Catecholamines Increase in the Urine of Non-Segmental Vitiligo Especially During Its Active Phase
Abstract
Neural factors appear to play a major role in the pathogenesis of vitiligo. To investigate the possible correlation between vitiligo and peripheral monoaminergic system activity, we used high-pressure liquid chromatography and electrochemical detector methods to evaluate the basal urine excretion values of catecholamines [norepinephrine (NE), epinephrine and dopamine (DA)], their relative metabolites [3-methoxy-4-hydroxyphenylglycol (MHPG), normetanephrine (NMN), metanephrine (MN), vanilmandelic acid (VMA) and homovanillic acid], as well as 5-hydroxyindoleacetic acid (5-HIAA), in 35 healthy subjects and in 70 patients, suffering from non-segmental vitiligo at different stages of the disease. Levels of NE, DA, NMN, MN, MHPG, VMA and 5-HIAA were found to be significantly higher in patients than in controls. The patients with progressive vitiligo (n = 56) presented increased urinary excretion values for all parameters (in particular, NE levels) than other patients. Interestingly, in patients at its more recent vitiligo onset (<1 yr), NE values were different to those of subjects affected from 1 to 5 yr and from 6 to 10 yr. This result was confirmed by the significant negative relationship detected between NE excretion values and disease duration. In both vitiligo and control groups, significant correlations were found between monoamines as well as between these monoamines and their metabolites. The increase in catecholamine turnover, mainly occurring at the onset of the disease, is probably due to the stress associated with the appearance of lesions. Moreover, considering that these compounds readily produce toxic free-radicals and that vitiliginous subjects have a defective free radical defence mechanism, they may also contribute to the disappearance of melanocytes in the early phases of vitiligo.
Abbreviations –
-
- 5-HIAA
-
- 5-hydroxyindolacetic acid
-
- 5-HT
-
- 5-hydroxytriptamine
-
- COMT
-
- catechol-O-methyltransferase
-
- DA
-
- dopamine
-
- DHBA
-
- 3,4-dihydroxybenzilamine
-
- E
-
- epinephrine
-
- HVA
-
- homovanillic acid
-
- MAO-A
-
- monoamino oxidase A
-
- MHPG
-
- 3-methoxy-4-hydroxyphenylglycol
-
- MN
-
- metanephrine
-
- NE
-
- norepinephrine
-
- NMN
-
- normetanephrine
-
- PNMT
-
- phenylethanolamine-N-methyl transferase
-
- VMA
-
- vanillylmandelic acid
Introduction
The aetiology of vitiligo is still controversial and several hypotheses have been proposed to explain the loss of melanocytes. These include: intrinsic genetic susceptibility acting as a predisposing factor in only a single subset of individuals (1), an autoimmune mechanism (2), an autocytotoxic destruction of melanocytes (3), an altered tetrahydrobiopterin homeostasis (4) and a neural hypothesis (5), singularly or in combination. The latter is supported by clinical, physiological, microscopic, ultrastructural, immunohistochemical and biochemical findings (5, 6). Moreover, the level of catecholamines, which is consistently released as a consequence of emotional and/or stressful events, is considered as being strictly related to the onset or worsening of the disease (7).
Different strategies have been adopted to study these neural markers and their metabolites. Morrone et al. (8) first reported that significant increases in the urinary concentrations of the catecholamine metabolites homovanillic acid (HVA) and vanillylmandelic acid (VMA) characterized the onset and progression of vitiligo, irrespective of the clinical type. Schallreuter et al. (9) noticed an increase in norepinephrine (NE) production in both plasma and urine. Successively, Chakraborty et al. (10) also evidenced higher urinary excretion values of indole metabolites in vitiliginous patients. Other studies on these markers have focused on cellular levels with increased production of catecholamines being observed in lesional keratinocytes (9, 11).
In previous studies, we noted that patients during a recent disease onset showed significantly higher concentrations of plasma monoamines and relative metabolites than long-term sufferers and subjects in a stable phase (12). The evaluations of monoamines and metabolites in plasma and urine are thought to provide different indications about the activity of catecholaminergic systems: the evaluation in the plasma reflects more accurately the actual activity and thus is more susceptible to momentary emotional events, the blood drawing included; while the evaluation in the urine, referring to a 24-h period of activity, gives more mediated information.
To further investigate the possible relationship between vitiligo and the activity of peripheral monoaminergic systems, we used high-pressure liquid chromatography and electrochemical detector (HPLC-ED) methods to measure the basal urine excretion values of catecholamines [NE, epinephrine (E) and dopamine (DA)], their related metabolites [3-methoxy-4-hydroxyphenylglycol (MHPG), normetanephrine (NMN), metanephrine (MN), VMA and HVA], as well as 5-hydroxyindolacetic acid (5-HIAA), the major metabolite of 5-HT in a larger group of patients.
Materials and methods
Subjects
Seventy patients of both sexes, male subjects (n = 33, mean age ± SE: 31.5 ± 2.6 yr, range: 11–59 yr) and females (n = 37, mean age ± SE: 34.1 ± 2.4 yr, range: 14–64 yr), suffering from non-segmental vitiligo (generalized n = 30, mean age ± SE: 30.3 ± 3.0 yr and acrofacial n = 40, mean age ± SE: 34.7 ± 2.1 yr) gave informed consent for their participation in the study. Fifty-six of those patients were in the active phase of the disease and 14 were in its stable phase. The activity, or progression, of vitiligo was defined as the development of new lesions or the extension of an old lesion over 3 months prior to examination (9).
The patients in active phase were subdivided into four groups according to disease duration: (a) <1 yr, (b) 1–5 yr, (c) 6–10 yr and (d) >10 yr. All the patients were free of medications for at least a week before participating in the study. Thirty-five healthy subjects, matched for sex and age (mean age ± SE: 30.5 ± 2.1 yr, range: 17–61), were used as controls.
Both patients and controls avoided food and beverages known to affect catecholamine metabolism (13) for at least 24 h both before and through out the sample collection period.
Urine Collection
Twenty-four hour urine specimens were collected to measure their catecholamines content (NE, E and DA), metabolite levels (NMN, MN, MHPG, VMA and HVA), and levels of 5-HIAA, which is the major metabolite of 5-HT.
The samples were refrigerated immediately after collection; a volume of 0.800 l and a creatinine value of 15 mg/(kg 24 h) were regarded as the minimum values acceptable for urine collection. Aliquots of urine (20 ml) were frozen at −80°C until assay.
Determination of Catecholamines and Metanephrines
Urinary levels of catecholamines (NE, E and DA) and metanephrines (NMN and MN) were evaluated by HPLC-ED according to the method described by Pagliari et al. (14), modified as in the following. In brief, urine samples were diluted using distilled water (1:5), with 1 ml of diluted urine being supplemented with 100 μl of 0.45 μM 3,4-dihydroxybenzilamine as the internal standard, acidified with 40 μl of 6 M HCl and then hydrolysed at 100°C for 30 min. After cooling and addition of 400 μl of 0.5 M phosphate buffer, the pH was adjusted to 6.5 with 5 M sodium hydroxide and the amines were adsorbed into a Bond Elut CBA cartridge (carboxylic acid resin, 1 ml; Analytichem Int., Harbor City, CA, USA), washed with 6 ml of methanol before use, and eluted with 1 ml of 1 M HClO4. A 50-μl aliquot of the eluate, diluted with mobile phase (1:1), was injected into the chromatograph and all the compounds were separated through an Ultrasphere XL ODS (70 × 4.6 mm, 3-μm particle size) column with an ODS precolumn (Beckman), using a mobile phase containing 25 mM potassium diydrogen phosphate, 0.65 mM sodium dodecylsulphonate, 0.26 mM ethylenediaminetetraacetic acid and 8% v/v acetonitrile buffered to pH 3.0 and delivered at a flow rate of 1.5 ml/min.
An electrochemical detector, ESA Coulochem Model 5100, equipped with conditioning and analytical cells (ESA, Bedford, MA, USA), was used at a nominal potential of +0.30 V, which was applied to the electrode of the conditioning cell, while +0.05 and −0.35 V were applied to the first and second electrode of the analytical cell, respectively. The sensitivity of the assay was 0.1–0.2 pmol/sample with an intra-assay variability of 5.4–6.2% and an interassay variability of 9.2–9.8%. Typical chromatograms of human urine obtained from vitiliginous patients at recent onset or stable phase of disease are reported in Fig. 1.
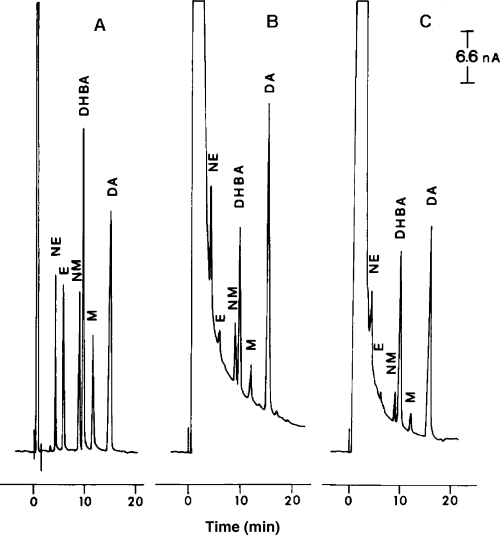
Typical chromatogram of human urine from vitiliginous patients. (A) NE, E, NMN, DHBA, MN and DA standards; (B) urine from a subject at recent onset of disease; (C) urine from a subject in the stable phase.
Determination of Neutral and Acid Monoamine Metabolites
Urinary metabolite concentrations, after purification by extractive procedures, were evaluated by HPLC-ED, according to the methods described by Santagostino et al. (15) for MHPG, and by Frattini et al. (16) for VMA, HVA and 5-HIAA.
Statistical Analysis
All statistical analyses were performed using the SPSS statistical package (SPSS, Inc., Chicago, IL, USA). One way variance analysis was carried out to evaluate differences in biochemical parameter values between controls and patients, who were grouped according to phases of vitiligo or according to disease duration for active vitiliginous patients. Student–Newman–Keuls (SNK) multiple comparison test (0.05 significance level) was performed ‘a posteriori’ when the F statistic was significant.
Student's t-test for unpaired data and the Pearson product–moment correlation (r) were also used.
Results
Urinary monoamine and metabolite levels were not significantly different between males and females (t-test for unpaired data), nor did they correlate with the subjects' age both in controls and in vitiliginous patients. In addition, no significant differences were found between the urinary values of subjects with generalized and acrofacial vitiligo. Therefore all vitiliginous patients were considered as a whole.
Concentrations of NE, DA, NMN, MN, MHPG, VMA and 5-HIAA were found to be significantly higher in patients than in controls. E and HVA also showed a trend towards increased levels in the patient sample (Table 1).
| Vitiligo patients n = 70 | Controls n = 35 | t-Value df = 103 | P-value | |
|---|---|---|---|---|
| NE | 74 ± 3 | 50 ± 2 | 4.92 | <0.001 |
| E | 6 ± 1 | 5 ± 1 | 1.97 | 0.052 |
| DA | 546 ± 41 | 295 ± 10 | 4.31 | <0.001 |
| NMN | 273 ± 17 | 163 ± 5 | 4.60 | <0.001 |
| MN | 183 ± 13 | 116 ± 3 | 3.47 | 0.001 |
| MHPG | 1448 ± 32 | 1350 ± 25 | 2.01 | 0.047 |
| VMA | 5638 ± 197 | 4458 ± 134 | 3.99 | <0.001 |
| HVA | 5233 ± 234 | 4637 ± 163 | 1.70 | NS |
| 5-HIAA | 7796 ± 378 | 5079 ± 137 | 4.99 | <0.001 |
- NS, not significant.
When the patients were subdivided according to disease phases and compared with controls, anova indicated significant differences between values of all the compounds examined, except for MHPG. ‘Post-hoc’ tests showed that: (a) urinary NE levels in patients in an active phase were significantly higher than for patients in a stable phase or in controls; (b) E, DA, NMN, MN and HVA levels were significantly higher in patients in an active phase than in controls; (c) VMA and 5-HIAA levels were significantly higher in patients in both an active and a stable phase than in controls (Table 2).
| Active phase n = 56 | Stable phase n = 14 | Controls n = 35 | F 2/102 df | P-value | |
|---|---|---|---|---|---|
| NE | 78 ± 3a,b | 60 ± 9 | 50 ± 2 | 16.02 | <0.001 |
| E | 6 ± 1a | 5 ± 1 | 5 ± 1 | 4.23 | 0.017 |
| DA | 570 ± 41a | 447 ± 121 | 295 ± 10 | 10.46 | <0.001 |
| NMN | 286 ± 19a | 223 ± 29 | 163 ± 5 | 12.56 | <0.001 |
| MN | 190 ± 16a | 156 ± 26 | 116 ± 3 | 6.77 | 0.0017 |
| MHPG | 1455 ± 30 | 1421 ± 111 | 1350 ± 25 | 2.11 | NS |
| VMA | 5653 ± 218a | 5576 ± 482a | 4458 ± 134 | 7.91 | <0.001 |
| HVA | 5458 ± 281a | 4337 ± 196 | 4637 ± 163 | 4.04 | 0.02 |
| 5-HIAA | 7916 ± 455a | 7314 ± 524a | 5079 ± 137 | 12.68 | <0.001 |
- ‘Post-hoc’ Student–Newman–Keuls test (significance level: P < 0.05): avs. control values; bvs. stable-phase values. NS, not significant.
Among the patients in an active disease phase (n = 56), those with more recent onset of disease (<1 yr; n = 16/56) showed higher urinary excretion values than other patients for all the parameters considered (Table 3). In particular, the statistical analyses indicated that NE values were significantly different to those of subjects affected from 1 to 5 yr as well as from 6 to 10 yr. These data were also confirmed by the significant negative relationship found between NE excretion values and disease duration (r = −0.38, n = 56, P < 0.01).
| <1 yr n = 16 | 1–5 yr n = 20 | 6–10 yr n = 12 | >10 yr n = 8 | F 3/52 df | P | |
|---|---|---|---|---|---|---|
| NE | 93 ± 9 | 76 ± 3a | 68 ± 5a | 67 ± 6 | 3.53 | 0.021 |
| E | 8 ± 1 | 6 ± 1 | 5 ± 1 | 6 ± 1 | 2.45 | NS |
| DA | 670 ± 84 | 496 ± 45 | 540 ± 70 | 603 ± 182 | 1.01 | NS |
| NMN | 343 ± 52 | 259 ± 20 | 270 ± 40 | 261 ± 36 | 1.19 | NS |
| MN | 241 ± 42 | 172 ± 18 | 185 ± 30 | 138 ± 17 | 1.80 | NS |
| MHPG | 1492 ± 68 | 1467 ± 56 | 1419 ± 30 | 1404 ± 60 | 0.40 | NS |
| VMA | 6019 ± 402 | 5905 ± 412 | 4976 ± 305 | 5304 ± 607 | 1.26 | NS |
| HVA | 6024 ± 604 | 5700 ± 495 | 5190 ± 498 | 4120 ± 375 | 1.68 | NS |
| 5-HIAA | 8754 ± 937 | 8100 ± 743 | 8031 ± 975 | 5610 ± 801 | 1.62 | NS |
- ‘Post-hoc’ Student–Newman–Keuls test (significance level: P < 0.05): avs. <1 yr values. NS, not significant.
In both vitiligo and control groups, significant positive correlations were found between monoamines (NE vs. E) as well as between these monoamines and their metabolites (NMN, MN or VMA). The main metabolites of dopaminergic and serotoninergic systems also correlated (Table 4).
| NE | E | DA | NMN | VMA | 5-HIAA | |
|---|---|---|---|---|---|---|
| NE | 1 | |||||
| E | 0.62 ** (0.59**) | 1 | ||||
| DA | 0.60 ** (NS) | 0.44 ** (NS) | 1 | |||
| NMN | 0.53 ** (0.61**) | 0.52 ** (NS) | 0.52 ** (NS) | 1 | ||
| MN | 0.60 ** (0.57**) | 0.74 **(0.40*) | 0.45 ** (NS) | 0.71 ** (0.45**) | 0.29 * (NS) | |
| VMA | 0.38 ** (0.49**) | 0.31 * (NS) | NS | NS (0.41*) | 1 | NS |
| MHPG | NS | NS | NS | NS | 0.40 ** (NS) | NS |
| HVA | NS | NS | NS (0.39*) | NS | NS | 0.70 ** (0.52**) |
- Patient values are in bold type; control values are in brackets. *P < 0.05; **P < 0.01; NS, not significant.
Discussion
Present data indicate that vitiliginous patients, irrespective of the type of distribution of depigmentation, had significantly increased levels of urinary monoamines and metabolites than healthy subjects, except for E and HVA. This increase was more pronounced in patients during the active phase for whom all the compounds evaluated, except MHPG, were significantly higher than in controls. In these subjects, NE excretion was also more elevated than in patients during the stable phase of the disease. In particular, the highest urinary concentrations of monoamine and metabolites were found in patients having a recent onset of the disease (<1 yr) (Table 3).
There are few data concerning the evaluation of catecholamines and their metabolites in the urine of vitiligo patients. For example, Morrone et al. (8), found urinary levels of HVA and VMA which were between four and 10 times higher in subjects at the onset of disease or with a progressive increase in both the number and/or size of previous lesions, than in controls. In our sample, the increase in the monoamines NE, E and DA and their catechol-O-methyltransferase (COMT)-derived metabolites NMN and MN was double in subjects with the highest excretion values (those at recent onset of the disease) compared with controls, and nearly half for the other metabolites, including VMA and HVA. Methodological differences between the studies could explain these results: it is well known that both physiological factors and dietary habits, such as coffee, tea, sodium intake, not to mention smoking and physical activity can modify the urinary excretion of catecholamines and metabolites (13). In Morrone's study, these factors were not explicitly considered and this may, at least partially, explain the elevated levels of final catecholamine metabolites observed. In addition, nearly one-third of the examined patients were affected by segmental vitiligo, a subtype of the disease in which some dysfunctions of the sympathetic nervous system in the skin may play an important role. Nevertheless, all our subjects were of the non-segmental type, where other mechanisms could prevail (17).
Recently, we observed that urinary excretion of NE was significantly increased in vitiliginous subjects of the non-segmental type during the active phase of the disease (18), which is a finding consistent with the increases of NE and some metabolites observed previously in the plasma of patients of the same type (12). In contrast, Schallreuter et al. (9) noted increased urinary levels of NE with vitiligo in only one-third of the patients (31%), while E-values were in the normal range. These authors, however, did not specify the phase of the disease: this could be the underlying reason for the low percentage observed in patients with increased NE excretion.
Could the faulty activity of some enzymes involved in catecholamine metabolism explain the increases in urinary levels observed in our study? Indeed, decreased activity by phenylethanolamine-N-methyl transferase (PNMT), which is the enzyme for conversion of NE to E, together with increased activity by monoamino oxidase A and COMT, the major metabolizing enzymes of catecholamines, have been found in the keratinocytes and melanocytes of vitiliginous subjects (9, 19, 20). In addition, an excessive de novo synthesis of (6R) 5,6,7,8 tetrahydrobiopterin(6-BH4), an essential cofactor of tyrosine hydroxylase, which is the key enzyme for the biosynthesis of catecholamines, was observed in epidermal extracts from lesional and non-lesional skin of patients with vitiligo. This resulted in the overproduction of NE in the keratinocytes of the same patients (9).
However, at present, there are no experimental data demonstrating that a significant part of urinary catecholamines or their metabolites is derived from specific cutaneous cells. Moreover, the alteration of a single metabolizing enzyme activity should modify urinary levels of compounds to varying degrees, i.e. if PNMT activity is diminished, then a decrease in E should match the increase in NE. Instead, our results seem to indicate that in the active phases of vitiligo, a quite generalized increase in urinary catecholamines and metabolites occurs, probably due to an increase in catecholamine turnover.
It is generally believed that levels of catecholamines and its metabolites in biological fluids are a reliable index of sympathetic and/or adrenomedullary activity (21). Hence, the moderate, albeit widespread, increases observed in our study support the hypothesis that the onset and active phase of the disease could be associated with increased activity by the sympathetic nervous and/or adrenomedullary systems. This inference is confirmed by recent studies on sympathetic skin responses (22), plasma NE and E, cutaneous blood flow and adrenoceptor response in stable-stage vitiligo (23). All these functions were not significantly different in the depigmented and normal skin of the same vitiliginous subject nor did they differentiate in healthy and vitiliginous subjects; only the patients suffering from the segmental type disease showed a significant increase in cutaneous α- and β-adrenoceptor response.
It is well known that stressful events are strictly associated not only with increased plasma catecholamine but also plasma corticosteroid levels. Indeed, altered plasma levels of these parameters were also observed in several stressful skin diseases such as psoriasis (24–26), cystic acne (27) and alopecia areata (28). In particular, higher serum corticosteroid levels and higher values of tyrosine aminotransferase (TAT) activity were found in vitiliginous subjects than in controls (10). Interestingly, TAT activity was higher in the newer rather than older cases. Because TAT activity also is increased in stressful situations (29) it appears reasonable that in the early active and progressive phases of vitiligo, a stress status, probably as a reaction to the appearance of the lesions, may be present. Moreover, the gradual decrease in monoamine and metabolite levels [including 5-HIAA, as already noted by Roychowdhury and Chakraborty (30)] and significant negative relationships between the excretion values of some monoamines and metabolites and the duration of vitiligo observed in our study indicate that the psychological impact of the disease fades over time. Thus, higher levels of urinary catecholamines found in the active phases of non-segmental vitiligo seem to be a consequence rather than a cause of the disease.
Nevertheless, an increase in catecholamine release from autonomic nerve endings might contribute, in the early phase of disease, to the impairment of melanocytes by both direct inhibition of tyrosinase activity and induction of TAT activity. TAT in vivo causes tyrosine deamination and yields p-hydroxyphenylpyruvic acid which in turn is a TAT inhibitor (10). In addition, catecholamines are easily oxidated by different oxidative systems with the formation of quinones, semiquinone radicals and oxyradicals, such as hydrogen peroxide, which is toxic for melanocytes (31, 32). Thus, links with the ‘biochemical’ theory (the defective recycling of (6R)-l-erythro 5,6,7,8 tetrahydrobiopterin leading to defective catecholamine biosynthesis and the formation of micromolar levels of hydrogen peroxide, which is toxic for melanocytes) (4) and with the autoimmune theory (where a damaged melanocyte triggers an autoimmune reaction bringing on its own destruction) (2) can be noted.
Even if the variations observed in catecholamine turnover are quite slight, it should be kept in mind that vitiliginous melanocytes have an intrinsic defect in their oxygen radical protection system (33). Low catalase levels (in association with hydrogen peroxide accumulation), low glutathione peroxidase activity in patients up to 46 yr and reduced activity by several detoxifying enzymes 11 (34), have in fact been reported in the epidermis and blood of vitiliginous subjects.
In conclusion, a moderate but significant increase in some monoamines and their metabolites has been found in the urine of patients affected by non-segmental vitiligo. In particular, higher levels were observed in subjects at the recent onset of disease indicating that stress related to the appearance of lesions may play a role. However, considering that catecholamines readily produce quinones, semiquinone radicals and oxyradicals and that in vitiliginous patients free-radical defence mechanisms are faulty, an increase in monoamine discharge might contribute to melanocyte damage in the early phases of the disease.




