Simultaneous Orthotopic Liver and Kidney Transplant with Repair of Abdominal Aortic Aneurysm: Operative Timing
Abstract
In the case of coexisting abdominal aortic aneurysm (AAA) and liver/renal failure, the controversial issue is the timing of the AAA repair and the transplantation of the affected organs. The question is whether to repair the AAA first and perform the double transplantation at a later time, or to perform all three procedures in the same operative session. This patient was affected by hepatic/renal failure and had also developed AAA. We describe the operative strategies utilized to perform the cadaver donor and recipient operations in this setting. In our patient, a combined liver/kidney transplantation with simultaneous aneurysm repair using arterial allografts was successfully performed. In a patient affected by end-stage liver, kidney, and aneurysmatic disease, a simultaneous liver/kidney transplant and AAA repair may represent the safest and most efficient treatment solution.
Introduction
Liver and kidney transplantation are the therapies of choice for end-stage hepatic/renal failure (1–4). In the case of coexisting abdominal aortic aneurysm (AAA) and renal failure, the controversial issue is the timing of the AAA repair in the patients awaiting transplantation (5–7). The two possible options are either to perform first the renal transplant and then the abdominal aortic aneurism repair or vice-versa (8–11). Both options are associated with significant potential perioperative complications (1, 7). There have been reports of simultaneous repair of coexisting aneurysmal disease and kidney transplant (KT) (1, 12). The patient whose case is reported here was affected by hepatic and renal failure and had also developed aneurysmatic disease of the hepatic artery, abdominal aorta, and common iliac arteries. In our patient, a combined orthotopic liver and kidney transplantation with simultaneous multiple aneurysms repair using arterial allografts was successfully performed.
Case Report
A 41-year-old Hispanic female with end-stage renal disease secondary to glomerulonephritis (on hemodialysis for 3 years) and end-stage liver disease secondary to chronic hepatitis B was listed for simultaneous liver/kidney transplantation. At the time of the initial visit, there was no visible aortic dilatation on either ultrasound or CT. At 1-year follow up, a CT scan of the abdomen showed an infrarenal AAA measuring 3.7 cm in maximal diameter. The aneurysm continued into the left common iliac artery, where it measured 2.6 cm in diameter. Moreover, an aneurysm of the proper hepatic artery extending to the bifurcation was diagnosed. Six months later, in May 2002, a repeat CT of the abdomen showed an increase in the size of the AAA to 4.7 cm and of the left common iliac artery aneurism to 3 cm, while the right common iliac was now aneurysmatic with a diameter of 2.4 cm (Figure 1). At that time, the decision was made to perform the combined transplant and the aneurysms repair simultaneously.

3D CT-scan view of the abdominal aorta aneurism (AAA)*, left and right iliac artery aneurysms, and hepatic artery aneurysm (→). The diameter of the AAA, and left and right common iliacs was 4.7, 3.0, and 2.4, respectively.
Donor operation
On May 20, 2002, a young cadaver multiorgan donor became available. The technique of the procurement was modified according to the needs of our recipient. For the cold perfusion, the left hypogastric artery was cannulated instead of the abdominal aorta to preserve the arterial conduit. The cannula was then advanced to the standard position in the infra-renal aorta. The procurement of the liver and pancreas was performed in the standard fashion; the celiac and the superior mesenteric arteries were retrieved along with the respective organs on small aortic patches. The retrieval of the kidneys was modified, leaving the right kidney connected by the renal artery to the abdominal aorta. The left kidney was procured with a small aortic patch around the take-off of the left renal artery. The left iliac artery system was left in continuity with the abdominal aorta and the right iliac system was taken for the pancreas transplant arterial reconstruction. The entire thoracic aorta was procured in continuity with the abdominal aorta. Finally, the right common and superficial femoral arteries were procured in continuity for right iliac reconstruction.
Recipient operation
The patient was initially explored through a standard bilateral subcostal incision with upper midline extension. The liver transplant was performed first. The liver graft was prepared on the back table in the standard fashion. The hepatic artery aneurysm was resected after ligation of the gastroduodenal artery and division of the normal-appearing common hepatic artery with vascular stapler, just proximal to the aneurysm. Recipient hepatectomy with preservation of the inferior vena cava was then completed without difficulties. The liver graft was transplanted using the ‘piggy-back’ technique with end-to-side caval and end-to-end portal vein anastomozes. The arterial anastomosis was performed end-to-end between the donor celiac artery on the aortic patch and the recipient common hepatic artery. The bile duct was reconstructed with an end-to-end anastomosis.
On the back table, the right kidney graft was prepared in continuity with the abdominal and thoracic aorta. In the aortic graft, the orifices and the lumbar arteries were oversewn with 5.0 Prolene, while the orifices of the celiac and left renal artery were closed with thoracic aorta patches. Originally, the donor right common/superficial femoral artery was anastomozed end-to-end to the right common iliac artery take-off, in an attempt to reconstruct both iliac systems in the back table (Figure 2).
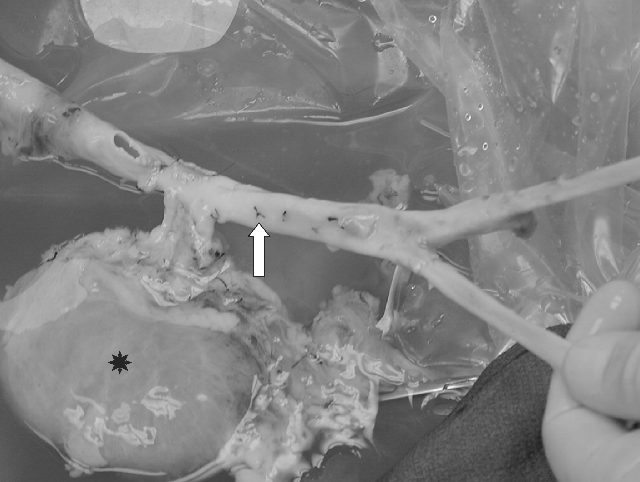
View of abdominal aortic allo-graft (→) with attached kidney (*) during the back table preparation before transplant.
At this time the midline incision was extended to the pubis. Proximal and distal vascular control of the infrarenal aorta and iliac aneurysms was obtained. Intra-operative view of AAA (Figure 3). Upon inspection of the size of the external iliac arteries bilaterally, it appeared evident that using donor iliacs for distal reconstruction would be not advisable because of the marked size mismatch. The plan for arterial reconstruction was therefore modified as shown in Figure 4. After proper clamping, the infrarenal anastomosis between the recipient abdominal aorta and the donor thoracic aorta, approximately 10 cm above the celiac artery take-off, was performed with 3.0 Prolene in a running fashion. Then the renal vein of the kidney graft was anastomozed to the recipient vena cava just cranial to its bifurcation. The kidney graft was promptly perfused moving the vascular clamp of the infrarenal aorta on the donor aortic conduit just distal to the take-off of the right renal artery. Subsequently, the distal anastomosis between the distal donor abdominal aorta and the right common iliac artery of the recipient, just above the take-off of the right internal iliac artery, was performed, allowing re-perfusion of the right leg. At this point the remaining segment of proximal donor thoracic aorta was anastomozed end-to-side to the conduit at the level of the orifice of the superior mesenteric artery. The donor thoracic aorta was then tailored at the appropriate length and anastomozed end-to-end to the left common iliac artery of the recipient just above the take-off of the left internal iliac artery. Finally, after re-perfusing the left leg a standard dome uretero-neocystostomy was performed. The patient had an uneventful recovery with prompt renal and hepatic function. She experienced no postoperative complications and was discharged home in 10 days. She received induction therapy with Basiliximab on days 0 and 4 post-transplant and was initially maintained on a combination of tacrolimus, mycophenolate mofetil, and steroids. She is currently 8 months post-transplant, in excellent condition with normal liver and renal function, and is maintained on tacrolimus and low-dose prednisone. A follow-up abdominal CT scan with vascular reconstruction shows no recurrent aneurysmal disease.
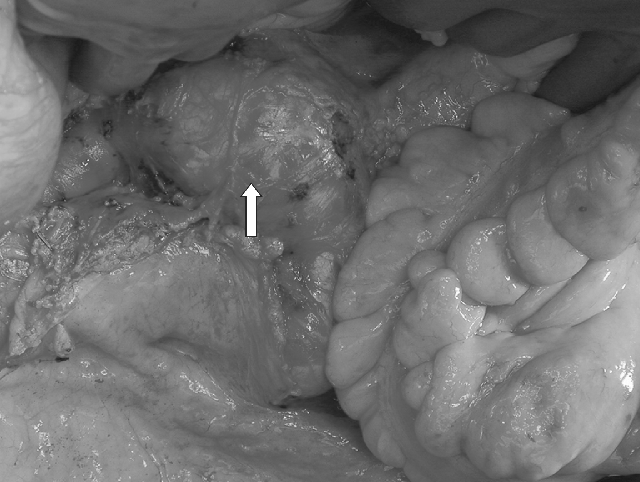
Intraoperative view (→) of abdominal aorta aneurism.
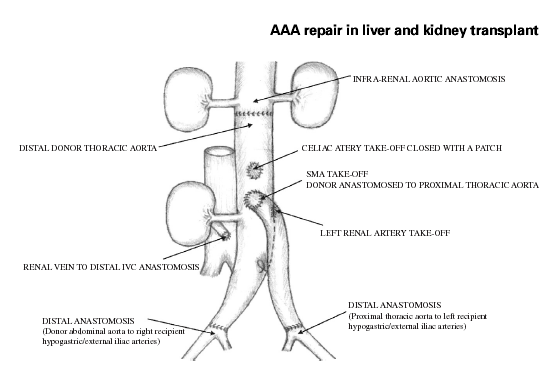
Schematic surgical reconstruction.
Discussion
The peculiarity of this case is the coexistence of failure of two organs and the aneurysm of the abdominal aorta, iliac, and hepatic arteries. The simultaneous transplantation of the liver and kidney is routinely performed with excellent results (1, 2, 3, 4, 13, 14). The transplantation of a kidney in patients with aneurysm of the iliac artery has also been performed by resection of the diseased vessel and implantation of the renal graft on the vascular graft (15). The simultaneous repair of an AAA with kidney transplantation has also been reported (1, 10, 16).
Our patient was in need of a simultaneous liver and kidney transplant. The question was whether to repair first the AAA aneurysm and perform the double transplantation at a later time, or to perform all three procedures in the same operative session. The argument against the first option is that the repair of an AAA in a patient affected by end-stage liver and kidney disease carries a higher risk of mortality and morbidity than in patients affected only by AAA (17–19). Moreover, the use of a vascular prosthesis could be associated with the potential risk of infection during the subsequent transplant procedures or later as a result of the immunosuppressive therapy (20). Lastly the repair of only the AAA would have left in place the hepatic artery aneurysm and the risk of its rupture. The possibility of performing the liver transplant first and the AAA and kidney transplant in a separate procedure at a later date was not entertained. There is in fact a reported increased risk of aneurysm enlargement and rupture in patients undergoing laparotomy for pathologies other than AAA (21, 22). A hypotensive episode during the AAA and kidney transplant procedure may cause hypoperfusion of the liver graft and even thrombosis of the hepatic artery. Aneurysm enlargement could also occur as a result of the effects of immunosuppressive therapy (23–27).
The option of performing all three procedures during the same operative session was then regarded as the most logical and safest for the patient.
The strategy was to plan the operation starting from the donor procedure. The necessity of obtaining a long segment of abdominal aorta in continuity with the iliac artery was solved by cannulating the proximal portion of one of the common iliac arteries, leaving the aorta and other iliac arteries intact. The retrieval of the kidneys was modified so as to leave the right kidney attached to the abdominal aorta by the renal artery. No large aortic patch was resected at the take-off of the celiac trunk and mesenteric and left renal artery, in order to facilitate the closure of each stump. The thoracic aorta was also procured.
The liver transplant was performed without modifying our standard ‘piggy-back’ technique and the arterial anastomosis could be performed end-to-end on perfectly healthy vascular tissue once the aneurysm of the recipient hepatic artery was resected. The second step of the operation was designed to keep the warm ischemia time of the kidney as short as possible and to guarantee repair of the aortic aneurysm and quick reperfusion of the lower extremities. Once the anastomosis between the recipient infrarenal aorta and the abdominal aorta of the donor had been completed, the renal vein was immediately anastomozed to the inferior vena cava, allowing prompt reperfusion of the kidney graft. This was possible just by moving the aortic clamp distal to the take-off of the right renal artery on the donor aorta. With both grafts reperfused, the vascular portion of the operation was easily completed, by performing the anastomosis between the common iliac arteries of the recipient and the donor iliac arteries on the right side and thoracic artery on the left. Lastly, the uretero-cystotomy was performed.
In the case of a patient affected by end-stage liver and kidney disease and aneurysmatic disease, a simultaneous liver/kidney transplant and AAA repair may represent the safest and most efficient treatment solution. This approach allows treatment of all pathologies at once, avoiding the problems associated with a re-laparotomy, and prevents the complications of the progression of end-stage organ disease or enlargement of the aneurysms.




