Links between rhinitis and asthma
Abstract
There is compelling evidence of a close relationship between the upper and lower airways in asthma and rhinitis. Rhinitis is present in the majority of patients with asthma, and a significant minority of patients with rhinitis have concomitant asthma. Similarities between the two conditions occur in the nature of the inflammation present in the target tissues. A common initiating step in the inflammatory process of allergic airways disease is the presence of immunoglobulin E providing an adaptor molecule between the offending allergen and inflammatory cell activation and mediator release. Differences in the two conditions arise largely from the structural differences between the nose and the lungs. In an asthmatic, concomitant allergic rhinitis increases healthcare costs and further impairs quality of life. The presence of rhinitis should always be investigated in children and young adults with asthma. Subjects with allergic rhinitis have an increased risk of developing asthma and may form a suitable population for secondary intervention to interrupt the ‘allergic march’.
Asthma and allergies, including rhinoconjunctivitis and atopic dermatitis, are common throughout the world, with a high burden of morbidity and cost. As the nasal and bronchial mucosa has similarities and most patients with asthma also have rhinitis (1–3), the concept of ‘one airway, one disease’ has arisen. However, not all patients with rhinitis present with asthma and there are some differences between the two conditions (4).
Epidemiological evidence
Relationship between rhinitis and asthma
Epidemiological evidence consistently demonstrates the frequent coexistence of asthma and rhinitis in the same patients.
Asthma is present in 20–50% of patients with rhinitis (5–8). In one study, 20 000 households were screened for symptoms of rhinitis and 84% (16 786 households) responded (9). Patients qualified as having perennial rhinitis if they had at least two rhinitis symptoms regularly for at least 6 months of the year, without any period of remission. Allergic associations were not looked for. The point prevalence of perennial rhinitis was 4.1%. Perennial rhinitis was strongly associated with a history of asthma [13.4% of patients with perennial rhinitis vs 3.8% of patients without; odds ratio (OR) 3.26].
The majority of patients with asthma present with symptoms of seasonal or perennial allergic rhinitis. However, it has been shown that perennial rhinitis is a risk factor for asthma independent of allergy. In epidemiological studies, rhinitis was found to occur in up to 80% of patients with asthma (7). In a group of 1245 subjects with documented asthma, 24% had seasonal allergic rhinitis only, 6% had perennial allergic rhinitis only and 22% were considered to have both (7). In some large clinical studies rhinitis is present in nearly all the patients with asthma; in one study over 95% had a history of allergic rhinitis and 73% had current seasonal allergies (10). Leynaert et al. analysed data from 34 centres participating in the European Community Respiratory Health Survey (7) using a random sample of 20–44-year-old subjects. Subjects were diagnosed with perennial rhinitis if they had any nasal allergies, including hay fever, and symptoms in the presence of animals, feathers (quilts, etc.) or in dusty areas of the house. Subjects with perennial rhinitis (n = 1412) were more likely to have concurrent asthma than control subjects (n = 5198). After adjusting for sex, age, smoking habit, family history of asthma, geographical area, and season at the time of examination, asthma was strongly associated with rhinitis not only among atopic subjects [OR = 8.1; 95% confidence interval (CI) = 5.4, 12.1] but also among nonatopic subjects (OR = 11.6; 95% CI = 6.2, 21.9). The strong association between perennial rhinitis and asthma in nonatopic subjects remained when the analysis was restricted to atopic or nonatopic subjects with immunoglobulin E (IgE) levels of 80 kIU/l or less. These results are consistent with the hypothesis that rhinitis is an independent risk factor for asthma. Data from northern Sweden also demonstrate the association between asthma and allergic rhinitis, and show that an adult with a family history of asthma or rhinitis has a three- to four-fold increased risk for developing asthma and a two- to six-fold increased risk for developing rhinitis compared with adults without a family history (11). The Copenhagen Allergy Study (12) investigated the frequency of asthma and rhinitis related to exposure to pollens, animal dander or mites. For people with pollen allergy, 41% of those with pollen-related rhinitis also had pollen-related asthma. Pollen-related asthma was almost nonexistent (0.1%) in those without pollen-related rhinitis. For the other allergens, the respective figures were 52 and 0.2% in subjects with animal dander allergy, and 41 and 1% for mite allergy. In all cases, therefore, more than 99% of subjects with allergic asthma also had allergic rhinitis. The risk of asthma among subjects with allergic rhinitis was calculated to be up to 300 times that among subjects without allergic rhinitis.
However, the results observed in developing countries may differ from those in western populations. A recent study showed that allergic rhinitis is far less common among asthmatic subjects in rural China than in asthmatic subjects in industrialized countries with a western lifestyle (13).
Rhinitis and nonspecific bronchial hyperresponsiveness
Many patients with allergic rhinitis have a physiological behaviour distinct from that of patients with asthma or normal subjects. They have increased bronchial sensitivity to methacholine or histamine (14, 15), especially during and slightly after the pollen season (16, 17). However, there are large differences in bronchial responsiveness between patients with asthma and patients with rhinitis that are not explained by the allergen type or IgE levels (18). In addition, the bronchial mucosa is much more responsive in asthma (compared with controls) than the nasal mucosa is in allergic rhinitis, probably for structural reasons (19).
Causative agents in rhinitis and asthma
Among the causative agents inducing asthma and rhinitis, some [e.g. allergens and aspirin (20)] affect both the nose and the bronchi. Most inhaled allergens are associated with nasal (5) and bronchial symptoms but in epidemiological studies differences have been observed. Although reservations have been expressed about the role of allergen exposure as the primary major cause of asthma (21), the prevalence of IgE sensitization to indoor allergens (house dust mites and cat allergens) correlates positively with both the frequency of asthma and its severity (22, 23). Alternaria (24, 25) and insect dusts (26) have also been found to be linked with asthma, but pollen sensitivity has not been found to be associated with asthma in epidemiological studies (27, 28). On the contrary, pollen sensitivity is always associated with rhinitis (5).
Occupational diseases represent an interesting model to study the relationships between rhinitis and asthma. Subjects with occupational asthma often report symptoms of rhinoconjunctivitis. The most common triggers of occupational asthma can also induce occupational rhinitis:
- •
Isocyanates (29, 30).
- •
Flour and grain. Bakers often present with rhinitis and asthma (31). In the 1970s and 1980s Swedish bakers were found to have a higher (× 2) risk of developing rhinitis than nonbakers (32). Grain handlers also present rhinitis, even in developing countries (33).
- •
Wood dust can induce rhinitis and asthma but the mechanisms for these reactions are still unclear (34, 35).
- •
Glutaraldehyde (36).
- •
Solder/colophony (37, 38).
- •
Laboratory animals (39–41).
- •
Resins and glues (37, 42, 43).
- •
Latex (44, 45).
- •
Persulphates (46).
Rhinitis is less pronounced than asthma with low molecular weight agents. However, rhinitis often appears before asthma in the case of high molecular weight agents such as those derived from small mammals (47–49), raw green beans (50), flour (51, 52) or latex (53, 54). In many patients, nasal symptoms occur before bronchial symptoms, suggesting that it may be possible to prevent the development of asthma. In addition, rhinitis caused by some low molecular weight agents is associated with or develops into occupational asthma (55–58), highlighting the importance of cessation of allergen exposure in occupational allergic rhinitis in order to prevent asthma.
’Bidirectional’ relationship between nasal and bronchial inflammation: bronchial challenge of rhinitis patients leads to nasal inflammation (and vice versa)
To analyse nasobronchial cross-talk, inflammation and the expression of adhesion molecules was studied in nasal and bronchial mucosa after allergen provocation. In one study, endobronchial allergen challenge induced nasal and bronchial symptoms as well as reductions in pulmonary and nasal function (59). In this study, the number of eosinophils increased in the challenged bronchial mucosa, in the blood and in the nasal mucosa 24 h after bronchial challenge (Fig. 1). Moreover, eotaxin-positive cells in the nasal lamina propria and enhanced expression of interleukin (IL)-5 in the nasal epithelium were found 24 h after bronchial challenge.
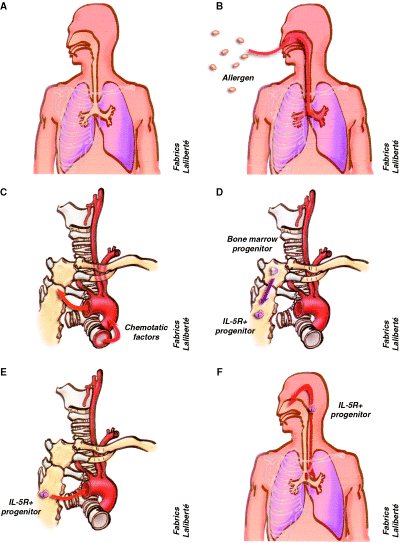
Local and systemic inflammation in allergic rhinitis. (A) anatomic links between the nose and lower airway, (B) inhalation of allergens leads to a local inflamatory reaction in the nose and bronchi due to an IgE-mediated reaction, (C) during the IgE-mediated inflammatory reaction chemokines, cytokines and other chemotactic factors are released into the circulation, (D) these factors act on the bone marrow to induce the differentiation of eosinophil/basophil progenitors, (E) the IL5 receptor-positive progenitor cells circulate into the peripheral blood, (F) and reach the upper and lower airways leading to the concept of systemic inflammation.
In a second study, bronchial and nasal biopsy specimens were taken before and 24 h after nasal provocation (60). At 24 h, an influx of eosinophils was detected in the nasal epithelium and lamina propria, as well as in the bronchial epithelium and lamina propria. Increased expression of ICAM-1, as well as increased percentages of ICAM-1+, VCAM-1+, and E-selectin+ vessels, were seen in both nasal and bronchial tissue of patients with allergic rhinitis. The number of mucosal eosinophils correlated with the local expression of ICAM-1, E-selectin, and VCAM-1 in these patients.
These studies show that allergen provocation of either the nose or the bronchi results in generalized airway inflammation (60).
Bronchial inflammation in rhinitis
Bronchial biopsies in rhinitis
Some studies have examined the bronchial mucosa in atopic nonasthmatic patients or in patients with allergic rhinitis. They have shown a slight increase in the size of the basement membrane (61) and a moderate eosinophilic inflammation (62) in patients with allergic rhinitis.
Natural exposure to pollen during the season provokes an increase in airway responsiveness in nonasthmatic subjects with seasonal allergic rhinitis and also induces inflammatory cell recruitment and IL-5 expression, leading to bronchial inflammation (63).
Bronchial allergen challenge in rhinitis
Patients with seasonal rhinitis who had no history of asthma developed a bronchoconstriction on endobronchial allergen challenge. Lavage carried out after challenge demonstrated the presence of pro-inflammatory mediators and cytokines as well as the recruitment of inflammatory cells (64, 65).
Pulmonary inflammation after segmental ragweed challenge was examined in allergic asthmatics and nonasthmatics (66). A total of 46 ragweed-allergic subjects took part in these studies. The subjects had normal or near-normal pulmonary function, were not on chronic medication, and were characterized by their skin sensitivity to intradermal ragweed injection, their nonspecific responsiveness to methacholine, and the presence (or absence) of a late asthmatic response after whole-lung antigen challenge. Contrary to expectations, the pulmonary inflammatory response, 24 h after segmental ragweed challenge, was the same in subjects with rhinitis as it was in subjects with asthma and rhinitis, as measured by numbers of total cells, macrophages, lymphocytes, eosinophils, and neutrophils in the bronchoalveolar lavage fluid (BAL). Thus, the lungs seem equipped to produce an IgE-mediated inflammatory response in allergic subjects whether asthma is clinically evident or not.
Thunderstorm-induced asthma
Bronchial challenge studies indicate that patients with nasal symptoms can only react if the allergen is properly administered into the airways. It may be argued that the doses of allergen inducing bronchial reactions are far greater than those naturally occurring during allergen exposure, but abnormal bronchial exposure appears to exist in thunderstorm-induced asthma (67–70). This has been associated with grass pollen allergy (67, 71, 72) and has recently been linked to greatly increased ambient concentrations of pollen grains caused by thunderstorm outflows (73). As the aerodynamic size of pollen grains ranges from 10 to 100 μm and only a fraction of them can be deposited into the bronchi, most patients only present with rhinitis. However, when exposed to water, pollen allergens are released in submicronic particles, the starch granules, which can reach the lower airways and induce asthma (74). An Australian study showed that subjects with a prior history of allergic rhinitis were at significantly increased risk of asthma exacerbations during thunderstorms (OR 6.01), confirming the close relationship between upper and lower airways (75).
Viral infection of the nose induces asthma and bronchial inflammation
A large number of asthma exacerbations, in both children and adults, are caused by nasal viral infections (76). Rhinoviruses are the major cause of common cold and a trigger of acute asthma exacerbations (77). Experimental upper respiratory (intranasal) rhinovirus infection increases airway hyperreactivity and late asthmatic reactions following allergen challenge (78). Rhinoviruses can also infect the lower airways during natural and experimental intranasal exposure (79–81). These findings suggest that asthma exacerbations may be partly induced through viral enhancement of lower airway inflammation following nasal viral infection.
Nasal and sinus inflammation in asthma
Similarities and differences between nasal and bronchial inflammation in asthma
In normal subjects, the structure of the airway mucosa shows similarities between the nose and the bronchi. Both nasal and bronchial mucosa are characterized by a pseudostratified epithelium with columnar, ciliated cells resting on a basement membrane. In the submucosa, vessels, mucous glands, structural cells (fibroblasts), some inflammatory cells (essentially monocytic cells, lymphocytes, and mast cells) (82, 83) and nerves are present.
There are also differences between the nose and the bronchi. The nose is richly supplied with a subepithelial capillary and arterial system and venous cavernous sinusoids. This rich vascularization is a key feature of the nasal mucosa and changes in the vasculature may lead to severe nasal obstruction (84). On the contrary, the bronchi are characterized by the presence of smooth muscle from the trachea to the bronchioles, accounting for the bronchoconstriction of asthma (85).
Recent advances in the understanding of the cellular and molecular biology of airways diseases have clearly shown that inflammation plays a critical role in the pathogenesis of asthma and rhinitis. The same inflammatory cells appear to be present in the nasal and bronchial mucosa (86). A growing number of studies show that the inflammation of nasal and bronchial mucosa is sustained by a similar inflammatory infiltrate, comprising eosinophils, mast cells, T-lymphocytes, and cells of the monocytic lineage (86–89). In addition, the same pro-inflammatory mediators (histamine and cysteinyl leucotrienes), Th2 cytokines (IL-4, IL-5, IL-13, GM-CSF) (86, 90–92), chemokines (RANTES and eotaxin) (93) and adhesion molecules (94–96) appear to be involved in both nasal and bronchial inflammation in patients with rhinitis and asthma.
However, there are major differences between the two sites. Although the bronchial and, especially, the nasal mucosa are exposed to the same environmental insults, epithelial shedding is more pronounced in the bronchi than in the nasal mucosa of patients suffering from both asthma and rhinitis (97). The intensity of the inflammation may not be identical. In patients with moderate–severe asthma, eosinophilic inflammation is more pronounced in the bronchi than in the nasal mucosa (97), whereas in patients with mild asthma inflammation appears to be similar in both sites. Moreover, eosinophilic inflammation of the nasal mucosa exists in asthma patients without any symptoms (98). However, the remodelling of the airways that can be found in the bronchial mucosa appears to feature less extensively in the nasal mucosa.
Sinus involvement in asthma
While the coexistence of asthma and rhinosinusitis has been recognized in the medical literature for many years (99), the question of whether rhinosinusitis is a precipitating factor for bronchial asthma is still debated. Current knowledge suggests that rhinosinusitis and asthma are linked by a common process that is mainly inflammatory, with eosinophils and airway epithelium having a central pathogenetic role. The association of chronic rhinosinusitis with asthma and allergy appears to be restricted to the asthmatic population with an extensive sinonasal disease, and the presence of peripheral eosinophilia in patients with rhinosinusitis indicates a high likelihood of extensive sinus disease (100). In a study assessing sinonasal involvement in patients with either mild–moderate or severe (corticosteroid-dependent) asthma, the proportion of patients with symptoms of rhinosinusitis was similar in both groups (74% in corticosteroid-dependent asthma and 70% in mild–moderate asthma) (101). All corticosteroid-dependent asthmatics had sinus abnormalities on computed tomography (CT) scan compared with 88% of the mild-moderate asthmatics. The clinical and CT scan scores were higher in the corticosteroid-dependent asthmatics, indicating a relationship between severity of asthma and the features of sinus disease. In both groups, the CT scan scores were correlated to the clinical scores (101). In another recent study examining sinus CT scans of patients with severe asthma, the same close relationship was observed (102). Moreover, in this study, it was shown that chronic sinusitis in severe asthma is related to sputum eosinophilia suggesting that the extent of inflammation in the nasal mucosa is related to lung function and inflammation in the bronchial mucosa in patients with severe asthma. The high prevalence of sinus disease in both groups provides further evidence of the close relationship between the upper and lower airways in asthma.
In contrast to patients with asthma, <10% of patients with chronic obstructive pulmonary disease (COPD) have nasal symptoms. Mucosal biopsies do not usually detect any nasal inflammation in these patients (103) and CT scans show few abnormalities. Thus, the nasal and sinus inflammation in asthmatics seems to be related specifically to asthma and is not a feature of all bronchial diseases (101).
Allergic inflammation is both local and systemic
Allergic responses involve a cascade of inflammatory events, which link reactive tissues in the target organ to the systemic disorder. Allergen-specific IgE antibodies together with mast cells and basophils play a central role in initiating the inflammatory cascade. In addition, two major mechanisms have been demonstrated to contribute to the increased number of eosinophils in the inflamed airways of allergic subjects: recruitment and persistence of inflammatory cells into the airways, and the presence of bone marrow progenitors in the inflamed airway tissues.
The role of IgE
Immunoglobulin E antibody production is part of the body's immune response to parasitic invasion, especially through the skin or mucous membranes (104). In allergic individuals, this protective mechanism is misdirected against noninvasive elements that come into contact with these surfaces. Allergic asthma and rhinitis are commonly associated with raised circulating levels of IgE (105), and the increased presence of total serum IgE is a risk factor for asthma even in nonallergic individuals (106). Following sensitization, IgE antibodies produced by B-cells bind to the high-affinity receptors on mast cells, basophils and some other cell types. Mast cell accumulation in the airway mucosa is an important pathophysiological event in allergic rhinitis and asthma as inhaled allergens impact the mucosal surfaces of the nose and/or lungs. The common initiating step in the acute processes of the allergic inflammatory cascade is allergen cross-linking of specific IgE molecules bound to the mast cells, which triggers the release of mediators such as histamine, leucotrienes and platelet activating factor (107). The immediate response depends on the structure of the target organ: typically, itching, sneezing, rhinorrhoea, and blockage in the nose (108), with bronchoconstriction and wheeze caused by smooth muscle contraction in the lungs.
Late phase allergic reactions, and chronic inflammatory changes in the asthmatic lung which involve T cells as well as mast cells and eosinophils, are less understood (107). Further involvement of IgE is implicated; however, through the array of pro-inflammatory cytokines derived from mast cells, including the Th2-type cytokines IL-4 and IL-13 that may account for localized B-cell IgE production in the airway tissues (109–111). In addition, antigen presenting and secondary inflammatory cells, including macrophages, dendritic cells, and eosinophils, are known to express the high-affinity IgE receptor in both asthma and allergic rhinitis (112–114).
Interestingly, it has been shown that IgE is produced in the local lymphoid tissues and locally in both the nasal and bronchial mucosa (115–116). There is a local synthesis of ɛ-germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure (117), a persistent IgE synthesis in the nasal mucosa of hay fever patients (118) and an increase in allergen-specific IgE in the BAL after segmental allergen challenge in atopic asthma patients (119). A local expression of ɛ-germline gene transcripts and RNA for the ɛ heavy chain of IgE was found in the bronchial mucosa in atopic and nonatopic asthmatics (111).
Bone marrow involvement
In patients with allergic diseases, allergen provocation can activate a systemic response that provokes inflammatory cell production by the bone marrow (120). There is considerable evidence in animal models and humans that the bone marrow plays an integral role in allergic inflammation (121). In response to allergen exposure in the airway, bone marrow (white blood cell) progenitors proliferate and differentiate, which leads to persistent increases in eosinophil numbers. Signalling between the lung and bone marrow after allergen exposure further suggests that allergy is a systemic disease. Although the nature of the signal-mediating activation of the bone marrow after airway allergen exposure is unknown, several pathways have been implicated, including allergen-induced haemopoietic growth factors, cell trafficking, and stimulation of resident bone marrow cells. A common thread in all these pathways is the importance of IL-5 (114).
After release and differentiation of progenitor cells, eosinophils, basophils and mast cells are typically recruited to tissues in atopic individuals. An understanding of the signalling process at the molecular level that leads to these interactions between the bone marrow and the target organ, especially the airways, may open up new avenues of therapy for allergic inflammatory disease (122, 123). Studies that support the critical involvement of the bone marrow in the development of eosinophilic inflammation of the airways indicate the systemic nature of these conditions.
In situ haemopoiesis
Another important mechanism, termed ‘in situ haemopoiesis’ (124), depends on the production of haemopoietic cytokines by inflamed airway tissues from patients with allergic rhinitis (125–127) and nasal polyposis (128). By generating a particular local ‘microenvironment’, these cytokines promote the differentiation and maturation of eosinophil progenitors that populate the nasal or bronchial mucosa (129, 130). It is therefore more likely that a truly systemic response to the application of inflammatory stimuli to the airway mucosa should be associated with an activation of the aforementioned mechanisms.
The ’allergic march’
Atopic dermatitis, asthma, and allergic rhinitis are common comorbidities. Skin symptoms and food allergy are generally the first allergic symptoms to occur in life. Asthma is often present by the second or third year but rhinitis appears to develop later, in particular pollen-induced rhinitis (131, 132). However, allergic rhinitis is not easily diagnosed in infants, making epidemiological studies difficult in young children. A recent study following 94 young children with atopic dermatitis showed a good prognosis for dermatitis (improvement in 87%) but an increased risk of developing asthma (in 43% of the children) or allergic rhinitis (in 45%) before 7 years of age (133). The risk of developing either form of respiratory allergy was especially high in children at an early age with specific IgE to a panel of foods. An increased risk of allergic airway disease associated with atopic dermatitis in early infancy has been shown in other studies (134), and it has been suggested that infants with early signs of atopic dermatitis and a family history of atopic disease are candidates for intervention to prevent the development of respiratory allergies.
In the International Study of Asthma and Allergies in Children (ISAAC), it was difficult to compare rhinitis and asthma in the younger age groups (6–7-year olds) as rhinitis often starts later in life than asthma (131–133). In adolescents in New Zealand, asthma is often more common than rhinitis, but in the same country lower prevalences of asthma are associated with lower prevalences of rhinoconjunctivitis (135). The age of onset of atopy may be an important confounding factor for the development of asthma and rhinitis or rhinitis alone. In an Australian study, it was found that atopy acquired in early life (before the age of 6 years) is an important predictive factor for asthma continuing into late childhood, whereas atopy acquired later was strongly associated with seasonal allergic rhinitis only (136, 137).
Allergic rhinitis as a risk factor for asthma
Asthma develops more commonly in patients with perennial rhinitis. The Children's Respiratory Study (6) showed that the presence of physician-diagnosed allergic rhinitis in infancy was independently associated with a doubling of the risk of developing asthma by 11 years of age. In adults, allergic rhinitis as a risk factor for asthma was shown in a 23-year follow-up of college students (138). Significantly more (10.5%) of the students originally diagnosed with allergic rhinitis went on to develop asthma compared with 3.6% of those who did not have rhinitis.
This study was recently confirmed by two other studies. A study examined risk factors for onset and remission of allergic rhinitis and asthma in Swedish adults (139). The onset of asthma was associated with allergic rhinitis (OR = 4.9), sensitization to pets (OR = 2.4), and smoking (OR = 3.0). The onset of asthma was strongly associated with allergic rhinitis among atopics (OR = 5.7), but the onset of asthma and rhinitis also tended to be related among nonatopics (OR = 3.5). A strong association between smoking and onset of asthma was found among nonatopics (OR = 5.7).
In a longitudinal community population, the extent to which rhinitis is an independent risk factor for adult-onset asthma was examined (140). Rhinitis was a significant risk factor for asthma (crude OR, 4.13; 95% CI, 2.88–5.92). After adjustment for years of follow-up, age, sex, atopic status, smoking status, and presence of COPD, the magnitude of the association was reduced but still highly significant (adjusted OR, 3.21; 95% CI, 2.19–4.71). After stratification, rhinitis increased the risk of development of asthma by about three times both among atopic and nonatopic patients and by more than five times among patients in the highest IgE tertile. Patients with rhinitis with persistent and severe nasal symptoms and a personal history of physician-confirmed sinusitis had an additional increased risk of asthma development. The authors concluded that rhinitis is a significant risk factor for adult-onset asthma in both atopic and nonatopic subjects.
It is, however, not clear whether allergic rhinitis represents an earlier clinical manifestation of allergic disease in atopic subjects who later develop asthma or if the nasal disease itself is a causative factor in asthma. Both are reasonable hypotheses, and the results of the study described earlier (7) – showing that perennial rhinitis is an important risk factor for asthma in atopic and nonatopic subjects – support both theories. As only a proportion of patients with allergic rhinitis develop asthma, it seems that the presence of an additional, as-yet unidentified ‘lung factor’ is a prerequisite. In clinical terms, additional criteria will be required to strengthen the power of a predictive tool for developing asthma (141).
Nevertheless, it will be interesting to know if treatment of a subject with allergic rhinitis can achieve prevention against the secondary development of asthma. A reduction in raised serum levels of IgE that are typically part of the atopic phenotype would be of interest in this regard, and anti-IgE may therefore be a candidate for this type of prophylaxis.
Rhinitis and asthma: a continuum of disease
There are similarities and differences between nasal and bronchial mucosa in rhinitis and asthma. It appears that most asthmatics also present with rhinitis, whereas only a fraction of rhinitis patients present with clinically demonstrable asthma (although a higher proportion of patients have nonspecific bronchial hyperresponsiveness). It seems that the epithelial-mesenchymal trophic unit exists from the nose to the bronchiolar-alveolar junction and that the same inflammatory cells are present throughout the airways, suggesting a continuum of disease.
However, there are differences in terms of exposure to allergens and noxious agents – the nose being more exposed than the lower airways. There are also major structural differences between the nasal and bronchial mucosa: the former has a large vascular supply whereas the latter has smooth muscle. Airway smooth muscle is of paramount importance in asthma owing to its contractile properties, but it may also contribute to the pathogenesis of the disease by increased proliferation (142), and the expression and secretion of pro-inflammatory mediators and cytokines (143).
It is therefore possible that the difference between rhinitis and asthma is that in the former there is an epithelial-mesenchymal trophic unit (144) whereas in the latter there is an epithelial-mesenchymal-muscular trophic unit (Fig. 2).
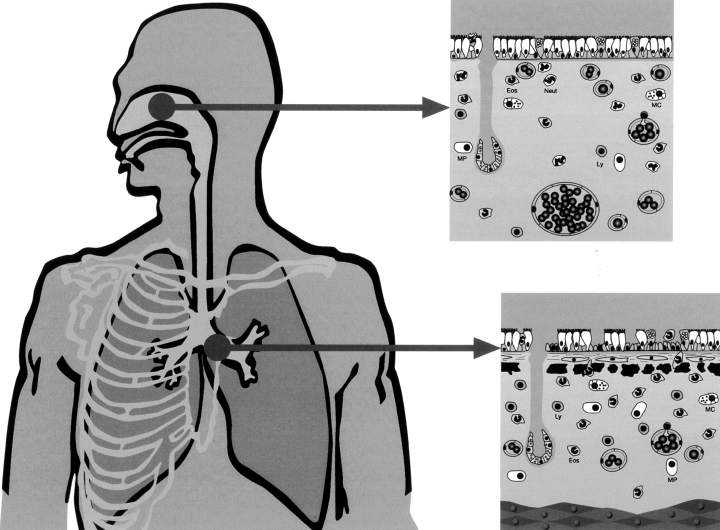
The same inflammatory cells are present in an epithelial-mesenchyma trophic unit from the nose to the bronchiolar–alveolar junction, however, there are structural differences. In rhinitis there are numerous blood vessels, which cause nasal obstruction whereas in asthma bronchial smooth muscle causes bronchial obstruction (149). Eos, eosinophils; Neut, neutrophils; MC, mast cells; Ly, lymphocytes; MP, macrophages.
Impact of rhinitis and asthma on quality of life
Quality of life has been found to be impaired both in patients with asthma (145) and in patients with allergic rhinitis (146), but the relative burden of these diseases has only been investigated in a population-based study (147). Answers to the SF-36 questionnaire were analysed from 850 subjects recruited in two French centres participating in the European Community Respiratory Health Survey. Both asthma and allergic rhinitis were associated with an impaired quality of life, and 78% of patients with asthma also had allergic rhinitis. Subjects with allergic rhinitis but not asthma (n = 240) were more likely than subjects with neither asthma nor rhinitis (n = 349) to report problems with social activities, difficulties with daily activities as a result of emotional problems, and poorer mental well-being. Patients with both asthma and allergic rhinitis (n = 76) experienced more physical limitations than patients with allergic rhinitis alone, but no difference was found between these two groups in the domains relating to social/mental health (Fig. 3). Asthma was not found to further impair the quality of life of subjects with allergic rhinitis for the domains related to mental disability and well-being.
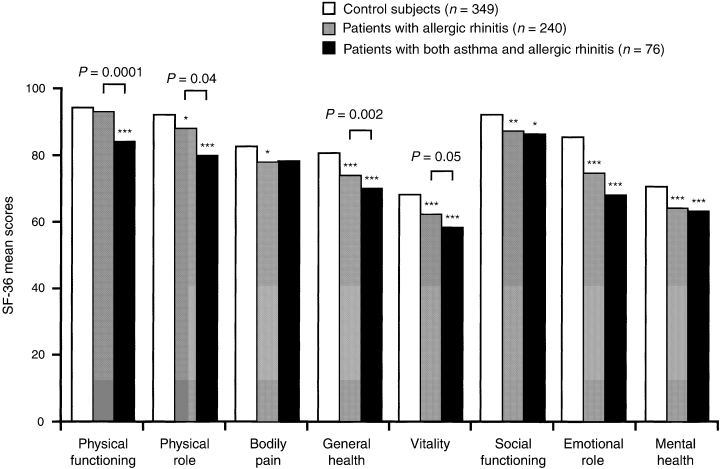
Results from the SF-36 questionnaire undertaken by 850 subjects participating in the European Community Health Survey, which illustrate the significant impact of allergic rhinitis and asthma on quality of life (147). Mean scores for SF-36 in control subjects (white bars, n = 349), patients with allergic rhinitis (grey bars, n = 240) and patients with both asthma and allergic rhinitis (black bars, n = 76). The first three concepts, on the left, are related to physical health; the last three, on the right, are primarily related to mental health; the other two concepts are related to both. The results of the Wilcoxon tests for comparisons between the different groups and control subjects are expressed with stars; no star indicates a P value > 0.05; one star, 0.01 ≤ P < 0.05; two stars, 0.001 ≤ P < 0.01; three stars, P < 0.001; the written P values correspond to the test between subjects with rhinitis alone and subjects with both asthma and rhinitis.
Asthma and rhinitis are common chronic conditions that affect adults of working age, but little is known about their relative impact on work loss and decreased productivity. Using random-digit telephone dialling, a population-based survey was carried out in adults aged 18–50 years in northern California. One hundred and twenty five persons with asthma (with or without concomitant rhinitis) and 175 persons with rhinitis alone were interviewed (148). Participation in any adult labour force as onset of the condition was lower among those with asthma (88%) than among those with rhinitis alone (97%) (P = 0.002). In contrast, among those still employed, decreased job effectiveness was more frequently reported in the rhinitis group (43 of 121; 36%) in comparison with asthmatics (14 of 72; 19%). It seems clear that both asthma and rhinitis negatively affect work productivity. Those with asthma are less likely to be employed at all, while among those in employment rhinitis is a more potent cause of decreased work effectiveness. The economic impact of asthma and rhinitis and related conditions may be underappreciated.
Therapeutic consequences
Although asthma and allergic rhinitis commonly occur together, treatments for one condition could potentially alleviate the coexisting condition.
Medications for asthma and rhinitis can be administered via local (intranasal, intra-ocular or inhaled (intrabronchial), oral and parenteral routes. There are advantages (and some drawbacks) to administering the drug directly into the target organ (149). Moreover, some drugs like cromoglycate or nedocromil are not absorbed when given orally and are only effective when administered locally. In patients suffering from asthma and rhinitis, local administration of drugs requires that they are given both nasally and bronchially and this may decrease compliance to treatment which is low in asthma and rhinitis.
Drugs administered locally
Glucocorticosteroids are the most effective drugs when used topically in the nose and the bronchi for the treatment of rhinitis and asthma. The intranasal treatment of rhinitis using glucocorticosteroids was found to improve asthma in some but not all studies (150). Symptoms (151, 152) and pulmonary function tests (151) were improved, and, exercised-induced asthma (153) or BHR (152, 154, 155) were reduced. These data suggest that treating nasal inflammation may help in controlling asthma. However, a number of aspects, such as the extent to which the pathophysiology of the two diseases overlaps and whether treating one will affect the other, still remain to be clarified.
Little is known about the effects of intrabronchially administered glucocorticosteroids on nasal disease. A study examined the effects of intrabronchially administered budesonide (avoiding nasal deposition of the drug) on nasal allergic disease in patients with seasonal allergic rhinitis, but without asthma (156). During the birch pollen season, budesonide reduced seasonal eosinophilia both in the circulation and in the nose along with an attenuation of seasonal nasal symptoms. Nasal and systemic anti-eosinophil actions are produced at commonly employed dose levels of orally inhaled budesonide.
Drugs administered orally
Orally administered drugs may have an effect on both nasal and bronchial symptoms. Oral H1 antihistamines represent the first line treatment of allergic rhinitis. Although some studies have found a modest effect of H1 antihistamines on asthma symptoms (157), these drugs are not recommended for the treatment of asthma (158). Leucotriene modifiers were shown to be effective in controlling symptoms of mild to moderate asthma and seasonal allergic rhinitis (159). Oral glucocorticosteroids are highly effective in the treatment of rhinitis and asthma but side-effects after long-term use are common.
Specific immunotherapy
The indications of specific immunotherapy in allergic asthma and rhinitis have been separated in some guidelines (160). This artificial separation has led to unresolved issues (161, 162) possibly because the allergen-induced IgE-mediated reaction has not been considered to be a multi-organ disease (163). It is therefore important to consider specific immunotherapy based on allergen sensitization rather than on the disease itself as most patients with allergic asthma also present rhinitis or rhinoconjunctivitis (149). Immunotherapy can also alter the atopic phenotype by restoring the normal equilibrium between Th1 and Th2 lymphocytes (164). This form of therapy is currently under investigation in subjects with allergic rhinitis as a means of prevention of secondary asthma, and initial results are encouraging (165).
Anti-IgE monoclonal antibody
The anti-IgE antibody, omalizumab, has been shown to be safe and effective in separate populations of patients with seasonal and perennial allergic rhinitis (166, 167) (Fig. 4), and in children and adults with moderate–severe allergic asthma (168–170). Studies are ongoing to assess its effectiveness in patients with comorbid allergic airway disease, and to investigate whether a course of treatment could reduce the risk of developing asthma. Its systemic activity and ability to reduce levels of IgE regardless of allergen specificity may be especially advantageous in these respects.
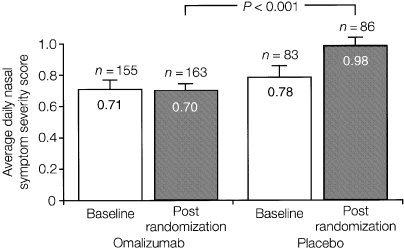
Results from a randomized, placebo-controlled parallel group study in 250 adults with seasonal allergic rhinitis illustrating the efficacy of the anti-IgE monoclonal antibody omalizumab. Average daily nasal symptom severity scores (with SEs) for the entire randomization period are shown for the intent-to-treat analysis. A highly statistically significant difference was found between omalizumab-treated patients and placebo-recipients (166).
Treatment of rhinitis reduces asthma severity
Two very recent studies showed that treating allergic rhinitis reduces health care utilization for comorbid asthma. In a first study, a retrospective cohort study was carried out with 1994–95 MarketScan claims data. The cohort was limited to patients with both allergic rhinitis and asthma, aged 12–60 years, who were continuously enrolled and had no evidence of COPD (171). The study sample population consisted of 4944 patients with allergic asthma, approximately 73% of who were treated for allergic rhinitis. The risk of an asthma-related event (hospitalizations and emergency department visits) for the treated group was about half of the untreated group.
In another study, Adams et al. attempted to find out whether treatment for rhinitis can reduce the risk for emergency department (ED) visits for asthma (172). A retrospective cohort was studied in members of a managed care organization aged >5 years who were diagnosed with asthma during October 1991 to September 1994. Of the 13 844 eligible persons, 1031 (7.4%) had an ED visit for asthma. The overall relative risk (RR) for an ED visit among those who received intranasal corticosteroids was 0.7 (95% CI, 0.59–0.94). When different rates of dispensing for intranasal steroids were examined, a reduced risk of ED visits was seen in those with >0–1 (RR, 0.7; 95% CI, 0.57–0.99) and >3 (RR, 0.5; 95% CI, 0.23–1.05) dispensed prescriptions per year.
These two studies show that treatment of nasal conditions, particularly with intranasal steroids, confers significant protection against exacerbations of asthma leading to ED visits for asthma.
Impact of rhinitis on asthma costs
Yawn et al. examined the incremental medical care costs of concomitant allergic rhinitis and asthma (8). For each member of a population-based asthma cohort, medical charts were used to record age at first diagnosis of asthma, the presence and age of any diagnosis of allergic rhinitis and the total, ambulatory and respiratory care-related costs of medical care. Yearly medical care charges were, on average, 46% higher for those with asthma and concomitant allergic rhinitis than for persons with asthma alone, after controlling for age and sex.
Conclusions
Rhinitis is a very common comorbidity of asthma and common mechanisms exist. An initiative in collaboration with WHO, termed Allergic Rhinitis and its Impact on Asthma (ARIA), has been developed to assess the association between asthma and rhinitis (149). It has recently been proposed that physicians should consider the diagnosis of rhinitis in all asthmatics (8). New therapies such as anti-IgE which act early in the allergic cascade, thereby preventing the initiation of the inflammatory process in both asthma and allergic rhinitis, may provide a promising option for future treatment of these conditions.




