Hematopoiesis leading to a diversity of dendritic antigen-presenting cell types
Abstract
Hematopoietic stem cells (HSCs) undergo expansion and differentiation, giving rise to all terminally differentiated blood cells throughout life. HSCs are found in distinct anatomical sites during development, and in adults, hematopoiesis occurs predominantly on the luminal side of the bone cavity in bone marrow. Millions of newly formed blood cells are generated per second to accommodate the short half-life of hematopoietic cells. For this to happen, HSCs must sustain their self-renewal capacity as well as their capability to commit and differentiate toward multiple cell lineages. Development of the hematopoietic system is finely regulated as the animal ages, so that it does not become exhausted or misdirected. This review covers aspects of hematopoietic development from the embryonic period through adult life in relation to development of dendritic cells. It also considers a role for HSCs in extramedullary sites and their possible role in myelopoiesis, with formation of tissue-specific antigen-presenting cells.
Early hematopoiesis
The hematopoietic and cardiovascular organ systems are the first to emerge during embryogenesis because the embryo requires a functional heart, vascular system and blood for survival and growth in the early postimplantation period. Blood cell development in the embryo depends on gastrulation and mesoderm formation. Mesodermal cells contribute to the heart, the aorta in the embryo proper, formation of hematopoietic cells in yolk sac and vascular interconnection in the embryo.1 Embryonic hematopoiesis in mice begins after gastrulation at embryonic day 6 (E6), when mesodermal cells commit to becoming hematopoietic cells.2 The sequential sites for hematopoiesis during embryonic development then include yolk sac, the aorta-gonad-mesonephros region, placenta, fetal liver, spleen and finally bone marrow (BM).3
Yolk sac, a bilayer structure of mesoderm and endoderm-derived cell layers, is the initial site for blood cell formation. The first hematopoietic precursors derive from mesoderm that gives rise to the hemangioblast, a bipotential precursor for blood and endothelium that enters the yolk sac to initiate primitive hematopoiesis across E7–E7.5. Blood cells produced at this time are primitive hematopoietic cells consisting mainly of large nucleated red blood cells.4 The presence of definitive hematopoietic progenitors then marks the start of a second wave of blood cell production on day E8.5.5 However, the microenvironment in the yolk sac does not support the differentiation of definitive hematopoietic progenitors. These therefore exit the yolk sac via the vitelline veins. Following transient appearance in the aorta-gonad-mesonephros region, hematopoietic stem cells (HSCs) are seen to colonize fetal liver passing through umbilical cord vessels of the placenta where they contribute to robust expansion and definitive hematopoiesis.
HSCs then appear in the fetal liver at E11.5 where they undergo proliferation and differentiation, with maximum expansion of HSCs across E15.5–E16.5, followed by a decline in cell numbers.6 Erythroid colony-forming unit and proerythroblasts prevail in early fetal liver, whereas myeloid and lymphoid progenitors accumulate at later stages. Modification of the liver microenvironment during fetal development occurs in preparation for HSC expansion and lineage differentiation,7 consistent with phenotypic changes in HSCs across different developmental stages.8
Bone development begins at E12.5 as mesenchymal condensations are observed. Briefly, mesenchymal progenitor cells first give rise to chondrocytes that create a cartilaginous framework for bone. Chondrocytes are later replaced by osteoblastic cells that build-up calcified bone through endochondral ossification to form the BM cavity.9 Vascular invasion into the bone then facilitates the circulation and seeding of HSCs. Clonogenic hematopoietic activity in BM can be found at ∼E17.5 and persists throughout postnatal life.10
Hematopoiesis in fetal spleen occurs from E13 until the first week of the postnatal period. Hematopoietic progenitors from fetal liver migrate to fetal spleen at E13–E14 and undergo proliferation and differentiation to give rise to mature blood cells. In contrast to fetal liver, fetal spleen does not have significant hematopoietic activity.11 Hematopoiesis in fetal liver depends on the expansion of progenitors, whereas hematopoiesis in fetal spleen relies on the immediate hematopoietic precursors derived from fetal liver that home directly to spleen. Special microenvironments or niches in fetal spleen appear to restrict or favor the development of particular blood cell lineages.12
Properties of HSCs
In adult humans, BM is the major hematopoietic organ producing >109 mature blood cells per day. Owing to the short half-life of mature cells, continuous production of cells depends on the ability of HSCs to self-renew and differentiate into all blood cell types.13 In the steady-state, specialized niches in BM provide an optimal microenvironment for maintenance of HSCs by regulation of their self-renewal and differentiative capacity, and conservation of their multipotency throughout cell division.14
Self-renewal is driven intrinsically by gene expression and modulated through HSC interaction with extrinsic cues in the environment. HSCs niches are crucial regulators that determine whether symmetric or asymmetric cell division occurs.15 During asymmetric division, HSCs forms a daughter cell and another HSC. Asymmetric division has been described for several tissue-specific stem cells, and several cell fate regulators, including Notch, HoxB4 and Sonic hedgehog, have been shown to have a role in the self-renewal process.16,17,18,19 Wnt signaling is important for self-renewal because induced expression of a frizzled ligand-binding domain, an inhibitor of the Wnt signaling pathway, leads to inhibition of HSC growth in vitro.20
Lineage commitment is the process by which HSCs become restricted in their differentiation and develop into a fully committed progenitor of a single blood cell Lin.21 All murine adult hematopoietic cells derive from the multipotential HSCs. This single cell type can restore the mature peripheral population of hematopoietic or blood cells in lethally irradiated recipient mice by BM transplantation. Murine HSCs were first characterized by their lack of lineage (Lin)-specific antigens, their expression of Sca-1 (stem cell-associated antigen-1) and c-Kit, and low expression of Thy-1.22 This subset was later classified as the Lin−Sca-1+c-Kit+ compartment (LSK). Within the HSC hierarchy, LSK represents a diverse group which can be classified as long-term self-renewing HSCs, short-term, or transiently, self-renewing HSCs and multipotential progenitors (MPPs).23,24
Long-term self-renewing HSCs have capacity for lifelong reconstitution of the hematopoietic system. In mice, they are highly enriched in the LSK-Thy1.1lo cell fraction that can be further purified as Flk-2–cells.25 The CD150, CD244 and CD48 signaling lymphocyte activation molecules have recently been found to discriminate HSC sub-populations such that LSKCD150+CD48−CD244−CD34− cells are defined as HSCs, LSKCD150−CD48−CD244+CD34− as MPPs and LSKCD150−CD48+CD244+ as more committed progenitors.26,27,28
A hierarchy of hematopoietic cell differentiation continues to be established using both cell surface marker analysis and in vitro colony-forming and functional assays. As multipotent progenitors, HSCs are present at the top of the hierarchy and possess high capacity for self-renewal and differentiation. Downstream on the hierarchy, HSCs lose their self-renewing ability and develop into MPPs that give rise to several lineages of blood cells.25 MPPs represent a heterogeneous population of stem/progenitor cells and remain under investigation in terms of their subsets and their differentiative potential.26,29,30,31
In terms of differentiation to give a diversity of antigen-presenting cells (APCs), the myeloid pathway is of particular interest. HSCs give rise to common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs) through a MPP.31,32 CMPs then differentiate to give rise to granulocyte-macrophage progenitors (GMPs) and megakaryocyte-erythroid progenitors (MEPs). GMPs are the progenitors of granulocytic and macrophage/monocytic cells, including neutrophils, eosinophils, basophils and monocytes. MEPs give rise to red blood cells and megakaryocytes. CLPs give rise to lymphoid and natural killer cell progenitors, followed by generation of mature T cells, B cells and natural killer cells. The Flt3+ subset of CMPs and CLPs gives rise to dendritic cells (DCs).33 Two further progenitors have recently been defined in relation to DC development (Figure 1). The earliest is the macrophage/dendritic progenitor (MDP), a myeloid dendritic progenitor that overlaps with the CMP and CLP, but not the GMP population.34,35 A downstream common dendritic progenitor (CDP) that responds to Flt3L has been identified as a specific progenitor of conventional (c)DCs and plasmacytoid (p)DCs.36,37 The relationship between CDPs and the previously defined CLP, CMP and MDP populations is still under investigation.
HSCs are distributed and not restricted to BM
BM primarily provides niches for HSC seeding and contributes the microenvironment, which supports self-renewal and differentiation. In the adult, HSC migration from BM into the bloodstream in the steady-state is important for maintaining homeostasis. Collection of stem cells for transplantation in a clinical setting takes advantage of this natural migratory phenomenon by enforcing the release, or by ‘mobilizing’ HSCs from BM, by infusion of chemotherapeutic drugs or cytokines such as granulocyte-colony stimulating factor (G-CSF). HSCs can then be collected from blood for stem cell transplantation. Adoptive transfer then leads to reconstitution of all hematopoietic cells after HSCs home to and infiltrate BM. One enigma is that HSCs introduced into the peripheral blood circulation can traffic back into lymphatic circulation and into BM, and therefore find their niche in this tissue (Figure 2).38 It is also clear that following intravenous infusion, HSCs localize in spleen and other extramedullary tissue niches (Figure 2).38
Several mechanisms are known to regulate HSC homing into niches. CXCL12 or stroma-derived factor-1 (SDF-1) and its receptor CXCR4 are master regulators of HSC migration through blood during embryonic development, consistent with reduction of myeloid progenitors in CXCR4−/− and CXCL12−/− mice.39,40 HSCs also use very late antigen-4 (VLA-4: integrin-α4β1) to localize themselves in contact with blood vessels in BM by binding to vascular cell adhesion molecule-1 (VCAM-1) expressed on BM stromal or endothelial cells (Figure 2).41 Migration of HSCs into extramedullary hematopoietic organs, such as spleen and liver, is also mediated by interaction of VLA-4 with VCAM-1, ahead of returning to the appropriate niche that secretes SDF-1 (CXCL12; Figure 2).41
Studies now show that HSCs can enter and circulate through the lymphatic system. The egress of HSCs from BM into extramedullary tissues depends on sphingosine-1-phosphate receptor (S1P1; Figure 2). HSCs also use S1P1 to migrate across lymphatic vessels, and therefore restore specialized myeloid cells in peripheral tissues.42 HSCs express toll-like receptors, and their co-receptors MD-2 and CD14, required for recognition of pathogen-associated molecular patterns such as bacterial lipopolysaccharide.43 Interaction of toll-like receptors with lipopolysaccharide signals myeloid differentiation in migratory HSCs localized in peripheral tissues. Lipopolysaccharide was found to amplify the differentiation of HSCs in local tissues, and to reduce the expression of S1P1 on HSCs, therefore leading to retention of HSCs within tissue sites.42
During inflammatory responses, the phagocytic activity of sinusoidal-lining macrophages is dramatically increased to remove invading pathogens. During inflammation, HSCs protect themselves from macrophage uptake by upregulating CD47, an immunoglobulin-like protein that interacts with integrins and thrombospondins to protect HSCs from reactive phagocytosis.44,45,46 CD47 interacts with its receptor, signal regulatory protein-α on macrophages and DCs, to prevent phagocytosis, therefore ensuring survival of HSCs during inflammation.44,45
Niches for hematopoiesis are distributed
Several niches have been described as sites for HSC maintenance including endosteum of bone and vascular niches in BM and spleen.47,48 In adult mice, the majority of HSCs reside within osteoblastic and vascular niches in BM where most hematopoietic activity occurs, whereas smaller numbers reside in vascular niches in other tissues.
BM niches contain specialized cells that provide membrane-bound and secreted growth factors to support HSC growth.49,50 Many studies have focused on the role of endosteal cells lining the inner surface of bone at the interface with BM in HSC maintenance.14,51,52,53,54 Endosteal cells differentiate into osteoblasts, which support cell-to-cell contact with HSCs, mediated through multiple adhesive interactions including homotypic interactions involving N-cadherin (Figure 2). Osteopontin (OPN) induces HSC retention and quiescence in the BM by binding to several integrins or to CD44, resulting in downregulation of Jagged1 expression on stromal cells and Notch1 expression on HSCs.55 Endosteal cells produce growth factors such as stem cell factor (SCF), which support HSC function and survival (Figure 2).56 Angiopoietin (Ang-1) and thrombopoietin (TPO) promote quiescence of HSCs, whereas stroma-derived factor-1 (CXCL12) regulates migration of HSCs within the BM.56,57,58 The endosteum also comprises the bone-resorbing osteoclasts, and a balance between osteoblastic and osteoclastic activities in the BM is important for development of HSCs.59
The highly vascularized nature of the endosteum is also consistent with endothelial cells having a critical role in regulation of HSC development in BM.50 Vascular niches are considered alternate sites for HSC maintenance. During embyogenesis, HSCs arise from progenitors located in perivascular sites, and HSCs in extramedullary tissues, such as liver and spleen, are located in sinusoidal or vascular areas in the absence of osteoblastic cells.
Lifelong maintenance of the HSC pool depends on protecting HSCs from premature exhaustion under conditions of stress. Quiescence in terms of cell cycle is a common property of niche-associated HSCs. Although HSCs divide infrequently, the entire HSC pool turns over every few weeks. In mice, dormant HSCs divide every 145 days or five times per lifetime.28 These multilineage long-term self-renewing cells create a silent reservoir of HSCs during homeostasis. Upon stimulation with granulocyte-colony stimulating factor (G-CSF), dormant HSCs enter cell cycle and then switch back to dormancy. HSCs can reversibly undergo self-renewal under conditions of stress.28 Quiescence is maintained by signaling within the niche, which induces the Tie-2 tyrosine kinase receptor on HSCs, which interacts with Ang-1 on osteoblasts,57 as well as the TPO/Mpl and Wnt/β-catenin signaling interactions, also important for HSC quiescence (Figure 2).60
Vascular conduits are the major highways by which hematopoietic cells and hematopoietic progenitors traffic to liver and spleen in adults.61,62,63 It is generally accepted that a small number of hematopoietic progenitors circulate through peripheral sites and then home back to the BM.64,65 Consistent with HSC migration, several studies have identified hematopoietic progenitors in heart.66,67,68 Indeed, c-Kit+ cells isolated in the heart after myocardial infarction have been reported to have a BM origin69,70,71 Hematopoietic progenitors from BM can give rise to microglia following transplantation into brain.72,73 After brain injury, it is possible that HSCs or other hematopoietic progenitors from BM may enter the brain and differentiate to become APCs. There are also reports of the presence of hematopoietic progenitors in peripheral tissue sites, such as kidney,74 skin75 and intestinal tract.76 Such progenitors can give rise to hematopoietic cells specific to each tissue site. Similarly, Langerhans cells in skin derive from self-renewing hematopoietic progenitors, which colonize the epidermis during embryonic development.77
Hematopoiesis leading to a distributed pattern of dendritic and myeloid cells in multiple tissues
Hematopoiesis leading to APC formation now appears to reflect a complex set of developmental pathways originating from progenitors in BM, leading to a diverse range of cells in different states of development within tissue sites, such as BM, liver, spleen and other lymphoid and non-lymphoid organs.78 DCs emerge from BM progenitors, but the exact progenitors that give rise to DCs and how they relate to known progenitors of lymphoid and myeloid cells in vivo are still under investigation (Figure 1). Recent developmental studies show that DC subsets and monocytes/macrophages are generated along a myeloid pathway.34,79 Data from parabiotic mice also support the hypothesis that lymphoid tissue DCs and monocytes share a common BM-derived MDP, identified as a Lin−Sca-1−c-KithiCD115+CX3CR1+Flt3+ subset also expressing CD34 and CD16/32.35,80 A more committed, distinct BM progenitor called the CDP has been identified as a Lin−Sca-1+c-KitloCD115+Flt3+ subset, which gives rise cDCs and pDCs (Figure 1).35,37 Data such as these support the concept that development of monocytes and macrophages is separated from that of DCs before these cell types migrate into peripheral lymphoid tissues.
Multiple DC subsets have now been identified in tissues around the body. Their immune capacity varies in terms of ability to take up antigen and presence of inflammatory stimuli. In thymus, DCs derive from an intrathymic lymphoid progenitor and represent a specialized subset important in creating a self-tolerant T-cell repertoire.78 In murine spleen, several subsets are recognized, including cDCs and pDCs (Figure 1), each phenotypically and functionally distinct. cDCs are small, non-granular cells comprising two subsets of CD8α+ and CD8α− cDCs.81 Counterpart cells can also be found in humans, free of infection and inflammation.82 The majority of DCs in spleen are CD8α− cDCs, with only ∼20% cDCs of CD8α+ phenotype.83 The CD8α+ cDCs are localized in the T-cell-rich areas or periarteriolar lymphatic sheath of spleen, whereas the CD8α− cDCs are found in the marginal zone. CD8α− cDCs can migrate into the T-cell zone upon activation with bacterial lipopolysaccharides.84,85 pDCs have strong capacity to secrete type-1 interferon-α upon viral or bacterial infection, and express CD11c, B220, CD36, CD4, CD68 and major histocompatibility complex-II (MHC-II) on their cell surface. The function of pDCs is linked to their expression of toll-like receptor-7 and -9, which detect viral nucleic acid in early endosomes.86
Monocytes can also be induced to differentiate in vitro under the influence of inflammatory cytokines, such as granulocyte-macrophage–colony stimulating factor (GM–CSF) and tumor necrosis factor-α (TNF-α) to give rise to monocyte-derived DCs. These have been commonly studied as a model DC type and used in intervention or immunotherapy against malignancies. However, DCs produced by this protocol represent inflammatory DCs and are distinct from steady-state cDCs. It is not yet clear whether monocyte-derived DCs correspond to any DC subsets in steady-state lymphoid organs.87 Other myeloid subsets described in spleen include tumor necrosis factor/inductible nitric oxide synthase-producing DCs88 and inflammatory monocytes.89
In general, DCs exist in peripheral tissues in an immature state and possess high capability for capturing and processing antigens from the local environment. After endocytosis of foreign antigens, these immature DCs in peripheral tissues migrate to lymphoid organs where they undergo antigen processing and cell maturation, whereby DCs upregulate co-stimulatory molecules, such as CD40, CD69, CD80 and CD86. Mature DCs possess high capacity for presentation of antigen to naïve T lymphocytes90,91 and have high capability for cross-presentation of endocytosed antigen for subsequent CD8+ T-cell activation.92 Presentation of antigenic peptides by DCs expressing appropriate co-stimulatory molecules results in activation of T cells for immunogenic responses, whereas antigen presentation without co-stimulation leads to T-cell activation for tolerogenic responses.93,94 A wide range of DC subsets are therefore formed that act as extremely important central controllers of tolerance and immunity.
Extramedullary sites for myelopoiesis of DCs
DCs can also develop within peripheral tissue sites, and this has clearly been shown for Langerhans cells.79 Cells of host origin were found several months after BM transplantation,77,79 indicating that skin Langerhans cells are derived from tissue-restricted myeloid progenitors. Thus, DC development from progenitors within peripheral tissue sites might be possible, given the distribution of HSCs within tissues and the potential for hematopoietic niches in multiple tissue sites. However, the extent to which this happens is not yet fully understood. In particular, spleen appears to support extramedullary hematopoiesis for the development of tissue-specific APCs. In humans and mice, steady-state spleen contains cDCs and pDCs, which are maintained by the replenishment of pre-DCs, or precursors of cDCs and pDCs, which are derived from progenitors in BM. We now present evidence that distinct dendritic-like cells appear to arise in spleen from endogenous self-renewing progenitors (Figure 1).78,95
In vitro studies from this lab showed that continuous long-term stromal cultures (LTCs) of spleen support production of distinct dendritic-like cells, which are large cells expressing CD11c, CD11b and MHC-I, but not MHC-II.96 As these cells resemble immature myeloid DCs, they were named LTC-DC.96,97,98,99,100 The phenotype of cells produced has remained stable over years of culture, with characteristic expression of CD11c, CD11b, CD80, CD86, MHC-I, CD205 but not MHC-II CD8α or B220.96,100 Gene expression studies have shown that LTC-DCs express genes encoding several cell surface molecules expressed by DCs.101,102 LTCs are distinct from other in vitro cultures for DC production in that stromal cells support DC production without addition of exogenous inflammatory cytokines, reflecting the capacity of the spleen stromal cells to support DC hematopoiesis.103 The continual production of immature myeloid DCs in LTCs led to the hypothesis that hematopoietic stem or progenitor cells are maintained within LTCs.104,105 Ongoing investigations have now supported that hypothesis.
Recently, it was shown that LTC-DCs have an in vivo counterpart cell, distinguishable from other DC subtypes on the basis of marker expression.104,106 These cells are distinct from cDCs by their higher endocytic activity, absence of MHC-II expression and their RelB-independent development.107,108 They are also phenotypically and functionally distinct from monocytes.106,109 The in vivo counterpart of LTC-DCs, termed ‘L-DCs’, has now been identified in both adult and neonatal spleen, and these cells possess highly endocytic activity, and can cross-present antigens to CD8+ T cells, with very limited ability to stimulate CD4+ T-cell responses.106,109 The inability of L-DCs to induce a helper T-cell response is likely due to the absence or low MHC-II expression on these cells.100 LTC-DCs and L-DCs are readily distinguished from monocytes, which cannot cross-present antigen to CD8+ T cells.105,106
Hematopoietic progenitors in LTCs and their counterparts in vivo have been investigated to substantiate the development of L-DCs as a distinct APC in the context of the spleen microenvironment. Based on the finding that a population of small cells is maintained in LTCs, which reflect Lin−c-kit+Sca-1+ progenitors,109 the question was raised as to whether HSCs or MPPs could serve the role as L-DC progenitors in spleen.95 HSCs derived from spleen95,104 and BM105 have now been shown to act as progenitors of L-DCs in LTCs. When these same subsets of HSCs from spleen or BM were adoptively transferred into lethally irradiated mice, there was a bias favoring production of L-DCs over other DC subsets in spleen.104,105 These data raise the possibility that APCs can develop in spleen from endogenous self-renewing HSCs and have tissue-specific function, perhaps related to blood-borne antigens. Further studies are underway to gain complete understanding of the development of this putative novel DC subset in the splenic context.
Conclusion
Indeed, there is much to be learned about tissue niches for HSCs and about tissue-specific hematopoiesis for production of APCs before their importance in tissue-specific inflammation and immunity can be interpreted and considered in terms of immunotherapy. Indeed, a role for self-renewing tissue-specific progenitors in production of tissue-specific APCs during the steady-state and inflammation can be justified in terms of tissue-specific immunity, reflecting some level of diversification and compartmentalization of the immune response.
ACKNOWLEDGEMENTS
This work was supported by project grant 585443 from the National Health and Medical Research Council of Australia to HO. SP was supported by a postgraduate scholarship from the Royal Thai Government.
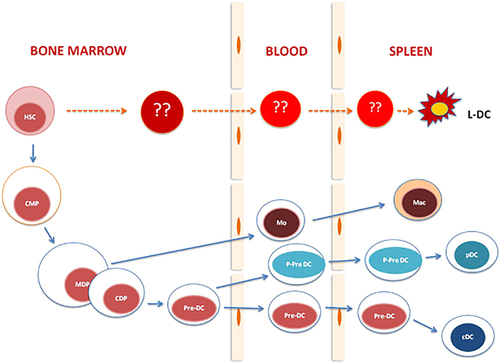
Myelopoiesis leading to DC development. The BM maintains HSCs. Myeloid cells are produced from a downstream CMP. These further differentiate to give rise to MDPs, which are precursors of monocytes, macrophages and DCs. Recently a CDP was shown to give restricted development of splenic DCs via a precursor detectable in both blood and spleen. A novel APC type has been characterized in murine spleen. These dendritic-like cells have been named ‘L-DCs’ and appear to derive by direct differentiation from HSCs in BM and spleen.
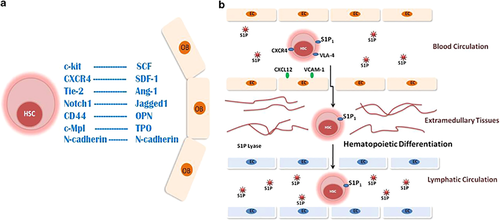
The BM microenvironment provides signals, which control HSC self-renewal, migration and quiescence. (a) HSCs migrate within the niche by interaction of SDF-1 (CXCL12) produced by osteoblasts (OB) with CXCR4 on HSCs. HSCs are maintained on the endosteal surface of bone through cell–cell interactions, including homotypic interactions involving N-cadherin, and OPN interaction with CD44 or integrins. OPN supports retention of HSCs in the niche by downregulation of Jagged-1 expression, which interacts with Notch-1 on HSCs. These interactions allow Tie-2 on HSCs to interact with Ang-1, and c-Kit to interact with SCF on the surface of osteoblasts. Ang-1 and TPO interactions with their receptors on HSC support HSC quiescence. (b) HSCs migrate from blood into extramedullary tissues by a sphingosine-1-phosphate (S1P) gradient. The interaction of S1P with its receptor (S1P1), in combination with other molecules such as CXCR4 and VLA-4 that interact with ligands on endothelial cells (EC) (CXCL12 and VCAM-1), facilitates HSC migration into extramedullary tissues. The S1P level in tissues is lower due to S1P lyase activity. This results in localization of HSCs within tissue niches for differentiation. HSCs can also enter lymphatic tissues including BM through the guidance of S1P.





