zuñiga 1987) |
| Perez-Farfante & Kensley (1997) | 30.0 | 10.0 | 0.0052 | Cited as Penaeus (Scelzo & Zuñiga 1987) |
| Farfantepenaeus californiensis | 27.0 | 9.3 | 0.0037 | This study |
Some evidence indicates that, for decapod crustaceans, there is a saturation point at which consumption becomes dependent on DO. This critical point, or incipient lethal limit (Fry 1971), separates the zone of respiratory regulation from the zone of respiratory dependence (Seidman & Lawrence 1985). Several authors (e.g. Egusa 1961; Broom 1970; MacKay 1974; Kramer 1975; Seidman & Lawrence 1985; Liao & Murai 1986) have reported critical DO points for shrimp, generally below 2 mg L−1. In the present study, a critical point was not defined, even for concentrations approximating 1.5 mg L−1 DO (30% saturation) at 55 g L−1. It seems that the incipient lethal limit for F. californiensis is probably below this level. This would agree with Egusa (1961), who defined a critical level for M. japonicus (between 0.7 and 1.4 mg L−1 DO), and MacKay (1974), who indicated that Litopenaeus schmitti (Burkenroad) shows a critical DO point at approximately 0.9 mg L−1.
Short-term determination of oxygen consumption provides valuable information relating to the changing response of organisms to environmental variations. However, Dall (1986) suggests that the routine rate of oxygen consumption should be defined over a larger experimental time (> 24 h) in order to incorporate factors such as circadian rhythmicity (Sacayanan & Hirata 1986) and metabolic scope (Bennet 1978). Other factors to be considered for future research include the molting cycle (Hewitt 1984), the maturation process, feeding and the effects of environmental factors such as light and temperature.
The increased rate of oxygen consumption at high salinities reflects the response of the organism to osmoregulatory and ionic imbalance (Kutty, Murugapoopathy & Krishnan 1971; Dalla Via 1978b). Increased energy requirements to fulfil basic metabolic functions (Qr) as salinity increases result in a reduction of the energy that can be diverted to growth (Qg). Consequently, the culture of brown shrimp at salinities over 35 g L−1 would probably result in reduced production, given the reduction in assimilated energy (Qa).
Acknowledgments
The authors wish to thank Dr Luis Martinez-Cordova and two anonymous reviewers for reviewing the manuscript. Thanks are also given to the technical personnel at CIBNOR who provided support to carry out the experimental trials.




