A note on group sizes of oribi (Ourebia ourebi, Zimmermann, 1783) from two contrasting sites in Zambia, with and without predation
Abstract
enThe oribi (Ourebia ourebi, Zimmermann, 1783) is a small species of antelope widely represented across open grasslands of sub-Saharan Africa. Although largely territorial, differences in group size and mating systems (monogamy/polygamy), have been linked to habitat conditions and population density. At high population densities, additional males are recruited to assist in territorial defence. Other factors which might impact on group size, include predation threat, especially at low population densities. In this study (1995–98), we recorded group sizes of oribi across two contrasting areas (c. 300 km2 each) of Zambia with (Kafue), and without (Bangweulu), large vertebrate predators. A total of 412 groups was recorded across the two sites, with mean sizes of 2.31 (n = 217) at Bangweulu and 2.33 (n = 195) at Kafue. The modal group size was two throughout (range 1–6). Groups were more variable in size and sex composition at Kafue than at Bangweulu, but there was no significant difference in estimates of population density between sites. Mean estimates were 2.02 and 1.90 km−2 for Bangweulu and Kafue, respectively. Sex ratios (males : females) were biased towards females (1 : 1.72 and 1 : 3.55, respectively) at both sites. Adult males were particularly scarce at Kafue. However, values of density and group size, were both unexceptional for the species. Whilst a modal group size of two is consistent with a monogamous mating system, variations in group composition and size were more difficult to explain. Adult females appeared to retain or recruit additional animals to groups, irrespective of the needs of territorial defence. This suggests that vigilance is an important function of groups on large open plains, especially in the presence of predators. By extension, reversed sexual dimorphism in oribi could reflect increased selection for vigilance duties in females. Predators had no apparent effect on population density, but oribi groups were more variable in size and sex composition in their presence. Adult males may be particularly vulnerable to predation when defending territorial borders at low population densities. However, further work is needed to define group dynamics in this species and to identify causes of mortality amongst adult males.
Résumé
frL'oribi (Ourebia ourebi, Zimmermann, 1783) est une petite espèce d'antilope largement présente dans les prairies ouvertes d'Afrique sub-saharienne. Bien qu'elle soit principalement territoriale, on a pu lier des différences dans la taille des groupes et dans le système d'accouplement (monogamie/polygamie), aux conditions de l'habitat et à la densité de la population. Lorsque la densité de population est élevée, des mâles supplémentaires sont recrutés pour aider à la défense territoriale. D'autres facteurs qui pourraient influencer la taille des groupes incluent la menace des prédateurs, spécialement si la densité de population est faible. Dans cette étude (de 1995 à 1998), nous avons relevé le taille des groupes d'oribis dans deux zones très différentes (env. 300 km2 chacune) de Zambie avec (Kafue) et sans (Bangweulu) grands vertébrés prédateurs. On a relevé un total de 412 groupes pour les deux sites, dont la taille moyenne était de 2.31 (n = 217) à Bangweulu et de 2.33 (n = 195) à Kafue. La taille modale du groupe était deux partout (allant de 1 à 6). Les groupes étaient plus variables en ce qui concerne la taille et le sex-ratio à Kafue qu'à Bangweulu, mais il n'y avait pas de différence significative des estimations de densité de population entre les sites. Les estimations moyennes étaient respectivement de 2.02 et de 1.90/km2 pour Bangweulu et pour Kafue. Le sex-ratio (mâles : femelles) était biaisé en faveur des femelles (1 : 1.72 et 1 : 3.55 respectivement) sur les deux sites. Les mâles adultes étaient particulièrement rares à Kafue. Cependant, les valeurs, tant pour la densité que pour la taille des groupes, n'étaient pas exceptionnelles pour l'espèce. Si une taille modale de groupe de deux correspond bien à un système de reproduction monogame, les variations de la composition de la taille des groupes sont plus difficiles à expliquer. Il semble que les femelles adultes maintiennent ou recrutent des animaux supplémentaires pour le groupe, quels que soient les besoins de défendre le territoire. Ceci laisse penser que la vigilance est un fonction importante pour les groupes qui vivent dans de vastes plaines ouvertes, spécialement lorsqu'il y a des prédateurs. Par extension, le dimorphisme sexuel inversé chez les oribis pourrait refléter une sélection accrue pour les devoirs de vigilance chez les femelles. Les prédateurs n'ont pas d'effets visibles sur la densité de la population, mais les groupes d'oribis ont une taille et un sex-ratio plus variables en leur présence. Les mâles adultes pourraient être particulièrement vulnérables à la prédation lorsqu'ils défendent les limites du territoire et que la densité de population est faible. Cependant, d'autres travaux sont nécessaires pour définir la dynamique du groupe de cette espèce et pour identifier les causes de mortalité des mâles adultes.
Introduction
The oribi Ourebia ourebi (Zimmermann, 1783) is a small (12–14 kg) species of antelope, widely distributed across open grasslands of Africa, south of the Sahara (Kingdon, 1982; Smithers & Skinner, 1991). The species is sexually dimorphic, with males bearing horns to 18 cm length (Arcese, 1994). Females are up to 2 kg heavier than males. Adult males defend territories throughout the year, by scent marking and by border ‘patrol’ (Gosling, 1986; Brashares & Arcese, 1999). Territories are stable with time, irrespective of the identity of resident male (Linn & Everett, 1992; Arcese, Jongejan & Sinclair, 1995). Scent marking at middens may also enable females to assess mate quality (Dunbar & Dunbar, 1980; Roberts & Dunbar, 2000). Calves are born prior to, or during, the rains in central Africa (Jeffery, Bell & Ansell, 1989; Jongejan, Arcese & Sinclair, 1991).
In spite of their territorial behaviour, various social structures and mating systems (monogamous/polygamous) have been proposed for oribi, depending on differences in resources and population density (Clutton-Brock, 1989, 1991; Thirgood et al., 1992, 1999; Arcese et al., 1995; Adamczak, 2000). At high population densities, additional males are recruited to assist in territorial defence (Rowe-Rowe, Everett & Perrin, 1992; Woodruffe & Vincent, 1994; Arcese, 1999). Males remain with females throughout the year even though they do not invest directly in the ‘care’ of their calf (Kleiman, 1977; Barlow, 1988; Brotherton & Manser, 1997). Both sexes could contribute indirectly to the protection of the group, through increased vigilance (Quenette, 1990; Baldellou & Henzi, 1992; Hannon & Martin, 1992; Cowlishaw, 1999). However, surprisingly little is known about the effects of predators on oribi (Mduma & Sinclair, 1994).
In this study (1996–98), we examined group sizes of oribi from two sites in Zambia with (Kafue National Park) and without (Bangweulu swamp region) large vertebrate predators. Lions, Pardus leo (Linnaeus, 1758), are common in the Kafue National Park (Mitchell, Shenton & Uys, 1965; NPWS/JICA, 1999; P. de Vere-Moss, pers. comm.) but are rarely seen at Bangweulu (Grimsdell & Bell, 1975). The main aim of this study was to evaluate the possible effects of predators on group size in oribi. Although both sites are noted for their diverse vertebrate assemblages, few quantitative data are available on oribi (Ansell, 1960; Bell et al., 1973; Simbotwe & Sichone, 1989; Sweet, 1996).
Materials and methods
The study was carried out in the Kalasa-Mukoso Flats region of the Lake Bangweulu floodplain basin (Kalasa-Mukoso village, 11°50′ S, 29°35′ E) and in Kafue National Park to the west of the Ngoma (15°54′ S, 25°57′ E) Research Station (Fig. 1). Both sites contain large areas of open grasslands subject to occasional fires during the dry season (Howard & Aspinwall, 1984). Field work was conducted between October and January (1995–96) at Bangweulu, and between July and December (1998), at Kafue. Seven village fishing camps occur within the Bangweulu region, but the area surveyed in Kafue, was largely uninhabited apart from the research station
Location of (a) Bangeweulu region, and (b) Kafue National Park in Zambia. Numbers and letters refer to main sampling areas at Bangweulu. Point counts made around sites A–E and transect counts around areas 1–9. Main villages indicated. Survey region at Kafue to the west of the Ngoma road
Regular surveys were carried out along transects (2 km2) during the morning and evening periods, using village tracks in Bangweulu and occasionally off-road at Kafue. All oribi seen (by telescope 70 × 40 mm) were recorded and plotted on field maps, using GPS and UTM co-ordinates. A 500 m strip (either side) was covered during counts from vehicles (Hirst, 1969; Burnham & Anderson, 1984). Extensive focal observations and ‘point counts’ (Mduma & Sinclair, 1994) were also made from five locations at Bangweulu (A–E, Fig. 1), to evaluate visibility, group membership and sampling errors (Sweet, 1996). All sites were chosen according to a random daily schedule. Where possible, animals were classified by sex and by age class (adults and juveniles). These subsets were used in the analysis of sex ratios. Groups consisted of animals dispersed at distances of less than 100 m from one another (see Arcese et al., 1995).
Data were entered into spreadsheets (available from C.R.G.) and coded by location (Bangweulu), habitat (Kafue), date, time of day, sex class together with the UTM co-ordinates. The main habitats represented at Kafue were grasslands (short, long, wet or burnt), ‘dambos’ and open woodlands, although the latter made up less than 5% of the total study area (NPWS/JICA, 1999). Site and habitat codes were used to sort data for analysis. Estimates of density were derived from total counts made from each sub-region (coded sites and habitats) surveyed. Areas were obtained from maps of the two sites. No attempt was made to record territorial boundaries, although most groups appeared to be restricted in their distribution (e.g. Linn & Everett, 1992). Single animals are included within the term ‘group’ for the purposes of analysis, but calves were excluded from all counts (see Arcese et al., 1995). All counts are considered to be fully representative as no additional animals were flushed from cover during detailed examination of habitats either at Kafue or at Bangweulu.
Results
Oribi were a regular feature of the faunal assemblages of the two sites surveyed. Estimates of density at Bangweulu ranged from 1.28 to 4.90 km−2 (F = 9.64, df = 4, 16, P < 0.001) with a mean of 2.02 km−2. At Kafue, the average density was 1.90 km−2 with a mean range of 1.30–2.45 km−2 (F = 1.20, df = 6, 20, P > 0.05). Estimates varied across Bangweulu with individual counts increasing with distance (1–8 km) to the nearest village (r = 0.669, df = 6, P < 0.05) (Sweet, 1996). By contrast, there was no obvious difference in density estimates between sites (habitats) at Kafue. Similarly, there was no difference in the pooled estimates between Bangweulu and Kafue (P > 0.05). The combined estimate for both sites was 2.0 km−2.
Individual groups varied in size from one to six across both Bangweulu and Kafue (Fig. 2). Group profiles were similar in both areas (χ2 = 6.85, P = 0.232 and G = 6.97, P = 0.222), with a modal group size of two (Fig. 2). There was no apparent difference in the derived mean group sizes (range in means 2.00–2.80) across sites at Bangweulu (F = 0.70, df = 8, 208, P = 0.687) nor effect of sampling date on group size (P > 0.05). By contrast, group sizes were more variable across Kafue. Mean values varied from 1.0 up to 2.9 (F = 2.97, df = 6, 188, P = 0.008) across habitats there. The largest groups (mean = 2.94) were found on the ‘short’ grasslands (n = 108) and the smallest (range 1.00–1.85) within woodland and dambo sites, respectively (n = 35). For other habitats, mean group size ranged from 2.54 up to 2.94 (n = 13 and n = 31, respectively, with a pooled S.D. = 1.118). When sorted by month (Fig. 3), the largest groups (mean = 2.94) were recorded in October at Kafue (F = 3.56, df = 5, 189, P = 0.004).
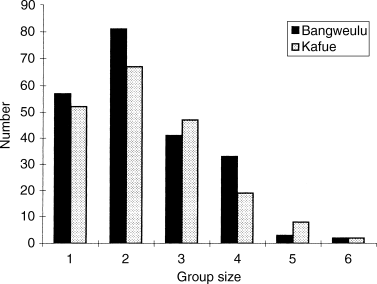
Distribution of group sizes of oribi at Bangweulu (n = 217) and at Kafue (n = 195)
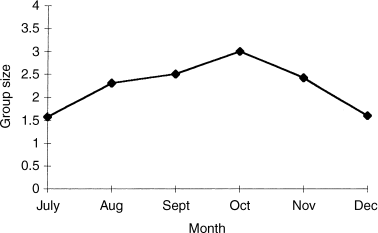
Mean size of oribi groups at Kafue with month (July–December)
The sex ratio was biased towards females for both sites. Thus for Bangweulu the combined sex ratio (males to females) was found to be 47 : 81, and for Kafue 75 : 266 (χ2 = 9.81, P < 0.01). This bias was particularly pronounced for individual groups of oribi (Fig. 2). For lone animals, 46.7% were males at Bangweulu and 32.4% at Kafue. For groups of two, 83.3% consisted of male–female pairs at Bangweulu, but only 4.0% were male–female pairs at Kafue. For groups of three, 88.8% were 1 male−2 female combinations at Bangweulu, but only 2.7% at Kafue. Similarly, 56.4% of groups of three at Kafue were all female groups as opposed to 11.1% at Bangweulu. For groups of four to six, more than 95% of groups contained either one or two males (both sites).
Discussion
Oribi were a conspicuous feature of the two areas of Zambia surveyed, confirming previous qualitative records (Ansell, 1960). However, estimates of population density were low compared with data from other areas of Africa, where values range from less than 2 km−2 up to 10 km−2 (Adamczak, 2000). Most of the higher values (7.8–9.3 km−2) come from the Serengeti region of Tanzania (Arcese et al., 1995). Reasons for these regional differences in density remain unclear, but may reflect a latitudinal cline in density and/or site conditions (Adamczak, 2000). There was no apparent difference in the average estimates of density for Bangweulu and Kafue, which suggests that ecological conditions were similar throughout (Linn & Everett, 1992; Arcese et al., 1995). Site differences at Bangweulu probably reflect disturbance, as most of the smaller counts came from areas close to village fishing camps. However, there was no evidence that oribi were exploited by villagers, as altenative game species were well represented across Bangweulu (Sweet, 1996). In terms of range size, each oribi group would, on average, have access to an area of about 1 km2.
A wide range of group sizes was recorded during the study, although a modal group size of two (Fig. 2) is consistent with a monogamous mating system. However, significant numbers of larger groups were recorded at both sites. These varied both in size and sex composition, especially at Kafue. Whilst variation in group size is common in larger, more mobile ungulate species, differences in group size amongst small, more sedentary species are more difficult to explain (Thirgood, 1996; Thirgood et al., 1999). During the current study multi-male groups were rarely recorded either at Bangweulu or at Kafue. Adult males were particularly scarce on Kafue. Groups were largest on those sites with limited cover at Kafue and they increased prior to the main calving season there.
These results suggest that a function of grouping in oribi may be concerned with vigilance duties. Where adult males were scarce, as at Kafue, females appeared to retain or recruit additional females, irrespective of the needs of territorial defence. There were no obvious differences in group composition across sites at Bangweulu. Thus, in the presence of predators, group size appears to be more responsive to site conditions, irrespective of the operant mating system.
If this is a correct interpretation, then reversed sexual size dimorphism seen in oribi may have arisen as a response to increased selection for body size in females, associated with a need to sustain vigilance on large open sites. As a sedentary species, the oribi would be particularly vulnerable to predation. Although both sexes may contribute to vigilance duties, males may be more committed to the defence of the territorial boundaries. Whilst it is difficult to account for the scarcity of males at Kafue, they may be subject to high predation pressures during the defence of territorial boundaries at low population densities (Prins & Iaason, 1989; Mduma & Sinclair, 1994).
Few field studies address the effects of predation on ungulates, but oribi are known to be taken by predators at Kafue (Mitchell et al., 1965). However, further work is needed to define the group dynamics of the species and to identify sources of mortality amongst males. Females appear to play a major role in determining group size in oribi irrespective of mating systems, at least at low population densities. As in many other ungulates, population responses to site conditions may arise, in part, from changes in behaviour of the female, rather than that of the male (Clutton-Brock & Albon, 1985; Mendoza & Mason, 1986; Baldellou & Henzi, 1992; Bowland & Perrin, 1995; Komers, 1996; Komers & Brotherton, 1997; Grant et al., 1992).
Acknowledgements
We are grateful to the National Parks and Wildlife Service (now ZWA) of Zambia, for permission to work in Kafue National Park and in Bangweulu. Thanks also to Rohlf Lindolm of WWF, Karen Dennis, F. Mupemo and S. Christian for assistance in the field and to various members of Greenforce who participated in the project. Also, for useful dicussions with D. Lloyd, W. Astle, P. Berrie, Peter de Vere Moss and Vera Adamczak. Helpful comments on the manuscript were provided by Dr P. Arcese and M. Hoult assisted with the preparation of figures. We acknowledge with thanks, the support given by the British Airways Assisting Conservation (BAAC) programme (flights for G.S).




