Recurrent glomangioma (“true” glomus tumor) of the middle ear and mastoid
Abstract
Objective
To review current literature and experience with glomangiomas, or true glomus tumors of the middle ear and mastoid as well asto report on the exceptionally rare case of a glomangiomastemming from the middle ear space with multiple recurrences.
Methods
Review of existing world literature and description of personal experience with rare cases of a glomangioma of the middle ear and mastoid.
Results
Review of existing literature revealed two cases of patients presenting with tinnitus and hearing loss refractory to medical management. Both patients were ultimately diagnosed with glomangioma on histopathology. Complete surgical excision is thought to be curative.
Patient
A 36-year-old woman presented with a rare case of a glomangioma of the middle ear presenting with unilateral hearing loss. She was noted to have a mass behind the tympanic membrane. Imaging revealed a diffuse mass filling the mastoid air cells. Imaging characteristics and histology were consistent with a glomangioma.
Intervention
Initial resection via mastoidectomy using a postauricular approach. The tympanic membrane was reconstructed with temporalis tissue. Follow-up revision tympanomastoidectomy was performed upon recurrence of disease. The chorda tympani were sacrificed due to tumor involvement. The incus and head of the malleus were removed to gain better access to the tumor. The ossicular chain was reconstructed with a Goldenberg Total Ossicular Prosthesis.
Main outcome measure
Recurrence of disease.
Follow-up
In the 67 months since her most recent surgery, there has been no evidence of recurrence by CT or physical exam.
Conclusion
Glomangioma of the middle ear represents an exceptionally rare entity that can present in a similar fashion to a paraganglioma.
Introduction
Paragangliomas of the head and neck are often inaccurately referred to as glomus tumors. A true glomus tumor, sometimes referred to as Barre–Mason syndrome, is aneoplastic process derived from the glomus bodies which consist of neuromyoarterial cells involved in controlling blood flow within the cutis to regulate temperature.1 These are rare tumors comprising less than two percent of soft tissue neoplasms.2 These tumors are primarily located within distal portions of the extremities; in particular, they are found in the nail beds where there is the highest density of glomus bodies.3 Classically, they are characterized by a triad of symptoms: hypersensitivity to cold, pinpoint pain, and pain out of proportion to the lesion.3 Intriguingly, when these tumors are found outside of the extremities, these symptoms are often not present.
In the subungual region, there is female and middle-aged predominance; however, other anatomic sites show no sex or age bias.1 Glomus tumors are rarely found in the head and neck region and, to date, have only been reported twice within the middle ear space.3-5 Furthermore, it is relatively unique for a glomus tumor to recur multiple times after complete excision.6 Herein, we report on the exceptionally rare case of a glomus tumor stemming from the middle ear space with multiple recurrences. Additionally, this patient has been followed for twenty years allowing for complete understanding of the natural course of the disease process.
Results
Review of literature
To date, there have only been two reported cases of glomangioma of the middle ear. In both cases, patients presented with symptoms of hearing loss and tinnitus refractory to medical management (Table 1). There was involvement of the middle ear, aditus ad antrum, and mastoid air cells with one case also reporting involvement of the external auditory canal. Imaging analysis was conducted using computer tomography (CT) and diagnosis was confirmed via histopathology. Complete surgical excision is thought to be curative. One case reports no sign of disease on CT scan during the most recent follow-up examination, 48 months after surgical resection.4
| Pt No. | Year | Age/Sex | Middle Ear | Aditusad antrum | Mastoid air cells | Hearing loss | Tinnitus | EAC |
|---|---|---|---|---|---|---|---|---|
| 1 | 20044 | 62/F | Y | Y | Y | Y | Y | Y |
| 2 | 20125 | 54/F | Y | Y | Y | Y | Y | NR |
| 3 | 2018∗ | 36/F | Y | Y | Y | Y | NR | NR |
- ∗:Presented case; EAC: external auditory canal; NR: not reported.
Case report
In August 1996, a 36-year-old female presented with two months of right aural fullness and subjective hearing loss refractory to medical management. Physical exam revealed bluish discoloration of the tympanic membrane and fluid/tissue filling the middle ear space. The tympanic membrane was noted to have poor mobility and tympanometry was Type B. Tympanocentesis revealed bright red blood. Magnetic resonance imaging (MRI) showed a diffuse process filling the mastoid air cells. Audiologic testing revealed mild to moderate right-sided conductive hearing loss. CT confirmed a diffuse process involving the middle ear space and mastoid without bony erosion. Fig. 1 interestingly, the magnetic resonance angiography (MRA) showed no evidence of increased vascular flow which is typically seen with paragangliomas. Although the MRA was not definitive, a diagnosis of glomus tympanicum paraganglioma was made based on all other imaging and physical exam findings.
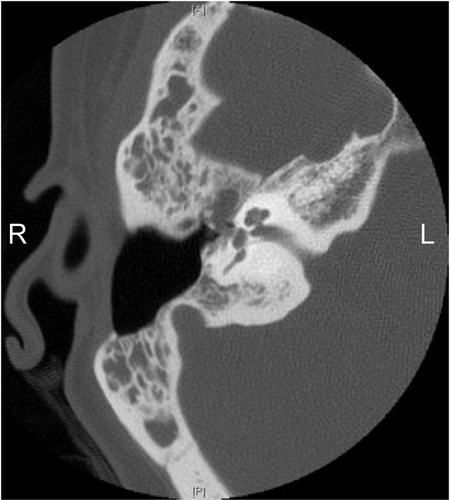
Axial CT imaging showing recurrence of the mass within the middle earcoming off the fallopian canal heading towards the Eustachian tube.
In September 1996, the patient underwent surgical resection via a postauricular approach. A soft friable mass was noted in the middle ear space with extensive involvement of the ossicles and extension into the Eustachian tube. Mastoidectomy revealed tumor extending into the antrum. The tumor was most adherent to the tympanic portion of the fallopian canal which also appeared to provide its vascular supply. It was removed with no dehiscence of the bone. All visualized tumor was removed and the ossicular chain remained intact. The tympanic membrane was reconstructed with temporalis tissue. The patient recovered from the procedure without complication and follow-up audiologic examination improved to mild conductive hearing loss. At the time, our pathology department interpreted the mass as being a highly vascular tumor of small round cells arranged in trabeculae with an ill-defined zellballen pattern consistent with a paraganglioma.
Routine follow-up was uneventful for two years until she developed intermittent pressure and pulsatile tinnitus. Physical exam revealed a red mass in the anterior TM. A CT scan demonstrated opacification anterior and medial to the malleus extending into the epitympanum concerning for tumor recurrence. There was no evidence of involvement of the jugular bulb as would be typical of a glomus jugulare (Fig. 2). In December 1998, she underwent revision tympanomastoidectomy revealing extensive recurrent tumor. The chorda tympani were sacrificed due to tumor involvement. The incus and head of the malleus were removed to gain better access to the tumor. Upon inspection, there was no residual tumor. The ossicular chain was reconstructed with a Goldenberg Total Ossicular Prosthesis. Follow-up audiologic examination showed amild to moderate right-sided conductive hearing loss.
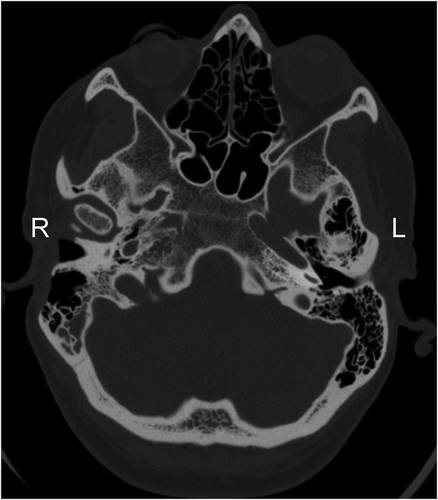
Axial CT imaging showing uninvolved and normal jugular bulbs bilaterally.
Specimens from both procedures showed a lesion with cellular proliferation admixed with a rich vascular network (Fig. 3). The tumor cells surrounding the vessels were rounded to slightly spindle with varying sized nuclei. However, none of the nuclei showed features of neuroendocrine differentiation. In the first recurrence, immunostains were performed on the tumor and showed negative results for chromogranin, S100, synaptophysin, cytokeratins; the tumor cells also showed negative results for vascular markers. Thus, the initial diagnosis of paraganglioma was incorrect. Further investigation showed the vascularity of the lesion and close association of tumor cells with vessels to be reminiscent of a true glomus tumor or glomangioma.
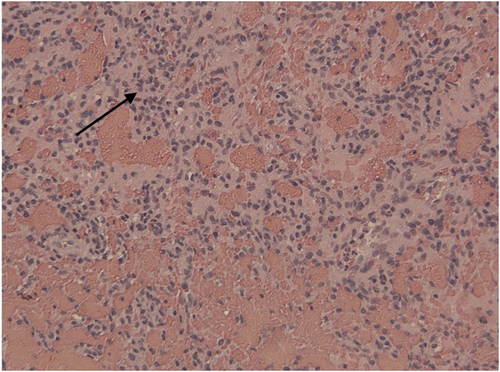
Tumor cells intervene among dilated red cell filled vascular channels. Arrow showing representative tumor cells Hematoxylin and eosin stain, × 150.
Routine follow-up imaging revealed soft tissue in the middle ear and she returned to the OR in June 2005 for resection of a cholesteatoma. Final pathology revealed a cholesteatoma as well as recurrence of the glomangioma. This pattern was repeated in 2010.
In the 67 months since her most recent surgery, there has been no evidence of recurrence by CT or physical exam. Her audiologic exam has been relatively stable with moderate right-sided conductive hearing loss.
Discussion
Glomus tumors were originally described by Masson in 1924 as benign vascular hamartomas; later, they were found to be benign mesenchymal neoplasms of soft tissue. The cells comprising the tumor are believed to be modified smooth muscle similar to those in normal glomus bodies.7 There are three categories of glomus tumor. The most common solid type is comprised of glomuscells with little smooth muscle and poor vascularity. The second most common glomangioma (current case) type has prominent vascularity and close association of tumor cells with vessels. The rarest glomangiomyoma type has prominent smooth muscle and vascularity.2 It is important to distinguish true glomus tumors from “glomus jugulare” tumors which arise in the region of the glomus body in the ear and are named for their location and not their histogenesis. Pathologic studies including immunohistochemistry indicate these tumors represent neuroendocrine lesions known collectively as paragangliomas. They stain positively for neuroendocrine markers, such as chromogranin A and synaptophysin with S100 highlighting the supporting or sustentacular cells.8
Malignant behavior is extremely unusual and is estimated to represent less than 1% of all glomus tumors.9 Glomangiomas, or glomuvenous malformations (GVM), make up about 20% of all glomus tumors and 5.1% of all venous anomalies.10 Pathologically, they may be misinterpreted as hemangiomas or venous/arteriovenous malformations depending on the degree of vascular proliferation.2
GVMs are thought to be sporadically congenital or familially inherited.10 Congenital lesions expand throughout childhood, whereas familial GVMs can appear spontaneously throughout life (often following trauma).10,11 Unlike in other anatomic sites, there is an increased frequency of sporadic GVMs compared with the familial form in the head and neck region.10 Sporadic GVMs are also more likely to be single and extensive.10 Studies report that 38%–64% of GVMs are familial in nature.10,12
A linkage disequilibrium was found in families with a history of GVMs involving chromosome 1p21-23.13 Further study identified the gene glomulin as uniformly mutated in all families studied.11 Glomulin appears to be involved in vascular maintenance; its expression is restricted to vascular smooth muscle cells which is consistent with its possible role in development of GVMs.14 Although numerous mutations were found in the different families, they all appeared to be loss of function mutations.15 Furthermore, in-situ hybridization of GVMs showed undetectable levels of glomulin and a single lesion was found to be doubly mutated possibly indicating that GVMs are inherited in a paradominant fashion and require a “second hit” to induce growth.11,15 It remains unclear if sporadic GVMs develop in a similar manner. Another area of inquiry is how GVMs develop in areas not known to have glomus bodies such as the middle ear as seen in this case. One intriguing hypothesis is that perivascular smooth muscles cells, which exists in the middle ear, can differentiate into glomus bodies which then acquire glomulin mutations or that glomulin mutations induce differentiation itself.2
Imaging can be effective for monitoring glomus tumors within the middle ear. Although we used CT scans of the temporal bone to monitor growth, Doppler ultrasound or MRI are the imaging modalities of choice as both can find lesions as small as 2 mm.1 Doppler, when appropriate, in one study had a 100% detection rate with no false negatives.16 Obviously, the location of this particular tumor in the middle ear made Doppler impossible. Lesions generally present on MRI with low signal intensity on T1 but often enhance with gadolinium.1 Rim enhancement around a hypointense focus on T1 with contrast is even more specific.1 On T2, these lesions generally show significant hyperintensity.1 MRI has similar sensitivity to ultrasound (97%), but has much poorer specificity (50%).17
Total surgical resection is the treatment of choice for glomus tumors. The tumors are not encapsulated and may show irregular borders leading to the possibility of recurrence if the nodule is not completely excised. Large case series show recurrence rates after complete excision ranging from 0% to 6.6%; however, all series focus on glomus tumors of the extremities.1,18 Nonetheless, complete resection is often difficult due to these lesions having unknown synchronous lesions.6 One group took advantage of the highly vascular nature of glomangiomas and used angiography to map the location of all synchronous lesions and postoperatively confirm the complete ablation of the lesions.6 Treatment outcome data is difficult to interpret due to the low number of cases in the head and neck. Treatment decisions are often based on inferences from other anatomic sites.
Conclusions
The review of existing literature revealed two cases of patients presenting with tinnitus and conductive hearing loss who were ultimately diagnosed with glomangioma of the middle ear. The presented case represents an unusual presentation of a rare disease, which proved difficult to control. Her primary presenting symptoms were conductive hearing loss and aural fullness due to the tumor's direct involvement of the middle ear. Though a rare entity, glomangioma should be included on the differential diagnosis in patients with glomus-like lesion in the middle ear.
Conflicts of interest and funding
The authors have no funding, financial relationships, or conflicts of interest to disclose.




