Reduced systemic vascular resistance is the underlying hemodynamic mechanism in nitrate-stimulated vasovagal syncope during head-up tilt-table test
Abstract
Background
Nitroglycerin (NTG) challenge during head-up tilt-table testing (HUTT) is often utilized to determine the etiology of unexplained vascular syncope. However, conflicting results concerning nitrate-induced hemodynamic changes during HUTT have been reported. The purpose of this study was to assess the determinants of presyncopal symptoms during NTG-stimulated HUTT.
Methods
We evaluated 40 patients with suspected vasovagal syncope. Beat-to-beat changes in blood pressure, heart rate (HR), cardiac index (CI), and systemic vascular resistance (SVR) during HUTT were measured with thoracic impedance cardiography and a plethysmographic finger arterial pressure monitoring device.
Results
None of the 40 patients complained of presyncopal symptoms during passive HUTT. However, after the administration of NTG 28 patients showed presyncopal symptoms (NTG+ group) and the remaining 12 patients did not (NTG– group). HR, CI, and the stroke index did not significantly differ between the two groups, whereas mean arterial pressure and SVR were significantly lower in the NTG+ group.
Conclusions
Presyncopal symptoms during NTG-stimulated HUTT are SVR mediated, not cardiac output mediated. This study challenges the conventional idea of a decrease in cardiac output mediated by NTG as the overriding cause of presyncopal symptoms during HUTT.
1 Introduction
Syncope is a syndrome in which a relatively short period of temporary and self-limited loss of consciousness is caused by a transient diminution of blood flow to the brain. The prevalence of syncope in the general population is reported to vary from 15% to 23% [1]. Vasovagal syncope is a transient loss of consciousness caused by systemic arterial hypotension resulting from reflex vasodilatation and/or bradycardia. It is the most common cause of syncope in the general population and is responsible for one-fifth of all syncope episodes [2].
The diagnosis of vasovagal syncope is usually based on a combination of careful history taking, clinical examination, and a surface electrocardiogram to exclude cardiac and non-cardiac causes of syncope [3], [4]. When the etiology of syncope is uncertain, head-up tilt-table testing (HUTT) is commonly used to reproduce symptoms in patients with suspected vasovagal syncope [3], [5]. Nitroglycerin (NTG) challenge during HUTT is often utilized to determine the etiology of unexplained syncope [5], [6]. NTG may facilitate syncope through various pathways, but the precise mechanism of its contribution to syncope remains unclear [7].
Traditionally, venodilation and the venous pooling of blood into the low extremities and splanchnic and mesenteric vasculature along with the subsequent reduction of left ventricular preload have been regarded as the main hemodynamic effects of NTG, while arterial dilatation is thought to play a smaller role [8], [9]. Some studies showed that sublingual NTG provokes a cardiac output-mediated vasovagal response that is not preceded by a fall in systemic vascular resistance (SVR) [10], [11]. However, conflicting results concerning NTG-induced hemodynamic changes have been reported. Several studies revealed decreases in SVR without a marked change in cardiac output (CO) or heart rate (HR) during NTG administration [12].
The purpose of this study was to assess the determinants of presyncopal symptoms and to compare hemodynamic patterns between patients with and without presyncopal symptoms during NTG-stimulated HUTT.
2 Materials and methods
2.1 Study population and head-up tilt-table test protocol
We evaluated 42 consecutive patients admitted to our hospital for syncope or presyncope between November 2012 and March 2013. All patient medical histories included at least one syncopal episode suspected to have been caused by vasovagal response in the last year. The exclusion criteria were an age of less than 18 years, a history of cardiovascular disease, carotid sinus syndrome, any disease that might affect the autonomic nervous system, and the use of medication that might affect the cardiovascular system.
HUTT was performed in accordance with current guidelines by a trained nurse [4]. Testing was performed between 9:00 AM and 1:00 PM in a quiet, temperature-controlled (23 °C) room [13].
Patients refrained from caffeine-containing products, smoking, and heavy meals for at least 6 h and from alcohol for at least 24 h prior to the test. After 10 min of supine rest, the table was tilted to 70°(passive tilt phase). If presyncopal symptoms did not occur during 20 min of passive tilt, 0.25 mg of NTG was administered sublingually and testing continued for another 20 min (NTG phase).
Presyncopal symptoms were defined as light-headedness, nausea, blurred vision, pallor, and sweating in association with hypotension and/or bradycardia. Impending syncope was halted by means of tilt-back. Patients were divided into two groups according to whether or not they experienced presyncopal symptoms after NTG administration.
2.2 Hemodynamic measurements
Hemodynamic parameters were determined with a thoracic bioimpedance cardiography (ICG) device (Niccomo™, Medis, Ilmenau, Germany), which records continuous changes in body electric impedance during the cardiac cycle. After rubbing and cleaning the skin with alcohol to achieve as low as possible skin-to-electrode impedance, two sensors with gel pads were carefully placed on each side of the thorax along the mid-axillary line and the two remaining sensors were placed on each side of the neck just above the clavicle. An alternating current of 1.5 mA (85 kHz) was applied, and the ICG signal was continuously displayed on the screen along with the electrocardiographic signal. Stroke volume (SV, mL) was computed every single heart beat and averaged over 15 heart beats to take physiological respiratory variations into account. The stroke index (SI, min/m2) was calculated based on SV and body surface area. CO (L/min) was the product of the estimated SV and HR. The cardiac index (CI, L/min/m2) was then calculated based on the SV, HR, and body surface area. Raw data were analyzed offline and all ICG measurements were performed with a single monitor using PC-version 1.9 of the Niccomo software. Beat-to-beat changes in systolic and diastolic arterial blood pressures were measured at every heart beat from the finger arterial pressure wave form by a plethysmographic method via the Finometer (Finometer® PRO, Finapres Medical System, Netherlands) and averaged. mean arterial pressure (MAP) was the true integral of the arterial pressure wave over one beat divided by the corresponding beat interval. HR (bpm) was obtained continuously by five-lead electrocardiographic monitoring. SVR (dyn s/cm5) was calculated as the MAP at heart level divided by the computed CO.
2.3 Study periods
-
Supine baseline: beat-to-beat data of the last 5 min in the supine position before head-up tilting were averaged.
-
After tilt: beat-to-beat data from the 5 min in the 70° head-up tilt position to the last minute before NTG administration were averaged (early steady-state circulatory adjustment). The first 5 min after head-up tilting were not included in order to avoid transition phenomena.
-
After NTG administration: beat-to-beat data of the last 5 min after NTG administration were averaged. If presyncopal symptoms occurred, the last 5 min before tilt back or counter-maneuvers were averaged.
2.4 Statistical analysis
Data were reported as mean and standard deviation for continuous variables and as percentages for categorical variables. For the testing of significance, between-group comparisons of continuous variables were analyzed by independent t-tests. Comparisons of categorical variables were generated by the Pearson χ2 test. Statistical comparisons were performed using SPSS, version 15.0 software (SPSS Inc.). All tests were 2-sided, and the results were considered statistically significant at a p-value <0.05.
3 Results
3.1 Study population
Of 42 patients, 2 were excluded because presyncopal symptoms were noted prior to the administration of NTG. The 28 (70%) patients who showed presyncopal symptoms after NTG administration were classified as the NTG+ group. Another 12 patients who had negative results were classified as the NTG− group. Table 1 lists comparative differences between the two groups. The NTG+ and NTG− groups did not differ significantly with regard to age, gender distribution, body mass index (BMI), smoking status, and history of hypertension or diabetes. The number of previous syncopal episodes was not different between the two groups (NTG+ vs. NTG−; 2.6±2.2 vs. 2.2±1.4; p=0.5). The presence of prodromal symptoms before syncope was similar between the two groups (NTG+ vs. NTG–; 70% vs. 75%; p=1.0) and nausea with sweating was the most frequently observed symptom. The occurrence of associated injury was similar in the NTG+ and NTG– groups (NTG+ vs. NTG–; 15% vs. 17%; p=1.0).
| Variables | NTG+ group (n=28) | NTG− group (n=12) | p-Value |
|---|---|---|---|
| Age (years) | 38±15 | 43±18 | 0.17 |
| Male (n, %) | 11 (39%) | 8 (66%) | 0.21 |
| Body mass index (kg/m2) | 23.8±4 | 24.3±3.7 | 0.18 |
| Smoking (n, %) | 4 (14.3%) | 3 (25%) | 0.13 |
| Hypertension (n, %) | 4 (14.3%) | 2 (16.7%) | 0.64 |
| Diabetes (n, %) | 1 (3.6%) | 1 (8.3%) | 0.51 |
| Syncopal episodes (n) | 2.6±2.2 | 2.2±1.4 | 0.5 |
| Presence of prodromal symptoms (n, %) | 19 (70.4%) | 9 (75%) | 1.0 |
| Presence of associated injury (n, %) | 4 (14.8%) | 2 (16.7%) | 1.0 |
- Data are expressed as the mean±SD or number (percentage). Body mass index (BMI)=Weight (kg)/Length2 (m2).
In the NTG+ group, HUTT after NTG administration was aborted after 3.5±1.8 min due to presyncopal symptoms and a progressive fall of blood pressure.
3.2 Hemodynamic data in the supine position before tilting
In the supine position before tilting, finger systolic pressure was higher in the NTG– group than in the NTG+ group (122±17 vs. 110±15 mm Hg; p=0.06). None of the remaining circulatory parameters including HR, LVET, CI, SI, and SVR differed significantly between the two groups in the supine position before tilting (Table 2). All patients were normotensive, with an average MAP of 86±13 mm Hg. In the supine position before tilting, the estimated CI was 2.9±0.5 and 2.8±0.7 l/min/m2 (p=0.27) and the estimated SVR was 1194±296 and 1228±416 dyn s/cm5 (p=0.32) in NTG+ and NTG− groups, respectively.
| Supine position | Passive tilt | |||||
|---|---|---|---|---|---|---|
| NTG+ (n=28) | NTG− (n=12) | p | NTG+ (n=28) | NTG− (n=12) | p | |
| Blood pressure (mmHg) | ||||||
| Finger systolic | 110±15 | 122±17 | 0.06 | 90±12 | 107±21 | 0.03 |
| Finger diastolic | 58±11 | 61±14 | 0.42 | 52±9 | 60±9 | 0.03 |
| Brachial systolic | 115±14 | 124±18 | 0.10 | 101±1 | 124±21 | 0.001 |
| Brachial diastolic | 60±11 | 63±14 | 0.47 | 55±12 | 66±9 | 0.05 |
| Mean arterial | 78±12 | 88±21 | 0.25 | 71±13 | 88±11 | 0.005 |
| Heart rate (bpm) | 67±11 | 67±9 | 0.87 | 82±17 | 70±14 | 0.04 |
| LVET (ms) | 308±47 | 308±52 | 0.99 | 271±41 | 248±43 | 0.16 |
| Cardiac index (l/min/m2) | 2.9±0.5 | 2.8±0.7 | 0.62 | 2.6±0.6 | 2.6±0.4 | 0.62 |
| Stroke index (ml/m2) | 46±11 | 42±11 | 0.27 | 35±9 | 35±6 | 0.54 |
| SVR (dyns/cm5) | 1194±296 | 1228±416 | 0.32 | 1186±346 | 1203±287 | 0.90 |
| SVRI (dyns/cm5/m2) | 661±226 | 661±223 | 0.99 | 642±256 | 647 ±169 | 0.36 |
- LVET, left ventricular ejection time; SVR, systemic vascular resistance; SVRI, systemic vascular resistance index.
3.3 Hemodynamic data during passive tilting
In the 70-degree head-up tilt position, all patients were asymptomatic. Both finger and brachial systolic and diastolic pressures as well as MAP were significantly decreased in the NTG+ group, along with a compensatory rise in HR in order to maintain CI (Table 2, Fig. 1A–C). In both groups, a fall in SI occurred upon moving from the supine to the tilt position (% fall of SI; NTG+ vs. NTG−; 29% vs. 21%; p=0.30). SVR was not significantly different between the two groups during passive tilting (NTG+ vs. NTG−; 1,186±346 vs. 1,203±287; p=0.90; Table 2, Fig. 2A and B).
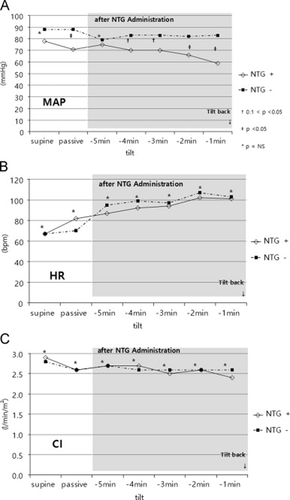
Changes in the average mean arterial pressure (MAP) (A), heart rate (HR) (B), and cardiac index (CI) (C) during three phases: supine baseline, passive tilt, and after nitroglycerin (NTG) administration. Particularly for the post-NTG administration phase, parameters during last 5min before tilt-back are presented. MAP significantly decreased in the NTG+ group along with a compensatory rise in HR in order to maintain CI.
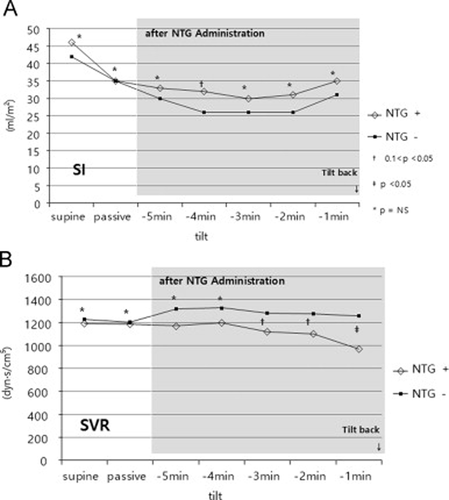
Changes in stroke index (SI) (A) and systemic vascular resistance (SVR) (B). SVR was significantly decreased in the NTG+ group after NTG administration, while SI was preserved.
3.4 Hemodynamic parameters after NTG administration
On average, peak HR in the entire cohort reached 2 min prior to the onset of presyncopal symptoms and exceeded 100 bpm in 19 patients. Peak HR was not different between the NTG+ and NTG− groups (107±26 vs. 103±13; p=0.54). Minute averages of circulatory data after NTG administration are shown in Table 3. The changes in circulatory variables during the last 5 min before tilt-back in both study groups are presented in 1, 2. In all patients, a further reduction of MAP along with an increase in HR was observed, with significant differences between the NTG+ and NTG− groups (66±15 vs. 82±13 mm Hg; p <0.001; Fig. 1A and B). During the last minute before tilt-back, all patients had marked hypotension, with a mean MAP of 60±15 mm Hg. The mean HR in the last minute prior to tilt-back was 101±23 bpm (range 32–158 bpm). HR fell below 60 bpm in 5 (18%) of the 28 patients in the NTG+ group. The estimated CI was 2.4±0.6 and 2.6±0.5 l/min/m2 in the NTG+ group and NTG− group, respectively (p=0.50). SI was further decreased after NTG administration in all patients, with a tendency toward a more pronounced decrease in the NTG– group (NTG+ vs. NTG−; 32±10 vs. 26±6; p=0.09). SVR after NTG administration was significantly lower in the NTG+ group than in the NTG− group (1079±268 vs. 1254±340; p=0.03).
| NTG+ group (n=28) | NTG− group (n=12) | p-Value | |
|---|---|---|---|
| Blood pressure (mmHg) | |||
| Finger systolic | 62±12 | 93±15 | <0.001 |
| Finger diastolic | 38±10 | 60±13 | <0.001 |
| Brachial systolic | 92±20 | 123±23 | <0.001 |
| Brachial diastolic | 53±14 | 71±11 | 0.001 |
| Mean arterial | 66±15 | 82±13 | <0.001 |
| Heart rate (bpm) | 84±32 | 99±12 | 0.14 |
| LVET (ms) | 209±23 | 226±48 | 0.341 |
| Cardiac index (l/min/m2) | 2.4±0.6 | 2.6±0.5 | 0.50 |
| Stroke index (ml/m2) | 32±10 | 26±6 | 0.09 |
| SVR (dyns/cm5) | 1079±268 | 1254±340 | 0.03 |
| SVRI (dyns/cm5/m2) | 612±193 | 683±261 | 0.04 |
- LVET, left ventricular ejection time; SVR, systemic vascular resistance; SVRI, systemic vascular resistance index.
4 Discussion
The precise mechanism by which nitrates increase the sensitivity of HUTT is not yet understood. We therefore tried to evaluate hemodynamic changes in subjects with nitrate-induced presyncope. Our data suggest that systemic hypotension in a vasovagal response induced by nitrates is the result of a decrease in SVR without a significant change of CO.
Small doses of nitrates have traditionally been considered potent venodilators, leading to decreased ventricular preload and CO with a lesser impact on arterial resistance, and this effect of nitrate compounds has generally been assumed to be the main mechanism of nitrate-induced vasovagal syncope [8]. Several studies that used pulsations of indirect finger arterial pressure to show a reduced CO in subjects with presyncope during nitrate-stimulated HUTT support this concept [9]-[11]. However, Koole et al. found no evidence of increased venous pooling during nitrate-stimulated tilt testing in patients with a history of vasovagal syncope using a radionuclide technique [14]. Moreover, two reports that used thoracic bioimpedance and echocardiography did not reveal a decreased CO after NTG administration [12], [15].
Consistent with our results, a recent study also suggested that the determinant of nitrate-induced presyncopal symptoms decreased SVR by means of thoracic bioimpedance [16]. These researchers examined the influence of body position on the response to NTG, and found that SVR decreased to a greater extent in subjects with presyncope than in the non-syncope group regardless of whether NTG was administrated in the supine position or upright position. They also observed a greater increase in aortic reflection time and decrease in pulse pressure in response to NTG in the presyncope group, which suggested decreased arterial resistance.
In this study, we evaluated beat-to-beat hemodynamic changes with ICG during 3 phases of HUTT: in the supine position, in the upright position, and during NTG administration. While passive tilting was insufficient to reproduce presyncopal symptoms, a marked decrease in MAP was observed in the NTG+ group and an HR increment that may be explained by the baroreflex mechanism was observed. After the administration of NTG, a more profound decrease in MAP with presyncopal symptoms was observed in the NTG+ group along with a minimal increase in HR. The relatively small increment of HR in the NTG+ group suggests that NTG can influence the autonomic response. Previous literature revealed that nitrate-induced vasovagal syncope is related not only to a vasodilatory effect but also to baroreceptor-mediated stimulation of autonomic tone and neurohormonal activation [7], [13]. NTG can stimulate sympathetic tone and induce vasovagal syncope by increasing cardiac contractility and subsequently triggering intraventricular pressure receptors, leading to the Bezold–Jarisch reflex [7]. However, we did not assess the effect of NTG on autonomic nervous tone and are unable to evaluate its contribution to presyncopal symptoms. Further studies of the role of baroreflex activity in vasovagal syncope are needed [13].
We found no difference in CI after NTG administration between the NTG+ and NTG– groups. This seems at variance with traditional concepts, according to which a decrease in venous return and subsequent decrease in CO are responsible for syncope. Notably, in our study, a steep fall in SVR, reflecting decreased peripheral arteriolar tone, was observed only in the NTG+ group, while CI was unchanged. These findings are strong evidence for the contribution of a decrease in SVR to nitrate-stimulated presyncopal symptoms, suggesting that arterial dilatation, instead of venodilation, may play a critical role in reduced SVR.
ICG, which simply and non-invasively measures changes in thoracic impedance generated by fluctuating blood volumes during the cardiac cycle, allows for the calculation of stroke volume, CO, and other derived parameters[17]. In recent years, the accuracy of ICG has been greatly improved by the advent of real-time beat-to-beat blood pressure monitoring, obtaining results equivalent to those of conventional invasive methods [17]-[19].
4.1 Limitations
There were several limitations in this study. First, the study population was small and further study is needed in a larger population. Second, the NTG+ group included thinner patients and a greater number of females. There were several reports that investigated the relationship between HUTT and gender. Wu et al. reported that the results of HUTT did not differ between males and females [20]. Pietrucha et al. also reported that there was no significant relationship between gender and the results of HUTT regardless of pharmacologic stressor [21]. However, the current study found that patients with presyncopal symptoms tended to be female and have lower blood pressure and lower body mass index, although this difference was not significant. Further study is needed to elucidate hemodynamic differences according to body physics and gender.
5 Conclusion
In conclusion, presyncopal symptoms during nitrate-stimulated tilt testing were explained by decreased SVR, while CO and SI remained unchanged.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors have declared that no conflict of interest exists.
Acknowledgments
None.




