Sodium thiosulfate protects human aortic smooth muscle cells from osteoblastic transdifferentiation via high-level phosphate
Abstract
Vascular calcification is recognized as a common complication in some patients, such as chronic renal failure. The purpose of this study was to investigate the role of sodium thiosulfate (STS) for the transdifferentiation of human aortic vascular smooth muscle cells into osteoblast-like cells induced by high-level phosphate. All human aortic vascular smooth muscle cells were divided into STS group 1 (treatment with STS) and STS group 2 (culture in a medium containing a high level of phosphate). STS group 1 included a normal group, a high-level phosphate group, and other subgroups based on treatment with different concentrations of STS. Cells of STS group 2 were cultured in a medium containing a high level of phosphate for 72 hours, and then divided into a high-phosphate control group and other subgroups based on treatment with different concentrations of STS. The mRNA and protein expressions of bone morphogenetic protein-2 (BMP-2), core binding factor α-1 (Cbfα-1), and matrix Gla protein (MGP) were detected. Meanwhile, calcium concentration and alkaline phosphatase (ALP) activation were measured. In STS group 1, the mRNA levels of BMP-2 and Cbfα-1 were elevated significantly in the high-level phosphate group compared with the normal group (p < 0.05). However, both gene expressions were attenuated in the STS-treated groups (vs. normal group, p < 0.05). MGP mRNA levels were reduced in the high-level phosphate group (vs. normal group, p < 0.05). In the STS-treated groups, mRNA expression of MGP was elevated compared to the high-level phosphate group (p < 0.05). In STS group 2, expression of MGP was enhanced significantly (vs. high-phosphate control group, p < 0.05) with both BMP-2 and Cbfα-1 reducing in the STS-treated groups (vs. high-phosphate-control group, p < 0.05). STS attenuates calcium concentration and ALP activation. It can reverse osteoblast differentiation of vascular smooth muscle cells and modulate the expressions of calcification-related factors.
Introduction
Vascular calcification, specifically arterial calcification, is recognized as a common complication in patients with chronic renal failure [1]. Coronary artery calcification is detected in more than 50% of patients on hemodialysis, and its presence is associated with all-cause and cardiovascular mortality [2]-[9]. A number of studies have suggested that high-level phosphate plays a key role in the occurrence and development of vascular calcification [10]-[12]. Although the research on metabolism of calcium and phosphate and vascular calcification has become a hot topic, there is still no effective treatment for vascular calcification.
Vascular calcification was previously considered to be a result of passive precipitation of calcium and phosphate ions. However, recent findings suggest that vascular calcification is an actively regulated process, which is induced via several interlinked mechanisms in which phosphate plays a key role in calcification [13], [14]. This process is characterized mainly by osteoblastic differentiation of vascular smooth muscle cells (SMCs) and dysregulation of endogenous calcification inhibitors [10], [12].
Sodium thiosulfate (STS) is a chelating and reducing agent. It has been used for the treatment of cyanide poisoning in humans for more than 100 years [15]. Recently, Pasch et al. [16] provided convincing evidence that STS prevents medial vascular calcification in uremic rats. STS has been shown to delay the progression of coronary artery calcification in patients on hemodialysis [17]. Although these results provide some scientific and clinic evidence favoring the use of thiosulfate, the mechanism of action is uncertain and needs to be clarified. It is hypothesized that the removal of calcium from precipitated minerals, thereby decreasing the calcification burden, and enhancement of the urinary calcium loss may contribute to the therapeutic effect of STS on calcification. However, whether STS modulates calcification-related factors in reducing phosphate-mediated vascular mineralization remains unclear. The present study aimed at investigating the effects of STS on high-level phosphate-induced osteoblastic differentiation of human aortic vascular smooth muscle cells (HASMCs).
Materials and methods
Reagents
HASMCs (6110) were purchased from ScienCell (Carlsbad, CA, USA). Smooth muscle cell medium (SMCM) was obtained from Yu Hengfeng Company (Beijing, China) and β-glycerophosphate from Rego Company (Shanghai, China). Polyclonal antibodies of Cbfα-1, bone morphogenetic protein-2 (BMP-2), and matrix Gla protein (MGP) were purchased from Boster Company (Wuhan, China) and secondary antibodies from Zhongshan Company (Beijing, China). Trizol reagent was obtained from Gibco (Tulsa, OK, USA). RNA was reversely transcribed by using ReverTraAce kit (Toyobo, Osaka, Japan). Bicinchoninic acid was purchased from Beyotime Company (Haimen, Jiangsu, China). Unless otherwise mentioned, all other analytical reagents were purchased domestically.
Cell culture
HASMCs were cultured in an SMCM at 37°C in a humidified atmosphere containing 5% CO2. All cells were divided into STS group 1 and STS group 2. STS group 1 was further divided into a normal group, a high-level phosphate group, and STS1000, STS2000, and STS3000 groups (treatment with STS 1000 mg/mL, 2000 mg/mL, and 3000 mg/mL, respectively). Cells of STS group 2 were cultured in a medium containing a high level of phosphate for 72 hours and then divided into a high-phosphate control, an STS-low, an STS-middle, and an STS-high group.
mRNA analysis of BMP-2, Cbfα-1, and MGP by real-time PCR
Primers were designed based on the published gene sequence from Genbank (National Center for Biotechnology Information, Bethesda, MD, USA). Primer sequences of BMP-2 were as follows: 5′-CGGTCTCCTAAAGGTCGACCAT-3′ (forward primer) and 5′-CGAACTTCCTGCGGCCCAGCT-3′ (reverse primer). The PCR product size was 120 bp. Primer sequences of Cbfα-1 were as follows: 5′-GCGCATTTCAGATGATGACACT-3′ (forward primer) and 5′-CAGTTCTGAAGCACCTGCCT-3′ (reverse primer). The PCR product size was 91 bp. Primer sequences of MGP were as follows: 5′-GAATCACATGAAAGCATGG-3′ (forward primer) and 5′-TCGCAAAGTCTGTAGTCAT-3′ (reverse primer). The PCR product size was 179 bp. Primer sequences of GAPDH as the internal control were 5′-CCTCAAGATCATCAGCAAT-3′ (forward primer) and 5′-CCATCCACAGTCTTCTGGGT-3′ (reverse primer). The PCR product size was 141 bp. Total RNA was extracted and analyzed using an ultraviolet spectrophotometer and by electrophoresis. Real-time PCR was performed using Bio-Rad iCycler and iQ Real-Time PCR systems (Veenendaal, The Netherlands). Genes were amplified by 40 cycles. Each cycle consisted of denaturation at 94°C for 5 minutes; annealing at 59°C (for BMP-2), 55°C (for Cbfα-1), or 57°C (for MGP) for 30 seconds; and extension at 72°C for 30 seconds. The PCR products were subjected to electrophoresis and scanned. The cycle threshold (ct) value is defined as the point at which the fluorescence generated within a reaction crosses the fluorescence threshold. The Ct value of each sample was calculated using the following formula: ΔCt = Ct(sample)−Ct(internal standard); ΔΔCt = ΔCt−ΔCt(control). The mRNA level was compared by calculating the values of 2−ΔΔct.
Detection of BMP-2, Cbfα-1, and MGP protein by Western blot
Total protein was extracted and was subjected to electrophoresis. The membrane was blocked with 5% milk in phosphate buffered saline (PBS) for 1–2 hours at room temperature. Then the membrane was immunoblotted with primary antibodies (1:400) to BMP-2, Cbfα-1, or MGP, and incubated overnight at 4°C. After incubation, the blot was washed three times with TBST and immunoblotted with the secondary antibody (1:20,000) for 1 hour at room temperature. The blot was exposed under chemiluminescence and analyzed by Quantity One software (version 4.2.2, Bio-Rad Laboratories, Hercules, CA, USA). The optical density of BMP-2 and Cbfα-1 was normalized to the optical density of β-actin.
Induction of transdifferentiation
HASMCs were routinely cultured in an SMCM. Cells were treated with 10 mmol/L of β-glycerophosphate to induce transdifferentiation. For the time-course experiment, the 1st day of culture in a medium with β-glycerophosphate was defined as Day 0.
Detection of calcium concentration
Calcium concentration was measured by a colorimetric method using methyl thymol blue. After treatment with STS, the cells were washed with hydrochloric acid for 24 hours. Then the supernatant was collected for the estimation of calcium content.
Alkaline phosphatase activation analysis
After treatment with STS, the cells were washed twice with precooled PBS; next, the cells were collected and total protein was extracted. After centrifugation at 3500 rpm for 15 minutes, the supernatant was collected for the detection of alkaline phosphatase (ALP) activation.
Statistical analysis
Data were presented as mean ± standard deviation. The significance of differences between all groups was evaluated using one-way analysis of variance with a post hoc Student–Newman–Keuls multiple comparisons test. Least significance difference (LSD) was used to compare between the two groups. Statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA), and p < 0.05 was considered to be statistically significant.
Results
mRNA expressions of BMP-2, Cbfα-1, and MGP
The mRNA expressions of BMP-2, Cbfα-1, and MGP were calculated using the formula of 2−ΔΔCt. In STS group 1, mRNA expressions of BMP-2 and Cbfα-1 on Day 2 were significantly elevated in the high-level phosphate group compared to that in the normal group (p < 0.05). However, both gene expressions were attenuated in the STS-treated groups, including STS1000, STS2000, and STS3000 groups (vs. high-level phosphate group, p < 0.05). The mRNA level of MGP was reduced in the high-level phosphate group (vs. normal group, p < 0.05). By contrast, mRNA expression of MGP was elevated in the STS-treated groups compared to that in the high-level phosphate group (p < 0.05). These findings suggest that a high level of phosphate can induce the transdifferentiation of HASMCs into osteoblast-like cells by upregulating the expressions of BMP-2 and Cbfα-1, while downregulating MGP expression. However, STS can inhibit such transdifferentiation of HASMCs by downregulating the expressions of BMP-2 and Cbfα-1 mRNA, while upregulating MGP mRNA expression (Fig. 1).
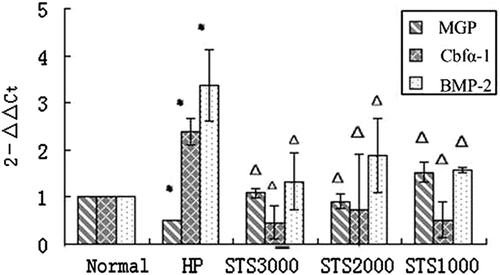
Changes in mRNA relative expressions of MGP, BMP-2, and Cbfα-1 in STS group 1. * p < 0.05, versus normal group. Δ p < 0.05, versus high-level phosphate group. BMP2 = bone morphogenetic protein-2; Cbfα-1 = core binding factor α-1; HP = high-level phosphate group; MGP = matrix Gla protein; STS = sodium thiosulfate.
In STS group 2, the expression of MGP was enhanced significantly (vs. high-level phosphate control group, p < 0.05) whereas that of BMP-2 and Cbfα-1 was decreased in the STS-treated groups (vs. high-level phosphate control group, p < 0.05). These findings revealed that STS can inhibit the expressions of BMP-2 and Cbfα-1 mRNA while upregulating MGP mRNA expression of HASMCs (Fig. 2).
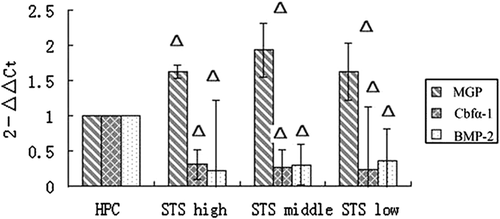
Changes in mRNA relative expressions of MGP, BMP-2, and Cbfα-1 in STS group 2. Δ p < 0.05, versus high-level phosphate control group. BMP2 = bone morphogenetic protein-2; Cbfα-1 = core binding factor α-1; HP = high-level phosphate group; MGP = matrix Gla protein; STS = sodium thiosulfate.
Western blot analysis of BMP-2, Cbfα-1, and MGP
In STS group 1, expressions of BMP-2 and Cbfα-1 on Day 3 were elevated significantly in the high-level phosphate group compared to that in the normal group (p < 0.05). However, both protein expressions were attenuated in the STS-treated groups, including STS1000, STS2000, and STS3000 groups (vs. normal group, p < 0.05). MGP expression was inhibited in the high-level phosphate group (vs. normal group, p < 0.05), whereas it was elevated in the STS-treated groups. These findings suggest that a high level of phosphate can upregulate the expressions of BMP-2 and Cbfα-1 while downregulating MGP expression. However, STS can reverse these processes (Fig. 3A and B).
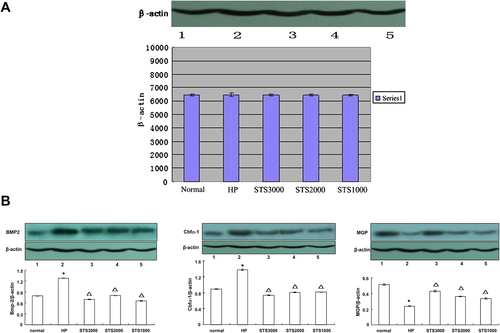
Expressions of β-actin, MGP, BMP-2, and Cbfα-1 in STS group 1. * p < 0.05, versus normal group. Δ p < 0.05, versus high-level phosphate group. BMP2 = bone morphogenetic protein-2; Cbfα-1 = core binding factor α-1; HP = high-level phosphate group; MGP = matrix Gla protein; STS = sodium thiosulfate.
In STS group 2, MGP expression on Day 3 was enhanced significantly (vs. high-level phosphate control group, p < 0.05) along with reductions of BMP-2 and Cbfα-1 in the STS-treated groups (vs. high-level phosphate control group, p < 0.05). These findings suggest that STS can reverse mineralization by modulating the expressions of BMP-2, Cbfα-1, and MGP (Fig. 4).
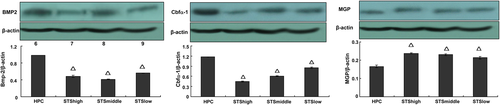
Expressions of β-actin, MGP, BMP-2, and Cbfα-1 in STS group 2. Δ p < 0.05, versus high-level phosphate control group. BMP2 = bone morphogenetic protein-2; Cbfα-1 = core binding factor α-1; HP = high-level phosphate group; HPC = high-level phosphate control group; MGP = matrix Gla protein; STS = sodium thiosulfate.
Attenuation of calcium concentration and ALP activation by STS
As shown in Fig. 5A and B, in STS group 2, calcium concentration and ALP activation on Day 3 were attenuated significantly after STS treatment (vs. high-level phosphate control group, p < 0.05).
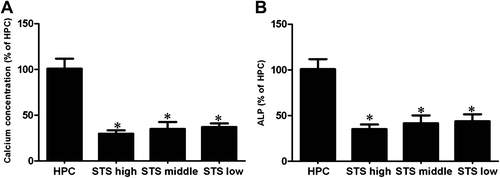
STS attenuates calcium concentration and ALP activation. * p < 0.05, versus high-level phosphate control group. ALP = alkaline phosphatase; HPC = high-level phosphate control group; STS = sodium thiosulfate.
Discussion
In vitro studies showed that both bovine and human vascular calcification can be induced in the presence of β-glycerophosphate. A high extracellular phosphate concentration leads to osteogenic differentiation of vascular SMCs and mineralization of the collagenous extracellular matrix produced by these differentiated cells [12], [18]-[20]. The increase of phosphate concentration in vascular smooth muscle cells results in the downregulation of smooth muscle-specific genes and upregulation of Cbfα-1, a central transcription factor involved in osteoblast and chondrocyte differentiation. An increase in the expression and activity of the transcription factor Cbfα-1 characterizes osteogenic phenotypic transition from SMCs [12]. Similar osteogenic differentiation of vascular SMCs has also been observed in human and animal models of vascular calcification. In the present study, we found that the expressions of osteoblast markers, including BMP-2 and Cbfα-1, were upregulated in HASMCs whereas the calcification inhibitor, MGP, was downregulated when cultured in a medium with a high phosphate concentration. More importantly, our study demonstrated for the first time that STS downregulates BMP-2 and Cbfα-1 induced by high-level phosphate. In addition, STS can also upregulate the expression of MGP, a calcification inhibitor, under high phosphate condition.
Accumulating evidence showed that BMP-2/Cbfα-1 signaling pathway plays a pivotal role in the process of vascular calcification. BMP-2 can induce the expression of Cbfα-1, and these two factors exert synergistic effects on vascular calcification. BMP-2, a member of TGF superfamily, is essential to the differentiation and proliferation of osteoblast and chondrocytes. The BMP-2-mediated in vitro differentiation reduces the expressions of SMC-specific markers. Moreover, exogenous BMP-2 can enhance calcium deposition in bovine vessel cells. The core binding factor Cbfα-1 plays an important role in the course of bone formation, differentiation of multipotent mesenchymal stem cells to osteoblast, osteoblastic differentiation, and osteogenic cells matrix gene expression; it has been recognized to be the earliest and most specific osteoblast marker. Our previous findings suggest that high-level glucose might evoke calcification of vascular smooth muscle cells by inducing osteoblastic differentiation and intracellular calcium deposition via the BMP-2/Cbfα-1 pathway in HASMCs in vitro [20]. The core binding factor Cbfα-1 has been reported to be present in the vascular calcified lesions of hemodialysis patients and nonhemodialysis Chroinc Kidney Disease (CKD) patients [21], [22]. Extracellular BMP signaling pathways are modulated by transient inhibitors. These soluble inhibitors are tissue specific. A combination of soluble BMP inhibitors with BMP can block the binding of BMP with its ligands.
MGP is a highly potent inhibitor of cartilage and vascular calcification. It is expressed mainly in cartilage and cardiovascular tissues. The MGP knockout mouse developed extensive aortic calcification spontaneously, and died at 6–8 weeks of age as a result of aortic rupture and subsequent internal hemorrhage [23]. This inhibitory effect of MGP has partly been attributed to its capacity to bind and inhibit BMP-2, a major regulator of Runx2/Cbfα-1 expression and a potent osteoinductive factor. MGP also abolishes BMP2 receptor binding and phosphorylation of Smad1, acting as an inhibitor of BMP2-dependent activation of Smads, critical cofactors that are involved in Runx2/Cbfα-1-dependent osteochondrogenic differentiation [24]. Sweatt et al. [25] showed that MGP binds to and inactivates BMP-2, a promineralization factor. MGP is considered to be a key inhibitor of BMP signaling pathways [26]. Transdifferentiation of aortic SMCs into osteoblast-like cells is observed in MGP-null mice in the presence of BMP. In atherosclerosis and Monckeberg's arteriosclerosis, MGP expression is found to be downregulated even prior to vascular calcification. Therefore, deficiency of BMP inhibitors such as MGP may contribute to the development of vascular mineralization.
STS has been prescribed for the treatment of cyanide toxicity for over a century. It has also been used for the prevention of cisplatin-induced side effects. Cicone et al. [27] first reported the application of STS in treating calciphylaxis. Further study also showed that STS can delay the progression of vascular calcification successfully in hemodialysis patients [17]. Some researchers demonstrated that STS probably latches onto calcium to form calcium thiosulfate complexes, a highly soluble complex that can be excreted easily [16], [28]. Moreover, another document showed that STS may induce pH reduction, followed by consequent increases in calcium excretion and negative calcium balance [16], [29]. Finally, STS is a strong antioxidant that can ameliorate oxidative insults in endothelial cells [16]. However, it is unclear whether STS can affect the transdifferentiation of HASMCs or modulate calcification-related factors in reducing phosphate-mediated vascular mineralization.
In the present study, in STS group 1, we found that the expressions of BMP-2 and Cbfα-1 were elevated significantly in the high-level phosphate group compared to that in the normal control group. However, both expressions were attenuated in the STS-treated groups. By contrast, MGP expression was increased notably in the STS-treated groups compared to that in the group treated with high-level phosphate. These findings suggest that STS can inhibit the transdifferentiation of HASMCs into osteoblast-like cells by downregulating the expressions of BMP-2 and Cbfα-1 while upregulating MGP expression mediated by phosphate. In STS group 2, MGP expression was enhanced significantly, whereas that of both BMP-2 and Cbfα-1 was reduced in the STS-treated groups. These results indicate that STS can inhibit the expressions of BMP-2 and Cbfα-1 while upregulating MGP expression of HASMCs. It is likely that STS can enhance the expression of MGP, and therefore inhibits the BMP2/Cbfα-1 signaling pathways and subsequent vascular mineralization. Meanwhile, in the present study, STS could attenuate calcium concentration and ALP activation (Fig. 5). However, more studies are warranted to elucidate the underlying mechanisms.
In conclusion, high-level phosphate can induce the transdifferentiation of HASMCs into osteoblast-like cells by enhancing the expressions of BMP-2 and Cbfα-1. Our study demonstrates for the first time that STS downregulates the expressions of BMP-2 and Cbfα-1 induced by high-level phosphate. In addition, STS can also upregulate the expression of MGP in a high-phosphate condition. These findings suggest that STS can reverse mineralization by modulating the expression of calcification-related factors. However, more studies are warranted to further elucidate the underlying mechanisms. Our findings offer a novel mechanism for explaining metastatic calcification and may lead to the development of new therapeutics aimed at reducing or preventing ectopic calcification.




