An Endoluminal Sleeve Induces Substantial Weight Loss and Normalizes Glucose Homeostasis in Rats with Diet-Induced Obesity
Abstract
To investigate the contributions of two surgical gut manipulations—exclusion of the proximal intestine from alimentary flow and exposure of the jejunum to partially digested nutrients—to body weight regulation and metabolism, we have developed a rat model of an investigational device, the endoluminal sleeve (ELS). The ELS is a 10 cm, nutrient-impermeable, flexible tube designed for endoluminal implantation. ELS devices were surgically implanted in the duodenal bulb of rats with diet-induced obesity. Body weight, food intake, stool caloric content, and glucose homeostasis were subsequently evaluated. ELS-implanted rats demonstrated a 20% reduction of body weight compared to sham-operated (SO) controls. ELS-treated animals consumed an average of 27% fewer kcal/day than SO, and there was no evidence of malabsorption. ELS treatment improved fasting glycemia and glucose tolerance after oral and intraperitoneal (IP) administration. ELS treatment enhanced insulin sensitivity, as demonstrated by decreased fasting and glucose-stimulated insulin levels and confirmed by calculation of homeostasis model assessment of insulin resistance (IR). These data suggest that selective bypass of the proximal intestine by ELS, with enhanced delivery of partially digested nutrients to the jejunum, mimics many of the effects of Roux-en-Y gastric bypass (RYGB) on body weight and glucose metabolism. Thus, ELS implantation may be an effective treatment for obesity and diabetes. Since the ELS device is amenable to endoscopic placement, it may offer a valuable alternative to more invasive surgical approaches in selected patients with obesity and its metabolic complications.
Introduction
The prevalence of obesity is increasing worldwide with profound health and economic consequences (1,2). Patients with obesity have substantially increased morbidity and mortality from obesity-related complications, including type 2 diabetes, cardiovascular disease, and several types of cancer (3,4,5). Although there are many therapeutic options for obesity, including pharmacologic, dietary, and behavioral approaches, the weight loss from these interventions is seldom durable. In contrast, surgical therapies commonly result in substantial and sustained weight loss, as well as improvement, and even resolution, of obesity-related comorbidities. Among the available gastrointestinal (GI) weight loss surgical procedures, Roux-en-Y gastric bypass (RYGB) is currently the operation of choice. Surgical reconstruction of the GI tract during RYGB likely alters mucosal exposure to ingested macronutrients so as to differentially modulate efferent gut signals that govern energy regulation and metabolism. The result is weight loss and improved metabolic control. The precise mechanisms of these effects are unknown, although evidence is accumulating that RYGB alters neuroendocrine function in the GI tract at multiple levels (6,7,8,9,10,11,12,13,14,15,16).
In recent years, several animal models, including ileal transposition, jejuno-ileal bypass, RYGB, and others, have been designed to investigate the physiologic effects of GI tract reconstruction during bariatric procedures (9,10,14,17,18,19,20,21,22,23). None of these models includes the entire complement of GI manipulations present in the RYGB, and some incorporate changes not present in the standard gastric bypass. Each surgical model is effective in reducing body weight and improving metabolism, although specific effects vary from model to model. These studies suggest that different components of RYGB contribute to its effects on energy balance and metabolism. To define the mechanisms by which RYGB regulates these processes, it is useful to consider this operation as comprising five GI manipulations or “components,” some combination of which results in the physiologic effects of the procedure: (1) isolation of the gastric cardia, (2) exclusion of the distal stomach from the alimentary flow, (3) exclusion of the proximal intestine from the alimentary flow, (4) exposure of the jejunum to partially digested nutrients, and (5) partial vagotomy (1). Identification of the component or combination of components responsible for the effects of RYGB on weight loss and metabolic control would help define the role of the GI tract in these functions and facilitate the development of more targeted and less invasive therapies.
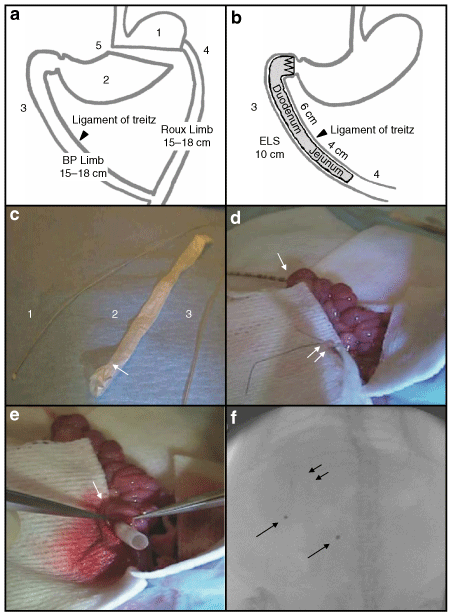
Roux-en-Y gastric bypass (RYGB) and endoluminal sleeve (ELS) placement in rats. (a) The five components of RYGB: isolation of the gastric cardia (1), exclusion of the distal stomach from alimentary flow (2), exclusion of the proximal intestine (3), exposure of the jejunum to partially digested nutrients (4), and partial vagotomy (5). (b) Schematic of the ELS device, in situ. The ELS enables the examination of two components of RYGB in isolation—(3) and (4). (c) Introduction catheter (1) and delivery tube (3) used to introduce the ELS, as described in the text. ELS device (2) is pictured with anchoring crown at the proximal end (single arrow). (d) ELS implantation (see text for full details). Introduction catheter is shown exiting from the duodenotomy and sutured to the ELS (double arrows). (e) The delivery tube, threaded over the anchoring crown of the ELS, is pictured extending from the duodenotomy (single arrow). (f) Fluoroscopic image of the ELS positioned within the duodenum. Anchoring crown is seen in the right upper quadrant (double arrows) with the radioopaque markers trailing distally (single arrows).
To explore the relative contributions of selective components of RYGB to body weight and metabolic control, we developed an indwelling, endoluminal device that mimics two of them in isolation. The endoluminal sleeve (ELS) is a nutrient-impermeable, flexible tube designed to be anchored in the duodenal bulb and to extend into the proximal jejunum (1). After implantation, ingested nutrients pass from the pylorus directly into and through the lumen of the ELS, isolating the mucosa of the proximal intestine from the nutrient-rich chyme (exclusion of the proximal intestine). As the flow of nutrients exits from the distal end of the ELS, the mucosa of the proximal jejunum is exposed to nutrients that have not been modified by either pancreaticobiliary secretions or the digestive and absorptive functions of the proximal gut (exposure of the jejunum to partially digested nutrients). Early experience with a similar device in a swine model and in human patients suggests efficacy of this approach for the treatment of obesity and diabetes (24,25,26,27,28). To explore the effects of ELS implantation on body weight and metabolic function in detail, we developed a rat model of the ELS device and a procedure for its safe and stable implantation. In this paper, we report that ELS implantation induces substantial weight loss and improves metabolic function in rats with diet-induced obesity, suggesting an important role of exclusion of the proximal small intestine and enhanced jejunal delivery of partially digested nutrients in the mechanism of action of RYGB.
Methods and Procedures
Animals
All experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Sprague-Dawley (Harlan, Indianapolis, IN) and Osborne-Mendel (OM; personal colony) were individually housed in a barrier animal facility. Animals were fed either regular rodent chow or a high-fat diet (HFD) that contained 35% calories from fat (Pharmaserv, Framingham, MA), as indicated in Study design and Results.
Device and implantation
The ELS is a flexible, polytetrafluoroethylene tube with an expandable metal anchoring crown at the proximal end (GI Dynamics, Watertown, MA). The tube is 10 cm long with radioopaque markers at 5 and 10 cm. After overnight fasting, rats were maintained on inhaled anesthesia throughout surgical implantation of the device. After releasing the intestine from the ligament of Trietz, two enterotomies were introduced, one immediately distal to the pylorus and the other 10 cm further down the small intestine. Next, an introduction catheter was threaded through the distal enterotomy and advanced retrograde to exit from the proximal duodenotomy. The flexible end of the ELS device was then sutured to the tip of the introduction catheter, and the ELS was pulled into the intestine by withdrawal of the introduction catheter. The duodenotomy was then repaired, and the crown of the ELS anchored to a surgical pledget situated within the subcutaneous compartment between skin and abdominal musculature. Finally, the distal enterotomy and laparotomy were repaired. The sham operation (SO) consisted of laparotomy with repair; release of the proximal intestine from the ligament of Trietz; duodenotomy with repair; and enterotomy with repair. Anesthesia time was standardized to 1.5 h for both ELS and sham implantation. After surgery, animals were provided with a liquid diet that was advanced as tolerated. All animals were placed on a solid diet no later than postoperative day 7. Starting during postoperative week (POW) 2, animals were evaluated by fluoroscopy on a weekly basis to ensure ELS retention. Animals were restrained with a translucent animal restrainer, without anesthesia, to obtain fluoroscopic images.
Study design
Sprague-Dawley rats were provided ad libitum access to the HFD from weaning to induce obesity, as previously described (29). Upon reaching a body weight of 675–700 g, rats were randomly allocated into two groups. ELS devices were implanted in one group (n = 8) whereas rats in the second group underwent the SO (n = 8). After postoperative recovery, animals were provided the HFD ad libitum, and body weight was assessed on a weekly basis. During POW 8, daily food intake and stool weights were measured on 7 consecutive days. Stool caloric content was determined by bomb calorimetry, and stool fat content determined by differential calorimetry with and without lipid extraction, as previously described (30). During POW 9 and 10, glucose metabolism was evaluated, as described below. Animals were sacrificed during POW 16, and necropsy was performed to confirm maintained positioning and patency of the ELS device. During the study period, one of eight implanted devices (12%) dislodged from the duodenal wall, migrated distally as detected by fluoroscopy, and was excreted into the stool; this animal was removed from the data analysis. In the other seven animals, the devices remained intact and stably positioned where implanted for the duration of the study.
To determine the effect of ELS implantation on acute body weight gain, ELS devices were implanted in lean, male OM rats. OM rats were used in this study because of their exaggerated and accelerated weight gain phenotype when provided ad libitum access to a HFD (31). To maintain a lean phenotype, OM rats were provided ad libitum access to regular chow from weaning. At 12 weeks of age, rats underwent either ELS implantation (n = 15) or SO (n = 15). In this experiment, ELS devices were implanted as previously described, except for the absence of the subcutaneous anchoring pledget. After postoperative recovery, animals were provided ad libitum access to the HFD. Body weight and fluoroscopic ELS retention was assessed on a weekly basis, and animals were sacrificed during POW 4 to confirm ELS positioning and patency. During the study period, the ELS device dislodged and migrated distally in a subgroup of ELS-treated OM rats, as determined by fluoroscopy. The observed migration, apparently facilitated by the lack of an anchoring pledget in this experiment, occurred in 7 of 15 animals (46%). These animals were followed prospectively for the duration of the study to determine the effect of ELS migration on acute body weight gain. The ELS device remained appropriately positioned and patent in 8 of 15 ELS-treated rats (54%) for the duration of the study.
Metabolic studies
Metabolic studies were performed after an 18-h overnight fast, unless otherwise indicated. Fasting blood glucose was measured in blood obtained by tail-stick, using a glucometer (LifeScan, Milpitas, CA). Glucose tolerance testing was performed after administration of glucose (1 g/kg body weight) by oral gavage or intraperitoneal (IP) injection, as indicated in the text and figure legends, and assessed using area under the curve (AUC) analysis of glucose excursion curves. Plasma insulin levels were determined by enzyme-linked immunosorbent assay (ELISA) (CrystalChem, Downers Grove, IL). Homeostasis Model Assessment (HOMA) values for insulin resistance (IR) and β-cell function (%B) were calculated as previously described (32). The acute insulin response was calculated as previously described (33). Insulin tolerance testing was performed after IP administration of insulin (Eli Lilly, Indianapolis, IN), 0.6 U/kg body weight, to ad libitum-fed rats and assessed using AUC analysis of glucose disappearance curves. Plasma total (acylated and deacylated) ghrelin levels were determined by radioimmunoassay (Phoenix Pharmaceuticals, Burlingame, CA).
Statistical methods
Data are presented as means ± s.e.m. Data were compared using Student's t-test, repeated measures ANOVA, and Mann-Whitney U-tests, where appropriate. The analysis was performed using SPSS 16.0 (SPSS, Chicago, IL).
Results
ELS induces weight loss in a rat model of diet-induced obesity
To determine the effect of the ELS on body weight, ELS devices were surgically implanted in the duodenum of obese Sprague-Dawley rats (1). A second group of animals underwent the same procedure omitting the ELS device (SO). Regardless of treatment (ELS or SO), all rodents exhibited an immediate postoperative loss of ∼15–20% of their preoperative body weight (2). By POW 2, ELS-treated animals weighed an average of 8% less than SO (583 ± 9.5 (ELS) vs. 616 ± 27 g (SO); P = 0.0001), a difference that increased to 20% (552 ± 12 (ELS) vs. 671 ± 27 g (SO); P = 0.001) by POW 7 (2). This difference persisted through the end of the experiment at POW 16 (P = 0.001; 2).
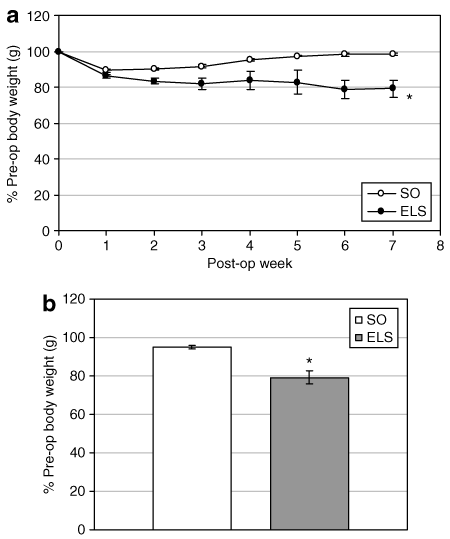
Endoluminal sleeve (ELS) implantation induces weight loss in a rat model of diet-induced obesity. ELS devices were implanted in obese, weight-matched Sprague-Dawley rats. Sham-operated (SO) animals underwent the same procedure, omitting the device. (a) Postoperative (post-op) body weight was followed on a weekly basis. (b) Body weights 16 weeks after surgery. *P < 0.05 vs. SO. Pre-op, preoperative.
To investigate the mechanism of the observed weight loss, we measured daily caloric intake and fecal caloric content during POW 8. ELS treatment reduced daily caloric intake by 28% (63.7 ± 11.4 (ELS) vs. 88.8 ± 6.2 kcal/d (SO); P = 0.001; 3, left). Stool quantity and bowel function was similar in ELS-treated and SO animals (data not shown). Daily stool caloric content was similar in the two groups (1.88 ± 0.1 (ELS) vs. 1.49 ± 0.3 kcal/d (SO); NS; 3, right). Ninety-eight percent of ingested calories were absorbed by the GI tract in both ELS-treated and SO animals, and animals in both groups absorbed >99% of ingested fat.
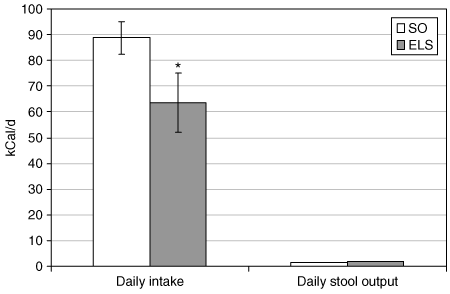
Endoluminal sleeve (ELS) implantation reduces calorie intake without causing malabsorption. Daily caloric intake (at left) and fecal caloric content (at right) were determined in ELS-treated and sham-operated (SO) rats, as described in Materials and Methods. *P < 0.05 vs. SO.
Ghrelin, the most potent peripherally active orexigenic hormone identified to date, has been implicated in regulating premeal hunger and meal initiation (6,34,35). Fasting ghrelin is unchanged or decreased after RYGB-induced weight loss, suggesting that this operation blocks or bypasses a compensatory hormonal (ghrelin) response that would tend to promote weight regain (6). Fasting ghrelin levels were unchanged after ELS-induced weight loss (4).
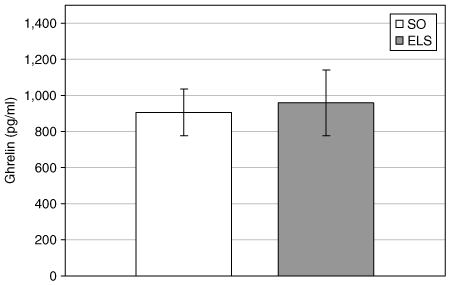
Ghrelin levels are unchanged after endoluminal sleeve (ELS)-induced weight loss. Plasma ghrelin (pg/ml) levels were measured in ELS-treated and sham-operated (SO) rats after an 18-h overnight fast.
ELS prevents acute weight gain in a rat model of diet-induced obesity
To determine whether the ELS can prevent acute diet-induced weight gain, ELS devices were implanted in 15 lean, OM rats. After postsurgical introduction of a HFD, a subgroup of ELS-treated rats (n = 8) failed to gain as much weight as the SO group, as demonstrated by a 12% lower body weight at POW 4 (P = 0.001 vs. SO; 5). Weekly fluoroscopy demonstrated that the ELS device was intact in all animals of this subgroup for the duration of the study. Necropsy confirmed maintenance of ELS position and patency. Interestingly, several ELS-treated rats (n = 7) exhibited initial weight loss followed by regain of weight to the same level as SO animals (5). Fluoroscopic evaluation during POW 2 revealed that the ELS had dislodged from the duodenal wall, migrated distally into the GI tract, and was eventually passed in the stool. Necropsy at the end of the study confirmed that the device had been extruded from in all animals in this subgroup. Presumably, the observed weight gain in these animals was due to ELS failure and early migration.
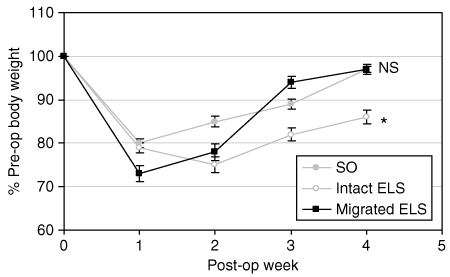
Endoluminal sleeve (ELS) implantation prevents acute diet-induced weight gain in lean rats. (a) ELS devices were surgically implanted in lean, Osborne-Mendel rats. Postoperative (post-op) body weight was followed on a weekly basis. In comparison to sham-operated (SO) rats (gray circles), ELS-treated animals with intact ELS devices gained 12% less weight (white circles). After an initial postoperative weight loss, ELS-treated animals in which the devices had dislodged and migrated (black squares) gained weight at the same rate as SO rats. *P < 0.05 vs. SO; NS, not significant vs. SO. Pre-op, preoperative.
ELS improves glucose homeostasis through peripheral insulin sensitization
ELS treatment substantially decreased fasting blood glucose (78 ± 8 (ELS) vs. 90 ± 2 mg/dl (SO); P = 0.001; 6) and insulin levels (0.57 ± 0.06 (ELS) vs. 1.11 ± 0.13 ng/ml (SO); P = 0.02; 6). Calculation of the HOMA-IR demonstrated a 55% improvement of peripheral IR by ELS treatment (2.67 ± 0.46 (ELS) vs. 5.94 ± 0.80 (SO) HOMA-IR units; P = 0.025; 6). Consistent with this observation, β-cell activity, as predicted by HOMA-%B, was decreased 36% after ELS treatment (236.8 ± 25 (ELS) vs. 367.2 ± 33 HOMA-%B units (SO); P = 0.028; 6). ELS-treated rats also exhibited improved oral glucose tolerance, as evidenced by a 40% decrease in the AUC analysis of glucose excursion (317.8 ± 25 (ELS) vs. 522 ± 37 (SO) AUC units; P = 0.008; 7, inset). Although the acute insulin response was unchanged after oral glucose administration in ELS-treated animals (7), glucose-stimulated insulin levels were significantly decreased (P = 0.006; 7. Notably, insulin tolerance did not differ between ad libitum-fed ELS and SO rats (7.
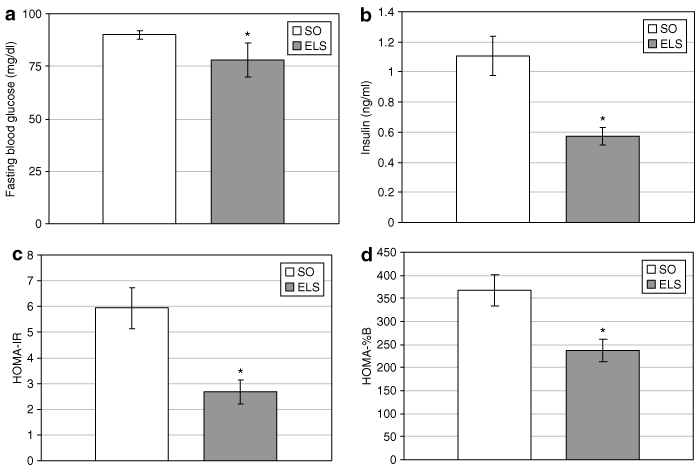
Endoluminal sleeve (ELS) implantation improves basal glucose homeostasis. (a) Fasting blood glucose and (b) insulin levels were measured in ELS-treated and sham-operated (SO) rats after an 18-h overnight fast. (c) Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated to evaluate the effect of the ELS on basal peripheral insulin resistance. (d) HOMA for β-cell function (HOMA-%B) was calculated to evaluate the ELS effect on pancreatic β-cell activity. *P < 0.05. vs. SO.
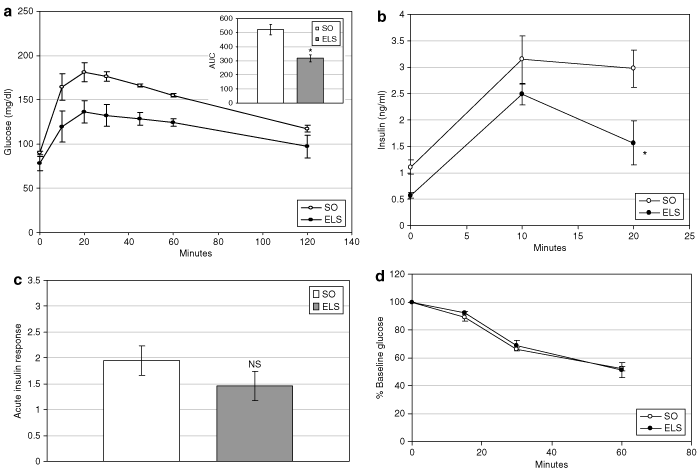
Endoluminal sleeve (ELS) treatment improves postabsorptive glucose homeostasis. (a) Glucose tolerance was evaluated in ELS-treated and sham-operated (SO) animals after oral administration of glucose (1 g/kg body weight). Glucose excursion curves (main graph) were analyzed by area under the curve analysis (inset). (b) Plasma insulin was measured after oral administration of glucose. (c) The acute insulin response (AIR) was calculated from postabsorptive circulating insulin levels after oral administration of glucose. (d) Insulin tolerance was evaluated using glucose disappearance curves after intraperitoneal administration of insulin (0.6 U/kg body weight) to ad libitum–fed ELS-treated and SO rats. *P < 0.05 vs. SO; NS, not significant vs. SO.
ELS improves glucose homeostasis independent of intraluminal nutrient passage
To determine whether the passage of endoluminal nutrients is required for the effect of ELS on glucose homeostasis, ELS-treated and SO animals underwent IP glucose tolerance testing. IP administration of glucose bypasses GI absorption and, presumably, the secretion of incretins and other neuroendocrine signals induced by luminal nutrients. ELS-treated animals demonstrated dramatically improved IP glucose tolerance, with an overall 43% decrease in the AUC of glucose excursion (368 ± 62 (ELS) vs. 643 ± 111 mg/dl AUC units (SO); P = 0.027; 8, inset).
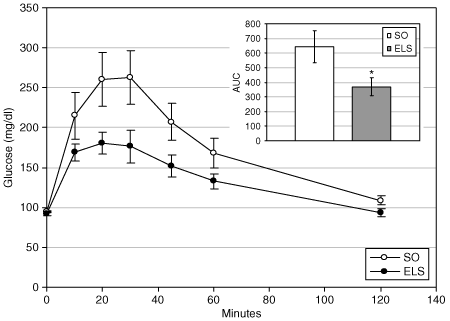
Endoluminal sleeve (ELS) implantation improves glucose homeostasis independent of postprandial passage of endoluminal nutrients. Glucose tolerance was evaluated in ELS-treated and sham-operated (SO) animals after intraperitoneal administration of glucose (1 g/kg body weight). Glucose excursion curves (main graph) were analyzed by area under the curve analysis (inset). *P < 0.05. vs. SO.
Discussion
Implantation of an ELS device in the duodenum and proximal jejunum mimics many of the effects of RYGB. In Sprague-Dawley rats with diet-induced obesity, ELS implantation causes modest weight loss and substantially improves glucose homeostasis. Presence of this device in the proximal intestine also prevents the development of diet-induced obesity in otherwise susceptible animals. Despite creating a barrier between ingested nutrients and the duodenal mucosa, the ELS does not cause significant malabsorption. Rather, the observed weight loss results primarily from reduced calorie intake. In addition, ELS implantation appears to improve glucose homeostasis by multiple mechanisms. Both fasting blood glucose and insulin levels decrease after this procedure, which is reflected in a 55% decrease in HOMA-IR and is consistent with significant improvement of basal peripheral IR. Because hepatic glucose output is the major determinant of the fasting glucose level, these data suggest that ELS implantation leads to decreased hepatic glucose output, likely as a result of improved insulin signaling in hepatocytes. Improved oral glucose tolerance with a concurrent decrease in glucose-stimulated insulin levels further underscores an overall improvement in peripheral insulin sensitivity. The similar effect of ELS implantation on IP glucose tolerance suggests a global improvement in glucose homeostasis that is independent of postprandial signals generated by endoluminal nutrient passage and provides additional evidence of overall improvement of insulin sensitivity. The improvement in glucose tolerance suggests that ELS implantation also enhances glucose disposal. We observed no effect of the device on insulin tolerance, which is largely a reflection of insulin-stimulated glucose disposal in muscle and adipose tissue. However, insulin tolerance testing is a relatively insensitive measure of peripheral insulin sensitivity, so the data are consistent with a modest effect of the device on glucose disposal in these tissues.
Although the ELS induces only about two-thirds as much weight loss as RYGB in the same strain of rats, the effect on glucose homeostasis is similar to that observed after RYGB (9,10,13,14,20,22). This observation suggests that manipulations of the small bowel leading to duodenal exclusion and enhanced delivery of partially digested nutrients to the jejunum are primary mechanisms by which RYGB alters glucose metabolism. In contrast, the more limited effect of ELS on body weight suggests that other components of RYGB (e.g., gastric manipulation and/or partial vagotomy) likely make a significant contribution to the weight loss induced by this procedure. The differential ability of ELS implantation to mimic some of the effects of RYGB on body weight and glucose homeostasis underscores the complexity of GI regulation of these metabolic functions. It suggests that different components of RYGB contribute to the myriad effects of this operation and that multiple regions of the GI tract participate in the normal regulation of metabolic function. By examining selected components of this complex operation in isolation, we have attempted to dissect the contributions of these manipulations to the regulation of body weight, food intake, and glucose homeostasis. The preferential effect of ELS on glucose homeostasis underscores the basic validity of this approach.
Of course, ELS is not merely a component of RYGB, and there are fundamental differences in the manner in which these two manipulations may affect GI anatomy and function. Although the ELS device prevents interaction between the nutrient stream and the mucosa of the proximal intestine, these regions of the gut are not completely bypassed as they are after RYGB. With ELS, nutrients pass through the lumen of the device and retain the opportunity to affect local mechanoreceptors and motor function. In addition, the presence of an endoluminal device in the duodenum (including the fixed anchor at the proximal end) may affect gastric emptying and gastric motility, two biological processes known to acutely regulate satiety (36,37). During fluoroscopic assessment of ELS position and patency, we have observed that gastric emptying appears somewhat slowed after implantation of this device (data not shown). Thus, a possible contribution of gastric motility effects to the observed weight loss, decreased food intake and improved glucose homeostasis cannot be excluded. Similarly, it is not known whether increased energy expenditure contributes to ELS-induced weight loss. We have observed that resting energy expenditure is increased after RYGB in rats, and pair-feeding studies suggest that this effect is a significant contributor to RYGB-induced weight loss (N. Stylopoulous and L.M. Kaplan, unpublished observations). Given the similar effects of ELS and RYGB on metabolic function, ELS implantation may also regulate energy expenditure.
Within the lumen of the proximal intestine, duodenal, pancreatic, and biliary secretions further digest nutrients previously emulsified in the stomach. The macromolecular components of this chyme are then capable of interacting with chemoreceptors and mechanoreceptors in the mucosa and bowel wall to generate neurohumoral signals of nutrient intake, composition, and availability (6,8,11,15,16,38,39). Candidate pathways mediating this communication include: (i) peptide hormones secreted by mucosal enteroendocrine cells; (ii) nonpeptide signals (e.g., lipids and carbohydrates) transported across the mucosal epithelium and released into the portal and lymphatic circulation; (iii) enteric neuronal circuits that communicate with other regions of the gut directly or via reflex arcs; and (iv) autonomic nervous pathways that communicate with the central nervous system. End-organ targets including other regions of the GI tract, the central nervous system (e.g., hypothalamus, brainstem, and reward centers), liver and pancreas normally coordinate a biological response to these gut-derived signals through the appropriate, compensatory regulation of energy intake, storage, and utilization (8,11,15,38,39). Implantation of the ELS device appears to alter the luminal microenvironment of the duodenum and proximal jejunum by segregating ingested nutrients both from luminal secretions and from the mucosa of the proximal intestine. Minimally digested nutrients and GI secretions are then delivered to the jejunum. As a result, nutrient binding to chemoreceptors of the proximal intestine is prevented, ingested nutrients passed beyond the region of the ELS can interact with regulatory chemoreceptors of the jejunum, and GI secretions untitrated by their usual substrates are exposed to the mucosa of the proximal small bowel. These alterations of the luminal environment likely modulate efferent gut signals to end-organ targets, thereby contributing to the observed effects of the device on feeding behavior, body weight, and glucose homeostasis.
The specific gut pathways activated in response to ELS-treatment are unknown. We and others have demonstrated that bypass surgery affects gut hormone secretion, particularly hormones implicated in the regulation of feeding behavior, satiety, glucose homeostasis, and the so-called “ileal brake” mechanism (9,10,17,19,20). Neutralizing antibodies to peptide YY reverse the inhibitory effects of jejuno-ileal bypass on food intake in thin rodents, suggesting a causal role for gut hormones in at least some of the effects of GI weight loss surgery (10). In addition, ghrelin, a potent peripherally active orexigenic hormone, has been implicated in a compensatory hormonal response to weight loss induced by food restriction or restrictive bariatric procedures (6). As with RYGB (6), ELS treatment does not lead to increased ghrelin levels. This finding suggests that weight loss induced by intestinal exclusion and/or accelerated jejunal delivery of ingested nutrients does not stimulate a compensatory stimulus to ghrelin secretion. To date, the roles of specific gut hormones in the physiological effects of RYGB or ELS have not been established. Because of the limited nature of ELS implantation, exploration of the relative effects of ELS and RYGB on hormonal and neuronal signaling from the gut will reveal specific contributions of the proximal small bowel to the regulation of appetite, body weight, and metabolic function.
In summary, exclusion of the proximal intestine from ingested nutrients and accelerated nutrient delivery to the jejunum induced by ELS mimic many of the effects of RYGB on body weight, food intake, and glucose homeostasis. The dramatic effects of ELS on glucose homeostasis suggest an important role for the duodenum and jejunum in the regulation of this metabolic function. The more limited effect of ELS implantation on food intake and body weight suggests that other components of RYGB, particularly manipulation of the stomach, likely provide a substantial contribution to GI regulation of body weight and ingestive behavior. The degree to which all of these regulatory effects are mediated through the pancreas, liver, or central nervous system remains unknown but is an exciting and interesting area for further study. In the long term, understanding these mechanisms will likely lead to new targets and approaches for the treatment of obesity and diabetes. More immediately, the efficacy of the ELS device in this rat model brightens the prospect that its endoscopically placed human analog will contribute to the treatment of these important disorders.
Acknowledgment
We thank Andy Levine for invaluable help in the development of the rat ELS model and Philip Davis and Lynn Wartschaw for excellent technical assistance. We also thank Morris White and Daniel Podolsky for their helpful review of the manuscript. These studies were supported by National Institutes of Health grants DK057478, DK046200, DK043351 (to L.M.K.); training awards DK007191 (to V.A.) and HD052961 (to N.S.); and a research grant from GI Dynamics (Watertown, MA).
Disclosure
The authors declared no conflict of interest.





