The cAMP Signaling Pathway has Opposing Effects on Rac and Rho in B16F10 Cells: Implications for Dendrite Formation in Melanocytic Cells
Abstract
A hallmark of melanocytic cells is their ability to form dendrites in response to growth factors and to ultraviolet irradiation. It is known that the cyclic adenosine monophosphate (cAMP) second messenger pathway stimulates melanocyte dendrite formation because agents that increase cAMP such as forskolin and dibutyrl cAMP induce dendrite formation in normal human and murine melanocytes and melanoma cell lines. The Rho family of guanosine triphosphate (GTP)-binding proteins regulates cytoskeletal reorganization in all cells tested and Rac and Rho have both been shown to regulate melanocyte dendrite formation. In this report, we analyzed the effect of cAMP on the activation of Rac and Rho and show that elevation of cAMP stimulates Rac and inhibits Rho in B16F10 cells. The Rho GTP-binding proteins have also been shown to either cross-activate or inhibit each other and in this report we show that Rac activates Rho in B16F10 cells. Microinjection of C3 botulinum exoenzyme toxin, an agent that specifically inactivates Rho or microinjection of constitutively active mutant Rac protein-induced dendricity in human melanocytes and in B16F10 and B16F1 murine melanoma cell lines. We conclude that cAMP-mediated dendrite formation in melanocytic cells is mediated through upregulation of Rac activity and downregulation of Rho activity.
Abbreviations –
-
- GFP
-
- green fluorescent protein
-
- GST
-
- glutathione-S-transferase (EDI)
-
- MSH
-
- melanocyte stimulating hormone
-
- dbcAMP
-
- dibutyrl cyclic AMP
-
- MC1
-
- melanocortin-1
-
- PKA
-
- protein kinase A
-
- Rho GDI
-
- Rho guanine nucleotide dissociation inhibitor
Introduction
Melanocytes are neural crest derived cells which form dendrites, actin and microtubule containing specialized cell processes that transport melanosomes for transfer to keratinocytes in response to hormones and ultraviolet (UV) irradiation. Melanosomes are lysosome-related organelles that contain a large complement of enzymes for the synthesis of melanin, a pigment that scatters light and therefore protects the skin from the carcinogenic effects of UV irradiation. Dendrite extension is a fundamental requirement for melanosome transfer. In melanocytic cells, hormones such as endothelin-1, nerve growth factor and α-melanocyte stimulating hormone (MSH) stimulate dendrite extension (1–6). These hormones activate distinct and overlapping signaling pathways including the cyclic adenosine monophosphate (cAMP) second messenger pathway [MSH and endothelin-1 (7–9)] and the protein kinase C and MAP kinase pathway [endothelin-1 (8, 9) and nerve growth factor (10)]. The ultraviolet irradiation activates multiple signaling pathways that stimulate melanocyte dendrite extension through upregulation of endothelin-1 production by keratinocytes (11) and by upregulation of the MSH receptor melanocortin-1 (MC1) on melanocytic cells (12–14). In human melanocytes, binding of MSH to the MC1 receptor results in elevation of cAMP through activation of adenylate cyclase (7, 15). The MC1 receptor is a member of a subfamily of G-coupled receptors with seven transmembrane domains (16, 17).
The Rho family of guanosine triphosphate (GTP)-binding proteins play a critical role in cytoskeletal organization, determination of cell polarity, cell–cell adhesion, cell cycle regulation, neurite outgrowth, apoptosis, exocytosis, and endocytosis [(18–24); for review see Ref. (25)]. Similar to other small GTP-binding proteins, Rho proteins alternate between active (GTP-bound) and inactive (GDP-bound) forms, which is controlled by regulatory associated proteins. In most cell types RhoA mediates stress fiber formation (26, 27), Rac1 mediates membrane ruffling and lamellipodia formation (28) and Cdc42 mediates filopodia formation (29). However, the effect of individual Rho proteins varies with cell types as in human epidermal carcinoma KB cells in which RhoA rather than Rac1 mediates membrane ruffling in response to hepatocyte growth factor and phorbol ester (30). Elucidation of the function of Rho proteins has been facilitated by the use of constitutively active mutants and dominant negative inhibitors. Bacterial toxins have also been useful, based on their ability to inactivate all Rho family members [Clostridium difficile toxin B (31, 32)] or to selectively inactivate Rho [C3 botulinum exoenzyme (33, 34)]. It was recognized early on that there exists a hierarchy of signaling among Rho proteins wherein activation of one protein leads to either the activation or inhibition of the other two proteins. Ridley et al. (28) showed in Swiss 3T3 cells that activation of Rac simulates lamellipodia formation followed by stress fiber formation. Activation of Cdc42 resulted in filopodia formation followed by lamellipodia formation. Based on these morphologic observations they concluded that a linear hierarchy exists of Cdc42 activating Rac which activates Rho. In N1E-115 neuroblastoma cells however, Rac antagonizes Rho effects and Cdc42 activates Rac (35). Using biochemical means to assess Rho protein activation, Rac has been shown to downregulate Rho in most cell types. This has been shown in NIH3T3 cells (36) and COS-7 cells (37).
We reported previously that expression of constitutively active Rac protein in B16F1 murine melanoma cells resulted in dendrite formation (38). Busca et al. (39) reported that Rho inhibition is responsible for dendrite formation in B16F10 cells. They showed that treatment of B16F10 cells with C3 botulinum exoenzyme stimulated dendrite formation and that constitutively active forms of Rho inhibited the ability of cAMP-elevating agents to stimulate dendricity. B16F1 and B16F10 cells are derived from a murine melanoma cell line; B16F1 cells have a low metastatic potential whereas B16F10 cells are highly metastatic (40). Because the cAMP second messenger pathway induces dendricity in melanocytic cells, we wished to determine the role of this pathway in mediating the activity of Rac and Rho and to determine the effect of these proteins in dendrite formation in human melanocytes and murine melanoma cells. We also sought to determine the hierarchical pathway of activation of Rac and Rho in melanocytic cells. We show that cAMP downregulates Rho protein activity but upregulates Rac activity. Through microinjection studies we show that both Rac and Rho control dendrite formation in melanocytic cells. Finally, we have determined that in melanocytic cells Rac activates Rho. We discuss these findings in the context of cAMP-dependent dendrite formation in melanocytic cells.
Materials and methods
Cell Culture and Reagents
The B16F10 and B16F1 murine melanoma cells were obtained from American Type Culture Collection (Rockville, MD, USA) and were maintained in Dulbeccos Minimal Essential Media (Gibco BRL, Gaithersburg, MD, USA) + 10% fetal bovine serum (Gibco BRL). Neonatal foreskins were obtained according to the University of Rochester's Research Subject Review Board. Human melanocytes were cultured in MCDB 153 (Gibco BRL) supplemented with 0.5% fetal bovine serum, bovine pituitary extract (15 μg/ml), phorbol ester (8 nM), basic fibroblast growth factor (1 ng/ml), insulin (5 μg/ml) and hydrocortisone (500 ng/ml). Supplements were obtained from Sigma Co. (St Louis, MO, USA). Forskolin, dibutyrl cAMP (dbcAMP), Nle4-D-Phe7-α-MSH [NDP-MSH; a potent synthetic analog of MSH (41)], and horse radish peroxidase-conjugated goat antirabbit and goat antimouse antibodies were purchased from Sigma Co. Monoclonal antibodies to Rac1 were purchased from Becton Dickenson Transduction Laboratories (Lexington, KY, USA); rabbit polyclonal antibodies to RhoA were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); rabbit polyclonal antibodies to Rho guanine nucleotide dissociation inhibitor (Rho GDI) were purchased from Zymed Laboratories, Inc. (San Francisco, CA, USA). Vitrogen was purchased from Cohesion (Palo Alto, CA, USA). The C3 botulinum exoenzyme was purchased from List Biological Laboratories (Cambell, CA, USA); L61Rac1-His protein (constitutively active Rac1) was purchased from Cytoskeleton Inc. (Denver, CO, USA). pGEX2TK plasmids expressing human p21-activated kinase binding domain 1B (PBD) or Rhotekin were a generous gift of Dr Collard (The Netherlands Cancer Institute, Amsterdam, the Netherlands) and have been described previously (42, 43). pGEX2TK plasmid expressing Rho GDI was a generous gift of Dr Alan Hall (University College London, London, UK).
Adenoviral Vectors
Recombinant adenoviral vectors capable of expressing constitutively active Rac (V12Rac), Rho (V14Rho) and Cdc42 (V12Cdc42) were a generous gift of Dr James Bambara (University of Colorado, Denver, CO, USA) and have been described previously (20). Each recombinant vector contains a complementary DNA (cDNA) for green fluorescence protein (GFP) for monitoring infection efficiency. Green fluorescence protein and Rho cDNAs were driven from separate cytomegalovirus (CMV) promoters.
Expression and Purification of Recombinant Proteins
Expression of recombinant proteins from BL21 Escherichia coli (Stratagene, LaJolla, CA, USA) containing plasmids was induced by the addition of 0.1 mM isopropylthiogalactoside for 4 h. Bacteria were harvested and resuspended in lysis buffer (20% Triton-X-100 in phosphate-buffered saline) and centrifuged at 4°C for 10 min at 20 000 g and the supernatant was incubated with glutathione-coupled agarose 4B beads (Pharmingen, San Diego, CA, USA). The GST-tag was removed from Rho GDI fusion protein prior to microinjection (see below) through incubation with thrombin protease solution. Thrombin was removed by incubation with p-aminobenzamidine agarose beads (Sigma Co.). Rho GDI protein identity was verified through Western blotting with anti-Rho GDI antibodies.
Affinity Precipitation of Cellular GTP-Rho and Rac
Equal numbers of B16F10 cells (approximately 2 × 107) were lysed in cold lysis buffer (50 mM Tris pH 7.5, 10 mM MgCl2, 0.2 M Nacl, 2% NP-40, 10% sucrose) on ice and disrupted using a French Press. Lysates were pre-cleared by incubating with glutathione beads. Either GST-PBD or GST-Rhotekin (10 μg/ml) were added to the cleared lysates in binding buffer (50 mM Tris, pH 7.5, 60 mM MgCl2, 80 mM Nacl, 1% NP-40) for 30 min at 4°C. GTP-bound proteins were captured by incubation with glutathione beads and pelleted. Beads were washed with binding buffer and GTP-bound protein was eluted with 2× Laemmli sample buffer. Samples were immunoblotted on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using standard procedures. To assess loading equality among different lysates a portion of each lysate was removed prior to the addition of GST-fusion protein and blotted for either Rac or Rho. Full range rainbow molecular weight markers were purchased from Amersham Life Sciences (Arlington Heights, IL, USA). Visualization of the immunoreactive proteins was accomplished with an enhanced chemiluminescence reaction (Amersham Life Sciences). Densitometry analysis was performed on digital images using NIH Image 6.2 software.
Microinjection
Human melanocytes, B16F1 or B16F10 melanoma cells were grown on vitrogen-coated acid washed glass coverslips at 2 × 105 cells/coverslip. Recombinant purified proteins (0.5 mg/ml) or C3 botulinum exoenzyme toxin (0.2 mg/ml) in water were microinjected into the cytoplasm using an Eppendorf microinjector (Brinkmann Instruments, Westbury, NY, USA) viewed on a Nikon Diaphot inverted microscope (Nikon, USA). Femptotips I (Brinkmann Instruments) were used and the injection pressure was 50–55 psi. Microinjections were performed within 10 min. During this time approximately 10–15 cells were successfully injected. To locate injected cells Oregon Green-conjugated dextran beads (Molecular Probes, Eugene, OR, USA) were coinjected at a concentration of 1 mg/ml. After microinjection cells were placed in their respective media and were fixed 1 or 18 h later in 4% formalin/phosphate-buffered saline. Images were captured with a Spot digital camera and post-processed using Adobe PhotoShop 5.5 (Spectra Services, Webster, NY, USA).
Results
The cAMP Second Messenger Pathway has Opposite Effects on Levels of Activated Rac and Rho
To assess the effect of the cAMP-signaling pathway on levels of activated Rac and Rho, cells were serum-starved for 1 h and were treated with forskolin (20 μM), which activates adenylate cyclase, dbcAMP (10−5 M), a cell permeable analog of cAMP, or NDP-MSH (10−8 M) for various time points. Activated Rac or activated Rho was then isolated from cell lysates using affinity purification with either GST-PBD or GST-Rhotekin which binds to activated Rac and Rho, respectively. To assess loading equality among different lysates, a portion of each lysate was removed prior to the addition of GST-fusion protein and blotted for either Rac or Rho. Representative blots are shown in 1-3. For densitometry analysis samples were compared with control samples after normalization for loading differences. Averaged results of densitometry analysis from three separate experiments are given in Tables 1–3. We chose to use B16F10 murine melanoma cells for these experiments because the large number of cells required for each isolation (2 × 107) makes the use of normal human melanocytes, which have a doubling time of approximately 48 h, difficult. Five and 10 min after treatment with forskolin, GTP-bound Rho was decreased (>60%) whereas GTP-bound Rac was increased over 200% in cell lysates (Fig. 1A,B and Table 1). After 30 min of forskolin treatment, GTP-Rho remained decreased (52%) compared with control cells, whereas GTP-bound Rac remained elevated at 30 min. Treatment with dbcAMP induced a reduction in GTP-Rho at 1 min (66%); levels of GTP-Rho remained depressed at 2 min and by 10 min GTP-Rho had returned to near control levels (Fig. 2 and Table 2). In contrast with Rho, GTP-bound Rac was increased over control levels at 1 min (25%) following treatment with dbcAMP and steadily increased at 2, 5 and 10 min with a 144% increase observed after 10 min (Fig. 2b and Table 2).
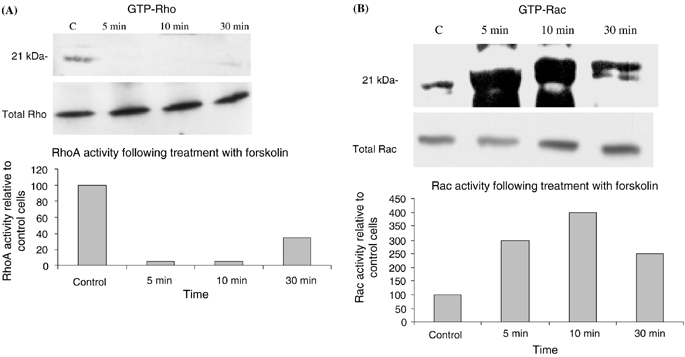
Forskolin induces a decrease in Rho and an increase in Rac activation. B16F10 cells were treated with forskolin (20 μM) and GTP-bound Rho (A) and Rac (B) were isolated by affinity purification as described in ‘Materials and methods’. GTP-bound Rho was decreased at 5 and 10 min and remained decreased at 30 min. In contrast, GTP-Rac was markedly elevated at 5 and 10 min and remained elevated at 30 min. Shown are representative blots of three separate experiments. Densitometry of the blot is shown as percentage expression compared with control cells.
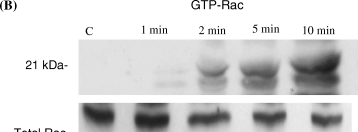
The cell permeable analog of cAMP (dbcAMP) induces a decrease in Rho activation and an increase in Rac activation. B16F10 cells were treated with dbcAMP (10−5 M) and GTP-bound Rho (A) and Rac (B) were isolated by affinity purification as described in ‘Materials and methods’. GTP-Rho was decreased at all time points tested compared with control cells. In contrast GTP-Rac was increased at 1, 2 and 5 min and remained elevated at 10 min. Shown are representative blots of three separate experiments. Densitometry of the blot is shown as percentage expression compared with control cells.
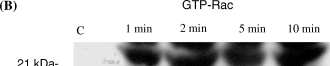
NDP-MSH, a potent analog of MSH, induces a decrease in Rho activation and an increase in Rac activation. Serum starved B16F10 cells treated with NDP-MSH (10−8 M) resulted in decreased levels of GTP-bound Rho (A) at all time points tested with a maximal inhibition at 5 min. In contrast, GTP-bound Rac is increased above control levels at 1 min and was persistently elevated at 10 min. Shown are representative blots of three separate experiments. Densitometry of the blot is shown as percentage expression compared with control cells.
| Time (min) | Percentage change in Rho-GTP from control ± SEM | Percentage change in Rac-GTP from control ± SEM |
|---|---|---|
| 5 | −67 ± 18 | +250 ± 25 |
| 10 | −67 ± 18 | +300 ± 23 |
| 30 | −52 ± 15 | +190 ± 22 |
- Results represent the average of densitometry readings from three experiments ±standard error of the mean (SEM).
| Time (min) | Percentage change in Rho-GTP from control ± SEM | Percentage change in Rac-GTP from control ± SEM |
|---|---|---|
| 1 | −66 ± 8 | +25 ± 15 |
| 2 | −70 ± 10 | +33 ± 10 |
| 5 | −41 ± 15 | +46 ± 12 |
| 10 | −12 ± 12 | +144 ± 10 |
- Results represent the average of densitometry readings from three experiments ±standard error of the mean (SEM).
| Time (min) | Percentage change in Rho-GTP from control ± SEM | Percentage change in Rac-GTP from control ± SEM |
|---|---|---|
| 1 | −42 ± 9 | +111 ± 20 |
| 2 | −55 ± 10 | +135 ± 10 |
| 5 | 0 ± 5 | +116 ± 15 |
| 10 | −45 ± 7 | +152 ± 15 |
- Results represent the average of densitometry readings from three experiments ±standard error of the mean (SEM).
Binding of MSH to the MC1 receptor results in an elevation in cAMP in normal human melanocytes and in B16F10 cells (7, 44) and treatment of cells with MSH or MSH analogs has been shown to induce dendricity in murine melanocytic cells (1, 2, 39). In B16F10 cells MSH has also been shown to stimulate protein kinase C activation (44). Treatment of serum-starved B16F10 cells with NDP-MSH decreased levels of activated Rho at 1 and 2 min (42 and 55%, respectively; Fig. 3A and Table 3). By 5 min GTP-Rho was undetectable. At 10 min levels of GTP-Rho remained depressed. In contrast NDP-MSH resulted in a marked increase in GTP-bound Rac at 1, 2 and 5 min (over 100% increase over control cells) and remained elevated at the 10-min time point (Fig. 3B and Table 3).
Rac Activates Rho in Melanocytic Cells
In many cell types activation of one member of the Rho family of GTP-binding proteins leads to activation or inhibition of other members of the family. To determine if Rac activates Rho, B16F10 cells were infected with Adeasy virus expressing either V12Rac or empty vector for 3 d at a multiplicity of infection (MOI) of 100. At this MOI approximately 70% of cells are infected as determined by green fluorescence protein (GFP)-fluorescence. We previously determined that expression of GFP shows >90% correlation with expression of recombinant protein using these vectors (45). Activated Rho was isolated using GST-Rhotekin and samples were blotted for Rho. Expression of V12Rac resulted in a marked increase in Rho activation compared with cells expressing empty vector (Fig. 4A). In contrast with Rac, expression of constitutively active Cdc42 in B16F10 cells did not result in Rho activation (Fig. 4B). Averaged results of densitometry analysis of three separate experiments showed that Rac induced a 125% increase in Rho activity compared with control cells. In B16F10 cells expressing constitutively active Rho, levels of Rac activation (assessed by pull down with GST-PBD) were not altered compared with cells expressing empty vector (data not shown). From this data we conclude that activation of Rho by Rac is unidirectional.
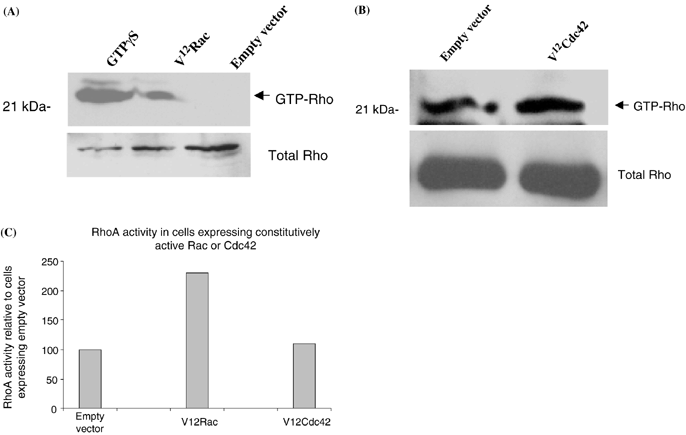
Rac activates Rho in melanocytic cells. GTP-bound Rho was isolated from cells expressing either V12Rac or empty vector and resolved on 15% SDS-PAGE (A). Western blots for Rho demonstrate a marked increase in GTP-bound Rho in cells expressing V12Rac compared with cells expressing empty vector. GTP-bound Rho was isolated from cells expressing either V12Cdc42 or empty vector and resolved on 15% SDS-PAGE (B). Western blots for Rho demonstrate equivalent levels of GTP-Rho in cells expressing constitutively active Cdc42 and vector-expressing cells. Densitometry of the two blots is shown as percentage expression compared with vector-expressing cells (C).
Role of Rho Proteins in Melanocyte Dendrite Formation
Interpretation of morphologic changes in cells exposed to constitutively active and dominant negative mutant Rho proteins is complicated by the possibility of compensatory pathways that may be activated in response to high levels of protein expression for a prolonged period of time. Similarly, toxins such as C3 botulinum exoenzyme require several days to permeate the cell membrane, which could potentially result in similar secondary effects. Microinjection of proteins and toxins into the cell cytoplasm avoids some of these pitfalls by allowing for rapid introduction of the protein or toxin into the cell and subsequent analysis of morphology within minutes or hours. To directly assess the effect of Rho proteins in human and murine melanocyte dendricity we microinjected cells with either recombinant Rho GDI protein, C3 botulinum exoenzyme toxin or recombinant V12Rac protein into the cell cytoplasm. Rho GDIs represent a class of proteins that inhibit the release of GDP from Rho proteins (46) as well as solubilize membrane-associated Rho proteins (47). C3 botulinum exoenzyme toxin specifically inhibits Rho through ADP ribosylation of Asn 41 in the effector region of the protein (33). Control cells were microinjected with Oregon Green and water. One hour and 18 h after microinjection, cells were fixed and examined. Results of these experiments are shown in 5-7.
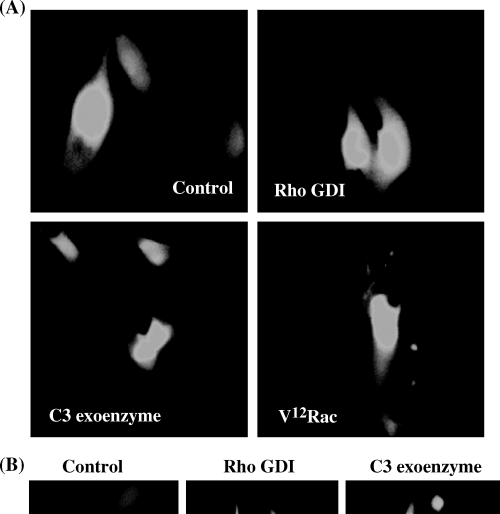
Effect of microinjection of Rho GDI, C3 botulinum exoenzyme toxin and V12Rac on dendricity of B16F1 cells (A) B16F1 cells are epithelioid and non-dendritic in culture as can be seen in cells microinjected with water. Rho GDI and C3 botulinum exoenzyme had no effect on dendricity 1 h following microinjection, however, microinjection of V12Rac induced a highly dendritic phenotype within 1 h. (B) Shown are representative photographs of B16F1 cells microinjected with either recombinant Rho GDI or C3 botulinum exoenzyme and examined 18 h later. Prolonged exposure to these agents induced an elongated and bipolar phenotype after 18 h. Bar = 13 μM.
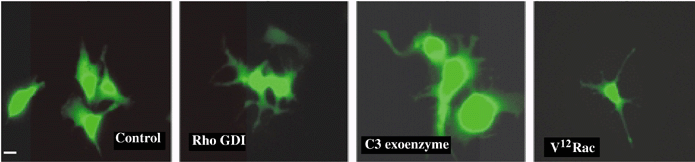
Effect of microinjection of Rho GDI, C3 botulinum exoenzyme and V12Rac on dendricity in B16F10 cells. B16F10 cells have short dendritic processes under normal culture conditions. In contrast with B16F1 cells, B16F10 cells became highly dendritic within 1 h of microinjection of Rho GDI and C3 botulinum exoenzyme. Microinjection of recombinant V12Rac also resulted in dendritic phenotype in microinjected cells. Bar = 12 μM.
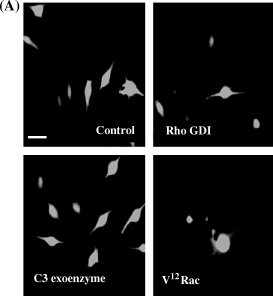
Effect of microinjection of Rho GDI, C3 botulinum exoenzyme and V12Rac on dendricity in human melanocytes. (A) For these experiments only bipolar non-dendritic melanocytes were microinjected, as can be seen in control cells. Microinjection of recombinant Rho GDI resulted in early dendrite formation 1 h following microinjection; cells microinjected with C3 botulinum exoenzyme showed dendrite extension in some, but not all cells at 1 h. Recombinant V12Rac induced a highly dendritic phenotype within 1 h. Bar = 15 μM. (B) Eighteen hours following microinjection of RhoGDI or C3 botulinum exoenzyme toxin virtually all injected cells were highly dendritic compared with control cells. Bar = 15 μM.
B16F1, B16F10 and human melanocytes showed cell-type specific differences in response to modulation of Rho protein activity. However, differences in responses consisted primarily of differences in time required to elicit a response. B16F1 cells are normally epithelioid in morphology and do not display dendrites as can be seen in control cells injected with Oregon Green and water (Fig. 5A). One hour following microinjection with either recombinant Rho GDI or C3 botulinum exoenzyme toxin little change in cell shape was noted. However, 18 h following microinjection of Rho GDI and C3 botulinum exoenzyme toxin B16F1 cells did begin to show elongated cell processes compared with control cells (Fig. 5B). In contrast, 1 h following microinjection of recombinant V12Rac protein B16F1 cells had become highly dendritic (Fig. 5A). B16F10 cells display short dendritic extensions under normal condition (Fig. 6). Introduction of Rho GDI or C3 botulinum exoenzyme resulted in a highly dendritic phenotype in virtually all cells within 1 h. Eighteen hours later cells maintained their dendritic phenotype (not shown). Microinjection of V12Rac induced the formation of increased numbers of short dendritic extensions, but to a lesser extent than in Rho GDI and C3 botulinum exoenzyme-injected cells. Human melanocytes exhibit a variety of morphologies under normal culture conditions. Only thin, bipolar cells were microinjected, as can be seen in control cells (Fig. 7A). One hour following microinjection of Rho GDI or C3 botulinum exoenzyme approximately 50% of the cells displayed short dendritic processes. However, 18 h following microinjection of Rho GDI or C3 botulinum exoenzyme virtually all cells were dendritic (Fig. 7B). Microinjection of V12Rac induced a dendritic phenotype within 1 h in approximately half of injected cells (Fig. 7A).
Discussion
The cAMP-second messenger system plays a critical role in melanocyte differentiation and melanogenesis. One of the earliest observations regarding the effect of cAMP-elevating agents on melanocytes was their ability to induce dendrite formation. Based on our earlier observation that activated Rac induced the formation of dendrites in B16F1 cells (38) and reports that Rho inhibition results in dendrite formation in B16F10 cells (39), we sought to determine the effect of cAMP on Rac and Rho activity. We addressed the role of Rac and Rho in dendrite formation in two murine melanoma cell lines and in human melanocytes through microinjection of activated mutant proteins or toxin inhibitors. Finally, we determined the hierarchy of activation of Rac and Rho in melanocytic cells. Using a method that specifically isolates activated Rho proteins, our results clearly show that elevation of cAMP in B16F10 cells has opposing effects on Rho and Rac activity. Through microinjection studies we demonstrate that while B16F10, B16F1 and human melanocytes show some temporal difference in dendrite formation in response to modulation of Rac and Rho activity, overall all three cells form dendrites in response to either Rac activation or Rho inhibition.
The observation that cAMP inhibits Rho and stimulates Rac in melanocytic cells is analogous to the situation in human breast carcinoma cells in which intracellular cAMP levels have been shown to stimulate Rac and inhibit Rho and result in increased cell migration (48, 49). We observed inhibition of Rho and activation of Rac following treatment with forskolin, dbcAMP and with NDP-MSH which supports a role for cAMP in regulation of these two proteins in melanocytic cells. Because MSH has been shown to elevate protein kinase C levels in B16F10 cells (44) we cannot exclude the possibility that the effects of MSH on Rac and Rho activity were mediated in part through changes in protein kinase C activation. Further experiments will be necessary to clarify this. However, the clear effect of forskolin and dbcAMP on Rac and Rho activity unequivocally points to a role for cAMP in Rac and Rho activation in B16F10 cells. Levels of GTP-bound Rac remained persistently elevated at the time points tested, whereas levels of GTP-bound Rho returned to normal or near normal during the same time frame. It is therefore possible that cAMP-induced melanocyte dendrite formation may be initiated by a rapid and transient decrease in Rho activity which results in loss of stress fibers and is maintained through persistent Rac activity, which stimulates lamellipodia formation and dendrite extension. Melanocytic cells appear to be similar to several other cell types in which the cAMP signaling pathway has been shown to inhibit Rho activity. Rho has been shown to be inhibited by agents that elevate cAMP in hepatoma cells (50). Dong et al. (51) showed that protein kinase A (PKA)-dependent phosphorylation of Rho inhibits binding of RhoA-associated serine/threonine kinase, a downstream effector of Rho. Laudanna et al. (52) showed that intracellular cAMP negatively modulates RhoA through inhibition of GDP/GTP exchange in lymphoid cells. Thus it appears that there are multiple mechanisms of Rho inactivation by cAMP. Intracellular cAMP has also been shown to inhibit Rho activity in endothelial cells (53). In combination with microinjection studies which show a role for Rac activation and Rho inhibition in dendrite extension we conclude that both cAMP-dependent activation of Rac and inhibition of Rho are involved in cAMP-induced dendrite formation. In this report we also showed that activation of Rac results in activation of Rho. This signaling hierarchy is similar to that reported in Swiss 3T3 cells but is different from that observed in other cells as well as neural crest derived cells, in which Rac has been shown to inhibit Rho (35). Because activation of Rho has been shown to inhibit dendrite formation in B16F10 cells (39), Rac-dependent Rho activation may serve as a mechanism to terminate dendrite extension.
To directly test the role of Rac and Rho in human melanocytes and B16F10 and B16F1 murine melanoma cells we microinjected agents that either specifically inhibit Rho (C3 botulinum exoenzyme), inactivate all Rho proteins (Rho GDI) or activate Rac (V12Rac mutant protein). While differences in the extent and timing of dendrite formation were observed among the three cell types in response to these agents, some general conclusions can be made. Human melanocytes and B16F1 cells had a delayed (18 h) response to Rho GDI and C3 botulinum exoenzyme, whereas the response of B16F10 cells was rapid (within 1 h). For all three cell types however, C3 botulinum exoenzyme and Rho GDI induced dendrite formation. For human melanocytes and B16F1 cells microinjection of V12Rac mutant protein induced dendricity within 1 h; the effect of V12Rac on dendricity of B16F10 cells was delayed (18 h), but increased dendrite formation could still be detected. Our results differ from those reported by Busca et al. (39) who failed to observe increased dendricity in B16F10 cells transfected with plasmids expressing V12Rac. In their study, transfection of mutant Rho protein-expressing plasmids was used over the course of 24–48 h, whereas we used microinjection and relatively short-term time points (1–18 h) for end-point analysis. In our experience long-term expression of mutant Rho proteins induces diverse morphologies in transfected cells (unpublished observations). Others have reported that long-term stimulation of Rho proteins may result in inhibition of the target protein through downregulation of their respective guanine nucleotide exchange factors (54). Therefore one possible explanation for the discrepancy between their study and the present study may be the result of the different techniques used to introduce mutant Rac proteins into cells. Rho GDI, which inactivates all three Rho proteins through sequestering them in the cytosol, also induced dendricity within 1–18 h. Based on this data it is possible that inhibition of Rho, rather than activation of Rac is the dominant mechanism underlying dendrite formation. However, the interaction of Rho GDI with Rho proteins is known to be modified by a variety of intracellular factors. For example it has been shown that PKA-dependent phosphorylation of RhoA and Cdc42 increases the ability of Rho GDI to extract RhoA and Cdc42 from membranes (55, 56). Phosphotidyl-inositol bis 4,5 phosphate (PIP2) has been shown to loosen complexes between Rho GDI and RhoA (57). Therefore it is possible that in our cell system Rho GDI may preferentially bind to Rho compared with Rac.
The results presented here support a model in which both Rac activation and Rho inhibition are involved in melanocyte dendrite extension and that the ability of cAMP to induce dendrites is a dual function of its ability to activate Rac and inhibit Rho. A dual role for Rac and Rho in melanocyte dendrite formation is highly analogous to neural cells in which Rac activation has been shown to stimulate neurite outgrowth and Rho inhibition has been shown to stimulate neurite retraction (35, 58, 59). Finally, because of the role of the cAMP second messenger system in melanocyte pigmentation and cell growth, cAMP-mediated pigmentation and cell growth may be mediated in part through Rac activation and Rho inhibition.
Acknowledgement– This work was supported by 1R01AR45427(GS).




