Usefulness of a wearable cardioverter defibrillator combined with catheter ablation for ventricular tachyarrhythmia storms after a myocardial infarction: A case report
Abstract
We report a case of a 60-year-old man with recurrent poly- and monomorphic ventricular tachycardia related to a recent myocardial infarction. Due to drug-refractory ventricular tachycardia despite complete revascularization, he underwent catheter ablation. Afterwards, he was fitted with a wearable cardioverter defibrillator. Three months later, no ventricular tachycardia had been recorded and an electrophysiologic study failed to induce an episode. Thus, wearable cardioverter defibrillators are useful bridging devices pending a final decision to implant a cardioverter defibrillator.
1 Introduction
Implantable cardioverter defibrillators (ICDs) are an established means of preventing sudden cardiac death from ventricular tachyarrhythmias [1]. However, current guidelines recommend deferring implantation of ICDs for 40 days or three months post-myocardial infarction (MI), depending on whether acute revascularization is achieved [1], [2]. Recently, wearable cardioverter defibrillators (WCDs) have emerged as a reasonable choice for patients in whom recovery of the left ventricular ejection fraction (LVEF) is expected during the post-MI chronic phase [3].
In this report, we describe a patient with recurrent ventricular tachycardia (VT) related to a recent MI, who was successfully managed with radiofrequency catheter ablation (RFCA) targeting the triggering ventricular premature contractions (VPCs) and the surrounding substrate in conjunction with a WCD during the acute waiting period. The WCD enabled reevaluation of the mandatory criteria for ICD implantation after three months of follow-up.
2 Case Report
A 60-year-old man with a history of diabetes mellitus and hypertension that had not been treated appropriately experienced crushing chest pain 10 days before admission accompanied by progressive dyspnea. ST segment elevation in the inferior leads and abnormal Q waves in the inferior and precordial leads were observed. Echocardiography revealed severe left ventricular (LV) dysfunction (LVEF 26.6%). Pulmonary congestion was noted on a chest radiograph.
On the 8th inpatient day, after his heart failure was stabilized, coronary angiography revealed total occlusion of the proximal right coronary artery (RCA) and 90% stenosis of the proximal left anterior descending (LAD) artery (Fig. 1A and B). Two days after angiography, the occluded RCA was successfully revascularized with a stent (Fig. 1C), and oral carvedilol at 2.5 mg/day was started.
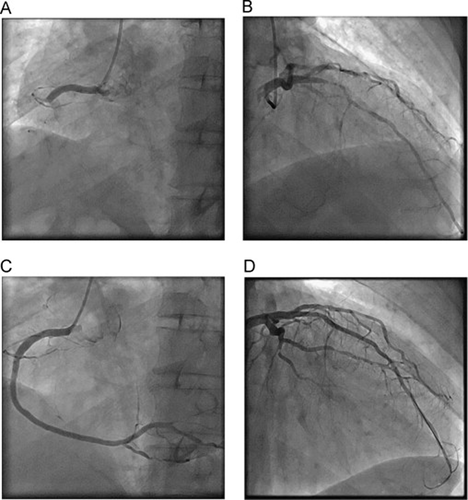
Coronary angiography—the first coronary angiography: (A) total occlusion of the proximal RCA (LAO view) and (B) LCA with severe stenosis at the proximal LAD (RAO-cranial view). Coronary angiography—after stent implantation: (C) angiography of the RCA (LAO view) and (D) angiography of the LCA (RAO-cranial view). RCA, right coronary artery; LCA, left coronary artery; LAD, left anterior descending artery; LCX, left circumflex artery; RAO, right anterior oblique; LAO, left anterior oblique.
On the 13th day, just before planned percutaneous coronary intervention for the LAD, he developed sustained monomorphic VT, which was terminated by 125 mg of intravenous amiodarone. However, he subsequently developed a hemodynamically unstable polymorphic VT requiring five electric cardioversions on the same day. Emergency coronary angiography revealed no subacute stent thrombosis or LAD occlusion; the LAD was urgently revascularized by stent implantation (Fig. 1D). Two days later, despite complete revascularization, he had another electrical storm in the coronary care unit and required seven electrical cardioversions that day. His hemodynamic status deteriorated significantly, necessitating management of his heart failure with deep sedation. During sedation, the dose of carvedilol was increased to 5 mg/day. After extubation, however, sustained VT occurred on the 23rd day, requiring electrical cardioversions twice that day.
A five-lead electrocardiogram showed recurrent VT triggered by the same morphologic VPC with a coupling interval of about 320 ms to the preceding QRS complex (Fig. 2). On the 25th inpatient day, an urgent electrophysiological study (EPS) and RFCA were performed.
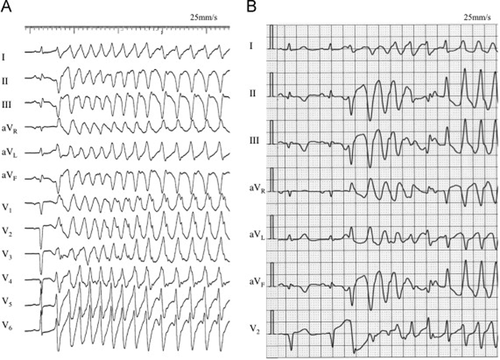
(A) Twelve-lead electrocardiogram recorded during emergency coronary angiography on the 13th inpatient day. (B) Five-lead electrocardiogram monitor recording on the 23rd day showing recurrent ventricular tachycardia. Those episodes were always initiated by the same morphologic ventricular premature contractions.
For mapping and pacing, multiple electrode catheters were positioned in the coronary sinus and right ventricle. CARTO® 3 System mapping technology (Cartosound, Biosense Webster, Diamond Bar, CA, USA) was used to create a three-dimensional reconstruction of the LV. An ablation catheter (NaviStar ThermoCool®, Biosense Webster, Diamond Bar, CA, USA) was then used to create a voltage map, revealing an area of low voltage (LVA) in the inferobasal wall. Discrete Purkinje potentials were observed along this LVA during sinus rhythm. During the EPS, VPCs were rare. However, on the mid-portion of the inferior wall of the LV, where there was a relatively viable region of the LVA, the local Purkinje potentials preceded the spontaneous targeted VPCs by 54 ms (Fig. 3). The pace map at that point showed nearly identical morphology to the target VPCs (11.5/12). Radiofrequency energy with a target temperature of 45 °C and maximum power of 40 W was delivered to the LVA to eliminate the trigger and modify the substrate of the ventricular arrhythmias. After the ablation, isoproterenol was intravenously administered and no target VPCs or VTs were recorded. To minimize procedure time, no VT induction was performed because of his unstable hemodynamic state.
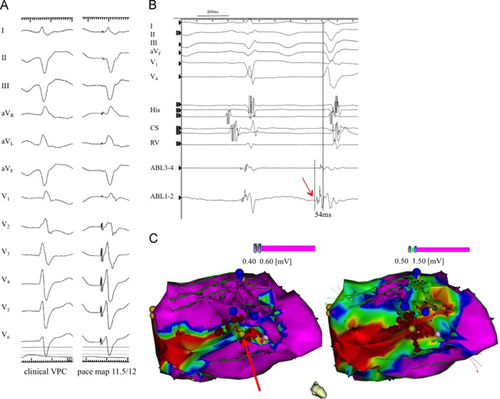
Electrophysiological study and radiofrequency catheter ablation. (A) Clinical VPC with almost the same morphologic features as those that initiated the ventricular tachycardia (left panel). Pace-mapping generated a morphological match (right panel). (B) Intracardiac electrocardiogram during the electrophysiological study. The red arrow shows a Purkinje potential preceding a VPC. (C) Electro-anatomic voltage map. The map delineates the low voltage area. The bright yellow tag shown by the red arrow represents the site where the pace map was recorded. The red tags show the sites to which radiofrequency energy was delivered. The dark blue tags represent the sites where discrete Purkinje potentials were recorded. VPC, ventricular premature contraction.
After the RFCA, neither sustained VT nor the VPCs that triggered the VT were recorded. The patient was fitted with a WCD (LifeVest, Zoll, Pittsburgh, PA, USA) on the third day post-ablation, and sotalol 80 mg a day was started. He was discharged from the coronary care unit the following day. During cardiac rehabilitation, the patient's medical therapy was dynamically adjusted. The dose of sotalol was temporarily increased to 160 mg a day, then gradually decreased, and finally stopped. He satisfactorily progressed in his rehabilitation protocol, and echocardiography revealed some improvement in the LVEF (from 26.6% to 45.2%). He was discharged on the 44th inpatient day with the WCD and on 5 mg of oral carvedilol daily, but without any antiarrhythmic drugs. His compliance was satisfactory, and no WCD shocks were recorded after discharge.
Three months after the WCD was placed, echocardiography showed further improvement in the LVEF (55.8%). The WCD was then discontinued and the patient was readmitted for a follow-up EPS. A standard programmed stimulation protocol [4] failed to induce sustained VT or any hemodynamic compromise. The patient was advised that ICD implantation was not mandatory. He agreed to follow-up without an ICD. Oral carvedilol was increased to 10 mg/day, and he was discharged. He is closely followed and his multiple coronary risk factors – diabetes mellitus, hypertension, and dyslipidemia – are appropriately managed to prevent any secondary acute coronary events. He has had no episodes of ventricular arrhythmia for eight months.
3 Discussion
We believe this is the first reported case of a patient in Japan successfully managed with a combination of RFCA targeting the triggering VPC and the substrate around it followed by preventative management with a WCD during the acute risk period. Our case highlights a specific example of need to exercise prudence in determining the indication for an ICD implantation.
Ventricular arrhythmias are the most common cause of sudden cardiac death related to an MI, especially in the early phase [5]. Although ICDs significantly reduce the incidence of sudden arrhythmic death in patients with LV dysfunction in the late post-MI phase [6]-[8], prophylactic ICDs implanted within the first 40 days after infarction do not reduce the overall mortality in high-risk patients [9]. Guidelines obtained from clinical trial data recommend against the implantation of an ICD in the early phase of an MI [1], [2]. A waiting period of 40 days or 3 months is recommended, depending on whether revascularization is performed. WCDs are external devices capable of automatic detection of ventricular tachyarrhythmias and defibrillation. It has recently been demonstrated that WCDs reduce the incidence of sudden cardiac death in post-MI patients during that waiting period [4]. In the early phase of an MI, patients with poor LV function (LVEF≤35%) are eligible for this device as “bridging therapy”. About 30% of patients with an MI have LVEF persistently ≤35%, which may deteriorate even more, but LVEF improves in about 70% [10]. Some MI patients do not fulfill the criteria for ICD implantation after the waiting period. WCDs “bridge” the time pending a final decision about ICD implantation.
Electrical storms in patients during the early phase of an MI are occasionally initiated by abnormal automaticity originating from damaged partially depolarized myocytes or Purkinje fibers [11]. Several studies have reported that RFCA targeting Purkinje potentials in the infarction border zone is an effective means of managing such drug-refractory, repetitive VT after an MI [12]-[14]. In our case, five-lead telemetry monitoring was helpful in identifying the target monomorphic VPCs triggering the VT, even though these VPCs occurred infrequently. Elimination of the triggering VPCs may not eradicate episodes of VT, which might still be inducible if the substrates persist. Guided by the voltage map, we delivered multiple RFCAs around the origin of the target VPCs. Although we did not strictly test the VT inducibility just after ablation, we demonstrated the non-inducibility of any VTs during the follow-up EPS, in association with significant recovery of the LVEF. As previously reported, the non-inducibility of VT as a procedural end-point of RFCA and a preserved LVEF are known predictors of recurrence after RFCA for an electrical storm [1], [15]-[17]. We believe that we achieved long-term prevention of VT in this patient by the substrate ablation and mechanical and electrical LV remodeling during the waiting period.
In this patient, ICD implantation as a means of secondary prevention of sudden arrhythmic death would be highly controversial. Nevertheless, follow-up of the patient without an ICD was appropriate because complete revascularization was achieved, the patient's hemodynamic and electrophysiological deterioration in the acute phase of MI was reversed, and an EPS showed that RFCA was effective in eliminating the triggering VPCs and the substrate of VT [5]. We believe that preventing secondary acute coronary events has much greater priority.
One limitation of our report is that it described a single case with a short follow-up. However, considering all the facts of the situation, it is reasonable to conclude that the RFCA was successful. Use of a WCD for 3 months can be bridging therapy pending a final decision about ICD implantation.
Conflict of interest
The authors have no conflicts of interest.




