Ex vivo ovine model for teaching open laryngotracheal surgery
Abstract
Objective
To develop an animal model for teaching open laryngotracheal surgical procedures.
Methods
The heads and necks from 5 pre-pubescent sheep were harvested after humane anesthesia. After 2–5 days to allow for rigor mortis to resolve, a specimen was supported with sandbags on an operating table. Operative procedures including tracheotomy, medialization laryngoplasty, anterior cartilage grafting, tracheal resection with primary anastomosis, and laryngectomy with closure of the pharynx were attempted.
Results
The ovine head and neck provided an accurate model for simulation of all attempted procedures. Ovine tissue resembled that of humans in mechanical properties and handling. Postsurgical endoscopy confirmed graft alignment.
Conclusions
The sheep head and neck provides an inexpensive, realistic, and safe model for surgical training for a variety of open laryngotracheal procedures. This is particularly relevant given the recent emphasis on surgical simulation and the relative rarity of some of these procedures in residency training.
Introduction
Surgical training in open laryngotracheal procedures is limited in most otolaryngology residency programs in the United States. Although, many programs provide adequate experience in tracheotomy and medialization laryngoplasty, few offer adequate training in laryngotracheal reconstruction or tracheal resection.1 The relative rarity of these cases and the increased use of endoscopic techniques has left a void in resident education.
Simulation has gained wide acceptance in medical student and resident education – providing trainees with first-hand exposure to emergency situations and complex procedures while minimizing risk to patients. This is particularly true for surgical training, where simulation allows students to acquire mechanical skills and teachers to document technical competence in a standardized fashion.2 Several models have been proposed for the experimental study of laryngotracheal stenosis including rodent, leporine, porcine, canine, and ovine.3-7 There has been limited use of animal models for surgical training in the head and neck where a porcine model for microlaryngeal surgery and cricothyrotomy has been proposed.8, 9
Our group recently described the use of a fresh, saline-perfused sheep head and neck model for surgical simulation.10 This article explores its use for teaching open laryngotracheal procedures.
Materials & methods
Tissue was collected from pre-pubescent sheep (n = 5; mean age: 4 months; mean mass: 28 kg) following humane euthanasia (100 mg/kg sodium pentobarbital solution) at the end of an in vivo, protocol approved by the Lewis Katz School of Medicine through its Institutional Animal Care and Use Committee (IACUC). These sheep were obtained from a Temple University and United States Department of Agriculture approved supplier, tested for Coxiella burnetii according to Centers for Disease Control and Prevention (CDC) protocol, and accepted into the University Laboratory Animal Resource facility of Temple University after full inspection by a licensed veterinarian to assure good health. No live animals were used in this study. Post-euthanasia, the head and neck of the sheep were disarticulated 4–6 cm above the sternal notch. The tissue was stored at 5 °C for 2–5 days.
Prior to working with fresh ovine tissue, study personnel were enrolled in the Temple University's Occupational Health Program and screened for Q-Fever antibodies. They wore appropriate personal protective clothing while handling the specimen. Sheep tissues were transported in double plastic disposable bags and all carcass material was treated as biomedical waste through a commercial waste management company.
The ovine head and neck preparation was supported in the supine position with sand bags. Standard soft tissue instruments were used to perform the procedures. Expired Montgomery™ silastic thyroplasty implants (Boston Medical Products, Boston MA) were used for medializations. Polydioxanone (PDS) suture (Ethicon US LLC, Somerville NJ) was used for the tracheal anastomoses after resection. Endoscopic evaluation of the anastomoses was performed with a Karl Storz™(Tuttlingen, Germany) adolescent Parsons laryngoscope through which a 4 mm 0° Karl Storz™ rigid rod endoscope was passed. A Karl Storz™ Tele Pack X system was placed at the foot of the operating table for image visualization and recording. Closure of the pharynx after total laryngectomy was performed using a 4-0 polyglactin 910 suture on a tapered needle (Ethicon US LLC, Somerville NJ).
Results
A total of 5 procedures were readily performed on the sheep head and neck preparations: tracheotomy with flap, medialization laryngoplasty with a silastic implant, anterior laryngotracheal cartilage grafting, tracheal resection with end to end anastomosis, and total laryngectomy with pharyngeal closure (Figs. 1-8). The ovine model proved highly realistic for open laryngotracheal surgery with the size of the larynx and trachea closely approximating that of an adult human. The trachea was quite pliable allowing for resection of segments yet still allowing tension free closure. The fresh soft tissues of the sheep have similar consistency, thickness and surgical handling to that of humans. In addition, their oropharyngeal anatomy easily allowed for laryngoscopy and bronchoscopy to assess the outcome of the procedures endoscopically. The fresh sheep had relatively strong rigor mortis in the first 48 h after sacrifice so it is best to use the preparation after that period of time.
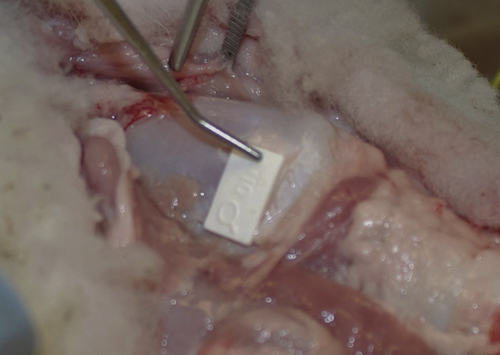
Right medialization laryngoplasty with silastic implant placed.
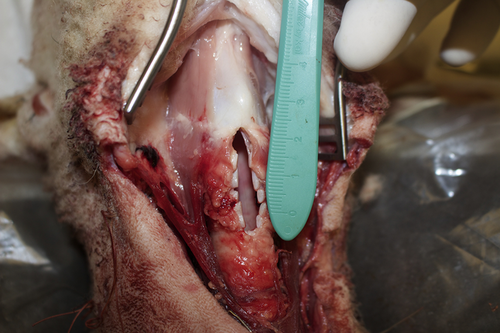
Anterior cricoid and tracheal divided.
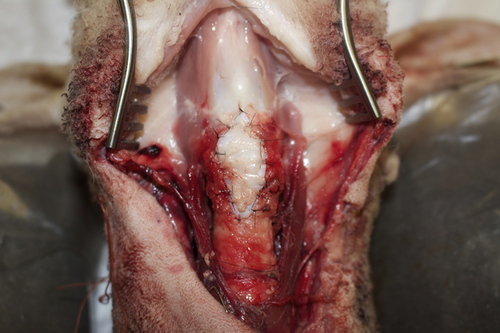
Anterior cartilage augmentation graft sewn in place.
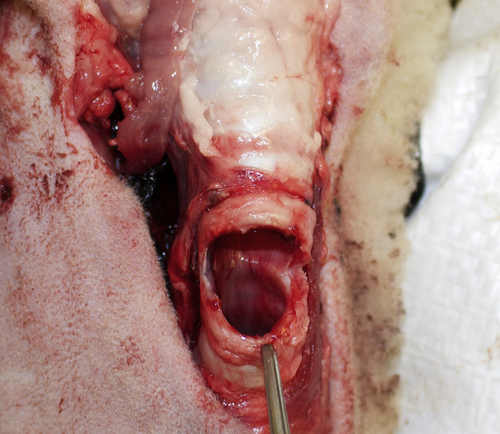
Tracheal resection superior cut completed.
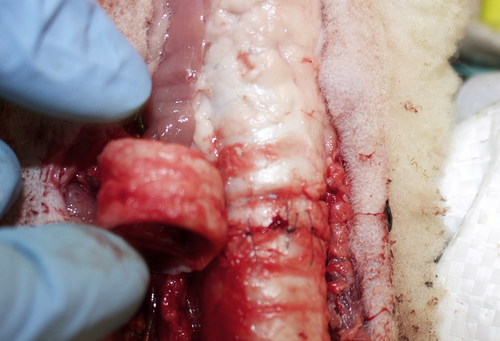
2 cm tracheal segment resected and re-anastomosed.
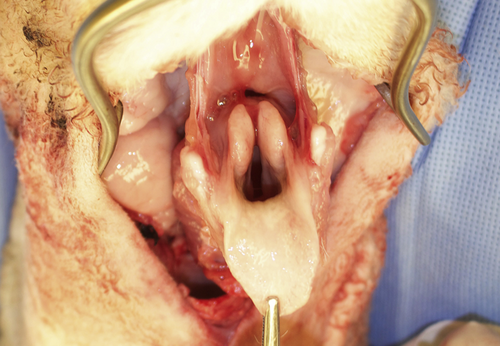
Total laryngectomy started with superior cut into pre-epiglottic space.
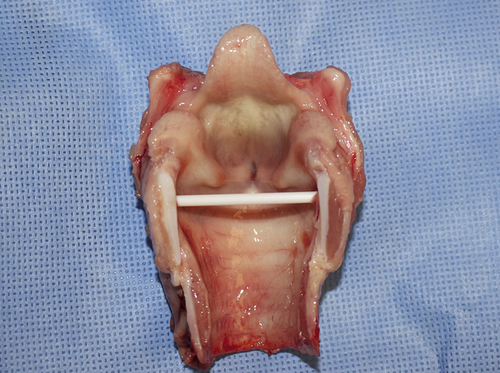
Posterior view of total laryngectomy specimen with posterior cricoid divided.
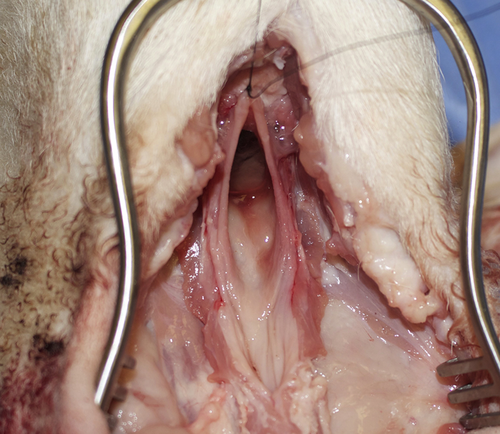
Pharyngeal defect post total laryngectomy for closure.
Discussion
Surgical simulation has become increasingly important in resident education. In fact, current Accreditation Council for Graduate Medical Education (ACGME) otolaryngology residency program requirements mandate that residents “must demonstrate knowledge of anatomy through procedural skills demonstrated in cadaver dissection, temporal bone lab, and/or simulation labs.”11 Human cadavers have been traditionally used in otolaryngology for teaching otologic surgery and bronchoscopy. Several authors have described using excised larynx models for learning laryngoscopy skills.12-16 Unfortunately, fresh human tissues are increasingly difficult to obtain and have the highest risk of disease transmission of any model.17 More recently, computerized simulators have been utilized for bronchoscopy and intubation as well as developing skills in endolaryngeal and percutaneous laryngeal procedures.18-22
Despite increased reliance on endoscopic procedures, open laryngotracheal operations remain important alternatives for the treatment of congenital and acquired airway disorders. Surgeons in training require exposure to the techniques of partial and total laryngectomy to assure the comprehensive management of laryngeal cancer. Unfortunately, residency experience in open surgical techniques has decreased because of the relative rarity of laryngeal cancer and a diminishing number of surgeons well-trained in these methods. Despite the use of different animal species as research models for laryngotracheal stenosis, there have been sporadic reports of their use as surgical training models.23
An ideal model for surgical simulation should be readily available, inexpensive, have a low risk of disease transmission, avoid undo ethical concerns, and accurately reflect normal human anatomy. While no single model is perfect, the ovine head and neck preparation satisfies many of these requirements.
We were fortunate to have access to a regular supply of sheep head and neck specimens through a shared tissue program at Temple University. For those lacking such a connection, farm-raised sheep can be obtained for the same purpose and are readily accessible around the world.24 Access to appropriate head and neck tissues usually requires a relationship with a slaughterhouse, as most sheep carcasses are delivered to meat markets without an intact head and neck. At approximately $4 per pound, each head and neck preparation would cost $20-$28.
Sheep laryngotracheal anatomy closely resembles that of humans.25 They have a large thyroid cartilage and distinct cricoid cartilage. The thickness and consistency of the thyroid cartilage is comparable to that of an adult female. The tracheal size is also similar in size and pliability as is the pharyngeal tissue which allowed for easy closure post laryngectomy.
Handling of fresh ovine tissue requires an understanding of zoonotic disease. Although sheep rarely transmit infections to humans, the main concern with using mature farm animals is Query Fever (Q fever), a treatable but potentially dangerous disease.26 Acute symptoms include fever, headaches, myalgia, cough, gastrointestinal distress, and chest pain. The causative bacteria, Coxiella burnetii reside primarily in the amniotic fluid and placental tissue of pregnant sheep. Human contamination with this may occur from aerosolized dried amniotic and placental material found in barnyard dust.27 Selection of tissues from male or prepubescent female sheep reduces the risk for Coxiella burnetii exposure and should be considered if a program chooses to use farm-raised animals.28
Conclusions
Simulation is an important component of surgical training for residents. The ovine model provides a realistic model for training in open laryngotracheal surgery. It closely resembles adult human laryngotracheal anatomy in size and consistency. It is inexpensive, readily available, and safe.
Funding
Supported in part by Department of Defense/Office of Naval Research grants N000141210810 and N000141210597.
Financial disclosures
None.
Conflicts of interest
None.
Acknowledgements
We would like to thank Marla Wolfson, Ph.D. for kindly donating head and neck sheep tissue following her non-survival lung study at the Center for Inflammation, Translational and Clinical Lung Research.




