Association between serum soluble urokinase-type plasminogen activator receptor and atrial fibrillation
Abstract
Background
Circulating soluble urokinase-type plasminogen activator receptor (suPAR), which can reflect immune activation and low-grade inflammation, may be a novel biomarker of cardiovascular disease.
Methods
We investigated the potential association between suPAR and the prevalence of atrial fibrillation (AF) by analyzing patients with either sinus rhythm, paroxysmal atrial fibrillation (PAF), or non-paroxysmal atrial fibrillation (NPAF), which indicates either permanent or persistent AF.
Results
Among 426 patients enrolled (mean age 71.4±9.2 years; 110 (25.8%) female), 310, 62, and 54 were diagnosed with sinus rhythm, PAF, and NPAF, respectively. NPAF was >10-fold more prevalent in the highest suPAR quartile (>3534 pg/mL; 32 (30.2%) of 106 patients) than in the lowest suPAR quartile (<1802 pg/mL; 3 (2.8%) of 107 patients). Logistic regression analysis showed that, as compared with the lowest suPAR quartile, the highest suPAR quartile was associated with NPAF with an odds ratio of 6.48 (95% confidence interval, 1.71–24.5) after adjustment for sex, age, log(eGFR), C-reactive protein, and systolic blood pressure. In multivariate receiver operating characteristic analysis to predict NPAF, the area under the curve (AUC) for the combination of age, sex, log(eGFR), and C-reactive protein was 0.777 (standard error [SE], 0.036); the addition of log(suPAR) slightly improved the prediction (AUC, 0.812; SE, 0.034, P=0.084).
Conclusions
Serum suPAR was associated with AF, particularly NPAF, as demonstrated by multivariate logistic regression analysis. Whether suPAR promotes or maintains AF should be investigated in further studies.
1 Introduction
Soluble urokinase-type plasminogen receptor (suPAR) is formed when the urokinase-type plasminogen receptor is cleaved from the cell surface during inflammatory stimulation [1]. Therefore, suPAR reflects immune activation and is emerging as a biomarker of renal dysfunction [2], [3], microalbuminuria [4], infectious disease [5], [6], and malignant disorders [7]. Several studies have suggested that suPAR may have a role in the development of certain disease conditions [8], noting that suPAR might represent a possible therapeutic target [9], [10].
Considering that impaired renal function and the presence of microalbuminuria substantially enhance the risk of cardiovascular disorders [11], [12], it is thought that suPAR may represent a novel biomarker for various cardiovascular disorders. In fact, suPAR levels may be elevated in patients with coronary artery disease, stroke, and heart failure [13]-[16]. We recently reported that suPAR is associated with a low left ventricular ejection fraction (LVEF) and increased plasma levels of brain-type natriuretic peptide (BNP) [17].
Decreased renal function and microalbuminuria, both of which were reported to be linked to suPAR [4], are also known to be associated with cardiac arrhythmias, such as atrial fibrillation (AF) and ventricular arrhythmia [18]-[20]. At present, however, data regarding the association between suPAR and the prevalence of AF is scarce. To this end, the present study examined whether serum suPAR was associated with AF among patients admitted to the Cardiology Department of Osaka Medical College Hospital.
2 Material and methods
2.1 Ethics statement
The current retrospective study was approved by the Ethics Committee at Osaka Medical College and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients or their guardians to participate in this study.
2.2 Study population
Between December 2013 and May 2016, 4128 patients were admitted to the Cardiology Department; among them, suPAR was measured in 525 patients after obtaining written informed consent to participate in this study. Of these patients, the patients who met the following conditions were excluded: (1) had undergone implantation of pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization therapy devices; (2) cardiac rhythm other than sinus rhythm or AF; (3) previous catheter ablation; (4) chronic hemodialysis, (5) lack of echocardiographic data; and (6) no plasma BNP determinations (Fig. 1). Following these exclusions, 426 patients were enrolled in the current study. Paroxysmal AF (PAF) and non-paroxysmal (i.e., permanent or persistent) AF (NPAF) were defined as described elsewhere [21]. “Overall AF” was defined as either PAF or NPAF.
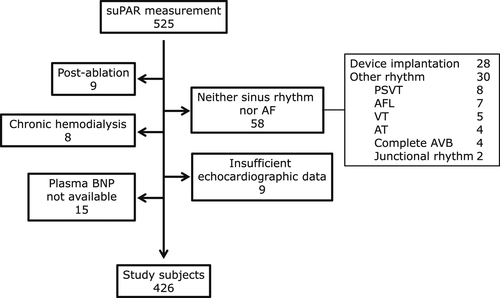
Flow diagram of patient enrollment. Abbreviations: AF, atrial fibrillation; PSVT, paroxysmal supra-ventricular tachycardia; AFL, atrial flutter; VT, ventricular tachycardia; AT, atrial tachycardia; AVB, atrioventricular block.
2.3 Laboratory analysis
Blood samples were collected in the morning after an overnight fast. Aliquots of serum and plasma were immediately obtained and stored at −80 °C until analysis. Serum levels of suPAR were measured using a kit (R&D Systems, Minneapolis, MN) according to the manufacturer׳s instructions. C-reactive protein (CRP) and BNP levels were measured by routine laboratory methods. Estimated glomerular filtration rate (eGFR) was calculated via the following Modification of Diet in Renal Disease equation for Japanese subjects: eGFR=194 × (serum creatinine)-1.094 × (age)-0.287 (×0.739, when female) [22].
2.4 Statistical analysis
Baseline characteristics were assessed with standard descriptive statistics. Data were expressed as mean ± standard deviation (SD), number (percentage) or median, and interquartile range (IQR). Spearman rank correlation testing was used to assess the correlation between two variables. SPSS statistical software (version 21.0; IBM Corp., Armonk, NY, USA) was used for data analysis. Multivariate receiver operating characteristic (ROC) analysis was performed using STATA 12 software (StataCorp LP, College Station, TX, USA).
3 Results
3.1 Patient characteristics
The mean age of the 426 participants who met the study criteria was 71.4±9.2 years, and 110 (25.8%) were women. The proportion of female patients did not differ significantly across the suPAR quartiles (Table 1). Anti-diabetic drugs were more frequently administered to patients with higher suPAR levels. As compared with patients in the lowest suPAR quartile, an elevated number of patients (>7-fold) were receiving loop diuretics in the highest suPAR quartile, which may, in part, be explained by the >12-fold greater prevalence of moderate or severe heart failure (New York Heart Association [NYHA] functional class III or IV) in this quartile. Among patients with sinus rhythm (n=310), PAF (n=62), and NPAF (n=54), respectively, 62 (20%), 19 (31%), and 45 (83%) were taking diuretic drugs (P<0.001, by χ2 test), and 42 (14%), 11 (18%), and 36 (67%) were diagnosed with heart failure of NYHA functional class III/IV.
| Variables | suPAR Q1 | suPAR Q2 | suPAR Q3 | suPAR Q4 | P |
|---|---|---|---|---|---|
| (n=107) | (n=106) | (n=107) | (n=106) | value | |
| Range, pg/mL | 513–1802 | 1812–2422.4 | 2422.7–3527 | 3534–26131 | |
| Women/men, n | 27 /80 | 23/93 | 29/78 | 31/75 | 0.637 |
| Age, years | 67.5±9.4 | 70.6 ±8.1 | 73.2±8.2 | 74.2±9.6 | <0.001 |
| Body mass index, kg/m2 | 23.9±3.3 | 23.1±3.1 | 23.7±3.5 | 22.7±3.3 | 0.026 |
| Systolic blood pressure, mmHg | 130±19 | 128±19 | 129±20 | 124±23 | 0.266 |
| Pulse rate, bpm | 71.7±15.3 | 72.0±14.3 | 74.5±15.8 | 78.0±19.6 | 0.020 |
| NYHA class III/IV, n (%) | 4 (3.7) | 8 (7.5) | 27 (25.2) | 50 (47.2) | <0.001 |
| Smoking status | |||||
| Never, n (%) | 45 (42.1) | 33 (31.1) | 29 (27.1) | 34 (32.1) | 0.199 |
| Former, n (%) | 53 (49.5) | 54 (50.9) | 59 (55.1) | 55 (51.9) | |
| Current, n (%) | 9 (8.4) | 19 (17.9) | 19 (17.8) | 17 (16.0) | |
| Medication | |||||
| ACE inhibitors/ARB, n (%) | 57 (53.3) | 57 (53.8) | 60 (56.1) | 58 (54.7) | 0.978 |
| Beta blockers, n (%) | 39 (36.4) | 43 (40.6) | 49 (45.8) | 57 (53.8) | 0.065 |
| Calcium channel blockers, n (%) | 47 (43.9) | 47 (44.3) | 56 (52.3) | 48 (45.3) | 0.570 |
| Any diabetic drugs, n (%) | 16 (15.0) | 28 (26.4) | 26 (24.3) | 42 (39.6) | 0.001 |
| Loop diuretics, n (%) | 9 (8.4) | 13 (12.3) | 26 (24.3) | 65 (61.3) | <0.001 |
| Thiazide diuretics, n (%) | 4 (3.7) | 6 (5.7) | 4 (3.7) | 15 (14.2) | 0.006 |
| Aldosterone antagonist, n (%) | 5 (4.7) | 7 (6.6) | 10 (9.3) | 33 (31.1) | <0.001 |
| Statin, n (%) | 62 (57.9) | 52 (49.1) | 58 (54.2) | 38 (35.8) | 0.008 |
- Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Patients with higher suPAR values exhibited a higher white blood cell count and CRP levels, and lower hemoglobin and serum albumin levels (Table 2) than that of patients with lower suPAR values. eGFR was significantly lower among patients in the higher suPAR quartiles. The prevalence of chronic kidney disease (i.e., eGFR < 60 mL/min/m2) among the first, second, third, and fourth suPAR quartiles were 32%, 48%, 57%, and 83%, respectively (P<0.001, by χ2 test). Patients with higher suPAR values had higher plasma BNP levels and lower LVEF, as reported previously [17]. Left atrial dimension was the largest among those with the highest suPAR levels. Patients with NPAF had a higher plasma BNP level (median, 332 pg/mL; interquartile range [IQR], 156–509 pg/mL) when compared with patients with sinus rhythm (median, 45 pg/mL; IQR, 45–115 pg/mL) and PAF (median, 68 pg/mL; IQR, 35–193 pg/mL) (P<0.001 by Kruskal Wallis test). The main cardiovascular conditions that resulted patients requiring hospital admission are shown in Table 3. Worsening heart failure was significantly more prevalent among higher suPAR quartiles.
| suPAR Q1 | suPAR Q2 | suPAR Q3 | suPAR Q4 | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | (n=107) | (n=106) | (n=107) | (n=106) | value | ||||
| Blood data | |||||||||
| White blood cell count, x103/µL | 5.28 | (4.42–6.45) | 5.90 | (4.58–6.92) | 6.40 | (5.02–8.01) | 6.07 | (5.22–7.49) | <0.001 |
| Hemoglobin, g/dL | 14.1 | (13.2–15.0) | 13.4 | (12.4–14.3) | 13.1 | (11.8–14.3) | 11.5 | (10.4–12.9) | <0.001 |
| Platelet count, x104/µL | 20.7 | (17.6–23.1) | 18.9 | (16.5–23.7) | 18.8 | (15.8–22.9) | 17.3 | (13.1–23.2) | 0.005 |
| Blood urea nitrogen, mg/dL | 16 | (13–19) | 16 | (13–20) | 18 | (14–23) | 24 | (18–31) | <0.001 |
| Serum creatinine, mg/dL | 0.83 | (0.68–0.98) | 0.84 | (0.73–1.01) | 0.93 | (0.75–1.20) | 1.18 | (0.93–1.66) | <0.001 |
| eGFR, mL/min/1.73 m2 | 67.9 | (57.7–76.9) | 61.2 | (51.3–75.5) | 56.8 | (41.4–67.5) | 42.2 | (29.0–55.2) | <0.001 |
| C-reactive protein, mg/dL | 0.08 | (0.03–0.15) | 0.08 | (0.04–0.21) | 0.18 | (0.06–0.55) | 0.25 | (0.09–0.98) | <0.001 |
| B-type natriuretic peptide, pg/mL | 31 | (14–58) | 44 | (23–113) | 80 | (33–236) | 185 | (62–475) | <0.001 |
| Echocardiographic data | |||||||||
| Left atrial dimension, mm (n=424) | 4.0 | (3.7–4.3) | 4.0 | (3.6–4.4) | 4.1 | (3.7–4.6) | 4.6 | (4.0–5.2) | <0.001 |
| LVDd, cm | 47 | (44–51) | 48 | (44–53) | 47 | (44–51) | 49 | (45–5.4) | 0.171 |
| LVDs, cm | 30 | (27–34) | 31 | (27–38) | 30 | (28–36) | 33 | (28–4.1) | 0.002 |
| LVEF, % | 66 | (60–72) | 65 | (56–71) | 64 | (54–69) | 60 | (42–68) | <0.001 |
| LVMI, g/m2 | 95 | (77–111) | 104 | (86–117) | 104 | (86–129) | 104 | (87–127) | 0.008 |
- Abbreviations: LVDd, left ventricular diastolic dimension; LVDs left ventricular systolic dimension; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
| suPAR quartiles | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Q1 | Q2 | Q3 | Q4 | |||||
| Admission diagnosis | |||||||||
| Acute myocardial infarction, n (%) | 1 | (0.9) | 3 | (2.8) | 6 | (5.6) | 3 | (2.8) | 0.259 |
| Unstable angina pectoris, n (%) | 6 | (5.6) | 8 | (7.5) | 6 | (5.6) | 2 | (1.9) | 0.303 |
| Worsening heart failure, n (%) | 5 | (4.7) | 14 | (13.2) | 28 | (26.2) | 58 | (54.7) | <0.001 |
| Stable angina pectoris, n (%) | 20 | (18.7) | 23 | (21.7) | 14 | (13.1) | 5 | (4.7) | 0.003 |
| Arrhythmic diseases, n (%) | 25 | (23.4) | 20 | (18.9) | 18 | (16.8) | 17 | (16.0) | 0.519 |
| Follow-up coronary angiography, n (%) | 28 | (26.2) | 22 | (20.8) | 18 | (16.8) | 11 | (10.4) | 0.025 |
| Pre-operative screening before non-cardiovascular surgery n (%) | 3 | (2.8) | 2 | (1.9) | 10 | (9.3) | 4 | (3.8) | 0.038 |
| Pre-operative screening before cardiovascular surgery n (%) | 7 | (6.5) | 13 | (12.3) | 12 | (11.2) | 8 | (7.5) | 0.409 |
| Arteriosclerosis obliterans, n (%) | 3 | (2.8) | 2 | (1.9) | 12 | (11.2) | 5 | (4.7) | 0.009 |
| Silent myocardial ischemia, n (%) | 23 | (21.5) | 20 | (18.9) | 20 | (18.7) | 20 | (18.9) | 0.946 |
3.2 Prevalence of overall AF, PAF, and NPAF across the suPAR quartiles
The prevalence of overall AF (P=0.002 by χ2 test) and NPAF (P<0.001) was highest among patients in the highest suPAR quartile (Fig. 2); however, the prevalence of PAF did not differ significantly across the four quartiles (P=0.085). The finding that the prevalence of arrhythmic disease did not significantly differ across suPAR quartiles (Table 3) suggests that the main reason(s) for admission among some patients with PAF or NPAF were not arrhythmia per se. In fact, “worsening heart failure” was one of the reasons for admission in 15 (24.2%) of 62 PAF patients and 42 (77.8%) of 54 NPAF patients, whereas “arrhythmia” was the one reason for admission in 49 (61.3%) of PAF patients, and 17 (21.3%) of NPAF patients.
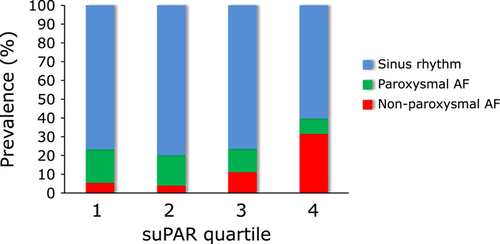
Prevalence of atrial fibrillation (AF) according to suPAR quartiles. The prevalence of overall AF (P=0.002 by χ2 test) and non-paroxysmal AF (NPAF) (P<0.001) was significantly higher among patients in the highest suPAR levels.
Next, we analyzed the association between suPAR and AF prevalence via logistic regression analysis. In the non-adjusted model (model 1), the highest suPAR quartile was found to be positively associated with NPAF and overall AF (Table 4). Following multivariate adjustments for sex, age, and low (eGFR) (model 2), the association between suPAR and NPAF, but not overall AF, remained statistically significant. After additional adjustment for CRP and systolic blood pressure (model 3), the highest suPAR quartile remained significantly associated with NPAF. Following further adjustment for plasma BNP level, the highest suPAR quartile was still associated with NPAF with an odds ratio of 5.94 (95% CI 1.56–22.7, P<0.01); however, after additional adjustments for diuretic use (loop or thiazide, model 4) or moderate to severe heart failure (NYHA III/IV) (data not shown), significance was abolished in the association between the higher suPAR and NPAF.
| suPAR Q1 | suPAR Q2 | suPAR Q3 | suPAR Q4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |||
| Dependent variable: NPAF | |||||||||
| Model 1 | 1 | (ref) | 2.08 | (0.51–8.54) | 4.79* | (1.33–17.3) | 15.0** | (4.42–50.8) | |
| Model 2 | 1 | (ref) | 1.79 | (0.43–7.41) | 3.37 | (0.91–12.5) | 8.21** | (2.22–30.4) | |
| Model 3 | 1 | (ref) | 1.62 | (0.39–6.77) | 2.96 | (0.79–11.1) | 6.48** | (1.71–24.5) | |
| Model 4 | 1 | (ref) | 1.26 | (0.28–5.69) | 2.16 | (0.54–8.60) | 2.97 | (0.74–11.8) | |
| Dependent variable: overall AF | |||||||||
| Model 1 | 1 | (ref) | 0.82 | (0.43–1.55) | 0.90 | (0.48–1.70) | 2.21** | (1.23–3.98) | |
| Model 2 | 1 | (ref) | 0.75 | (0.39–1.46) | 0.72 | (0.37–1.40) | 1.45 | (0.73–2.89) | |
| Model 3 | 1 | (ref) | 0.73 | (0.38–1.43) | 0.70 | (0.36–1.38) | 1.37 | (0.68–2.79) | |
| Model 4 | 1 | (ref) | 0.66 | (0.33–1.32) | 0.58 | (0.28–1.17) | 0.85 | (0.40–1.81) | |
- Model 1, non-adjusted; model 2, adjusted for sex, age and log(eGFR); model 3, adjusted for variables used in model 2 plus systolic blood pressure, and CRP; model 4, adjusted for variables used in model 3 plus diuretic use. * and ** indicate p<0.05 and P<0.01, respectively, versus the lowest suPAR quartile. Abbreviations: OR, odds ratio; CI, confidence interval; ref, reference.
When log(suPAR) was used in the place of suPAR quartiles, log(suPAR) was associated with NPAF in model 3 with an odds ratio of 1.93 (95% CI 1.29–2.88, P<0.01) per one log(suPAR) basis, and the addition of plasma BNP level or moderate to severe heart failure to the variables used in adjusting model 3, log(suPAR) was associated with NPAF with an odds ratio of 1.68 (1.15–2.64, P<0.01) and 1.46 (0.96–2.23, P=0.076) per one log(suPAR) SD basis, respectively.
3.3 Multivariate ROC analysis
In multivariate ROC analysis, the area under the curve (AUC) to predict NPAF, for a combination of age, sex, log(eGFR), and CRP (model 1), was 0.777 (standard error [SE], 0.036); further addition of log(suPAR) incrementally improved the prediction (AUC, 0.812; SE, 0.034; P=0.084; Fig. 3). The AUC to predict NPAF for a combination of model 1 plus diuretic use was 0.869 (SE, 0.027); in this case, the further addition of log(suPAR) did not improve the prediction (AUC, 0.869; SE, 0.029, P=0.997).
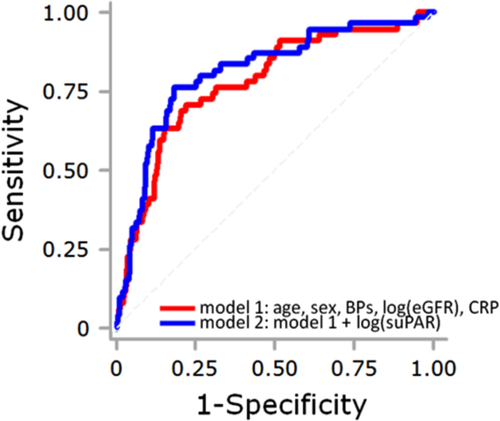
Receiver operating characteristic (ROC) analysis for the prediction of non-paroxysmal atrial fibrillation (NPAF). The red line indicates the ROC curve used to predict NPAF for a combination of age, sex, log(eGFR), systolic blood pressure and CRP (model 1). Purple line shows the ROC curve used to predict NPAF for model 1 plus log(suPAR) (model 2). The area under the ROC curve tended to increase in model 2 (0.812), as compared with model 1 (0.777, P = 0.084).
4 Discussion
The findings of the current study demonstrated that serum suPAR was significantly associated with the prevalence of AF, particularly non-paroxysmal AF. Although this association remained significant after multivariate adjustment including renal function and CRP, further adjustment for diuretic use or for the presence of moderate to severe heart failure abolished the statistical significance, indicating that the observed association may be explained, at least in part, by the higher prevalence of AF among heart failure patients. In ROC analysis, the addition of suPAR improved the prediction of NPAF, suggesting the utility, albeit marginal, of suPAR as a biomarker or a potential therapeutic target for NPAF among patients with cardiac conditions.
What would be the possible mechanisms, if any, linking suPAR and NPAF? Like CRP, suPAR is considered to be a marker of low-grade inflammation [7], and has been reported to be associated with cardiovascular events and coronary artery disease [13], [23], [24]. Although the association between suPAR and heart failure [1], [16], [17], a condition in which enhanced immune activation and inflammation may also have a role [25], has been reported in several studies, minimal information is currently available regarding the association between suPAR and arrhythmic disorders, in which enhanced inflammation may also have a role [26].
In a prospective cohort study of the general population in Sweden, Borne et al. examined the potential association between suPAR and the incidence of heart failure and AF [1]. They found that the incidence of AF among individuals in the highest suPAR tertile was significantly greater than that among those in the lowest tertile, with a hazard ratio of 1.39 after adjustment for age and sex. However, significance was lost after further adjustments for various variables, including systolic blood pressure, diabetes, cystatin C, and N-terminal pro-BNP (NT-proBNP). Considering that N-terminal pro-BNP may be increased in patients with AF, whether NT-proBNP or BNP should be entered as an independent variable may require discussion. In the current study, however, the association between suPAR and NPAF remained significant after further adjustment for plasma BNP, suggesting the possibility that the association between suPAR and AF may, in part, be independent of heart failure. Log(suPAR) was only significantly associated with NPAF after adjustment variables in model 3 plus moderate to severe heart failure. Therefore, we should examine this point through future studies that involve a larger number of study patients.
Sonmez et al. have recently reported that several fibro-inflammatory markers that are associated with AF incidence are also correlated with arterial remodeling, which is assessed by the left atrial volume index (LAVI) [27], although how these fibro-inflammatory markers are associated with left atrial remodeling remains unknown. It has recently been shown that arterial remodeling, which is related to AF incidence [28], is associated not only with increased interstitial fibrosis but also with an increase in the number of immune cells in the left atrium [29]. The relationship between chronic low-grade inflammation and left atrial remodeling may be further supported by the finding that individuals with high CRP have left atrial enlargement, even without AF [30]. Taken together, enhanced fibrosis [31] and immune activation and chronic inflammation [29] may explain the association observed between suPAR and AF. Whether study participants with sinus rhythm that have higher suPAR levels are more likely to have a greater LAVI should be investigated in our study population in future studies.
The current study has some limitations. Firstly, owing to its cross-sectional nature, the study cannot provide information on the causal or resultant nature of the relationship. Whether patients with elevated suPAR levels were more susceptible to AF incidence or whether the occurrence of AF increases serum suPAR levels should be determined in future longitudinal studies. Secondly, further adjustment for diuretic use abolished the significant association between suPAR and NPAF (Table 4, model 4); however, NPAF patients exhibited higher BNP levels and were more likely to be diagnosed with NYHA functional class III/IV; therefore, diuretic use may, in part, be a consequence of heart failure aggravated by the presence of NPAF, which would suggest that entering diuretic use as an independent variable would lead to over-adjustment of the model. On the other hand, in ROC analysis without diuretic use, inclusion of suPAR led to a slight, yet significant, improvement in NPAF prediction. Therefore, whether suPAR represents a truly useful biomarker in NPAF should be investigated in future, particularly longitudinal, studies. Thirdly, among the study population, those who had been subjected to catheter ablation were excluded (Fig. 1). Although the number of these patients was relatively small (n=9), it may have led to an underestimation of the prevalence of AF among the study population and thereby have influenced the results obtained. Lastly, suPAR levels may be associated with the lifestyles that are likely to have a relationship with the occurrence of AF [32], [33]. Additional, more detailed, data than the information obtained in the current study is required to assess the influence of lifestyles.
5 Conclusions
Among patients with cardiac issues, serum suPAR was associated with AF, particularly NPAF, and multivariate logistic regression analysis demonstrated that the association between suPAR and NPAF was independent of age, sex, systolic blood pressure, eGFR, CRP, and plasma BNP. Whether elevated suPAR has a role in the development of AF warrants further investigation.
IRB approval
-
1
- 1)
Date of IRB approval: 2011/05/22;
- 2)
Approval number, No. 892:
- 3)
Status of informed consent; written IC was taken
-
2
- 1)
Date of IRB approval: 2014/09/01;
- 3)
Approval number, RIN31:
- 4)
Status of informed consent; written IC was taken.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported in part by Grants in Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (no. 15K09106). We would like to thank Chieko Ohta, Yumiko Ohgami, and Megumi Hashimoto for their excellent technical assistance.




