How to diagnose and manage patients with cardiac implantable electronic device infections
Abstract
Over the last two decades, there has been a surge in the number of Cardiac Implantable Electronic Device (CIED) implantation. These devices improve the quality of life and survival among certain cardiac patients. However, this benefit might be affected by device complications and one of the most important ones is CIED infection as it carries significant morbidity and mortality. CIED infection can present as a device pocket infection or endovascular infection and its diagnosis could be challenging. In general, management of CIED infection involves device removal and antibiotic therapy and requires collaboration between different clinical teams. Future efforts and research should focus on measures to prevent the occurrence of this outcome.
1 Introduction
Over the last few decades there has been a significant increase in the number of patients receiving cardiovascular electronic implantable cardiac devices [1], [2]. This was the result of large body of evidence showing the important role of these devices in improving both the quality of life and survival among patients with heart disease [3], [4]. In the United States, it is estimated that about 4.2 million patients underwent implantation of a permanent pacemaker (PPM) or implantable cardioverter defibrillator (ICD) between 1993 and 2008 [5]. With an increasingly aging population, more CIEDs are implanted in older patients with more comorbidities [6]. With this growth in number of CIED implantation has come and increasing recognition of associated complications. One of these complications is CIED infection, and while its incidence remains low, the significant morbidity and mortality associated with this complication makes it one of the most serious complications of CIED implantation [7].
2 The magnitude of the problem
There has been a wide range of reported rates of CIED infection in the literature. The lack of a clear denominator, the inconsistency in defining CIED infection, and the different follow up durations used, make the understanding of the true incidence of CIED infection challenging. In general, it is believed that the risk of CIED infection after de-novo primary implantation is about 0.5% and is higher for device replacement or upgrade at around 1–7% [8]-[11]. While the incidence rate of CIED infection remains low, the rate of increase in reported CIED infections each year is alarming [12], [5], [13].
3 Pathophysiology and risk factors
Patient with CIED may get infected at time of initial implantation or during pocket revision for device change or upgrade [8], [2]. Device erosions are by default infected but most commonly are a secondary manifestation of slow growing underlying infection [8]. Pocket infection can track along the leads leading to endovascular infection. Less commonly, the pocket or the intravascular portion of a CIED can become infected as a result of hematogenous seeding during an episode of bacteremia [8], [2], [14]. The overlap between “pocket infection” and “endovascular infection” is large. Many patients with CIED pocket infection have positive blood cultures and even vegetation on the leads [7].
Most infections are monomicrobial but about 10% are polymicrobial [7]. Staphylococcal species with its ability to form biofilms account for over 80% of these infections [7], [15]. Recently there has been increasing trend toward methicillin-resistant Staphylococcal species [7], [9], [10], [15]-[17]. Poorly timed cultures or prior antibiotic use can lead to negative cultures despite clear clinical evidence of CIED infection [7]. Other microorganisms involved include Corynebacterium species, Enterococci, Gram-negative bacilli, fungi, anaerobes, Candida species, and mycobacteria [7], [15].
Different studies have identified different risk factors for developing CIED infections. These factors can generally be divided into three groups: patient-related, procedure-related and microorganism related and they are presented in Table 1.
| Patient related | Procedure related | Microorganism related |
|---|---|---|
| Renal dysfunction [28], [29] | Secondary procedures (device replacement or upgrade) as opposed to primary implantation [9], [28]-[31] | Bacteremia with Staphylococcal species carries higher risk for CIED infection than bacteremia with Gram negative bacilli [10], [32], [33] |
| Heart failure [28] | Presence of temporary pacemaker system at time of implantation [9] | |
| Fever within 24h prior to implantation [9] | Presence of more than one lead [31], [34] | |
| Diabetes [28] | ICD implant as opposed to PPM [10] | |
| Oral anticoagulation use [29] | Abdominal implantation [35], [36] | |
| Steroid use [34] | Early intervention for hematoma or lead dislodgement [9], [16] | |
| Physician experience [37] |
4 Diagnosis
Patients with CIED infections can have different presentations. Most patients present with pulse generator pocket infection manifested by inflammatory changes that involve the pocket itself (Erythema, tenderness, warmth, drainage, and erosion) [7], [15] (Fig. 1). These local findings can sometimes be accompanied by systemic signs and symptoms (fever, chills, nausea/vomiting etc.). Other patients may present with a device pocket that looks intact, but with combination of inflammatory signs and symptoms supported by positive blood cultures and imaging data (echocardiography or computed tomography) that suggest endovascular infection of the CIED (2, 3). Patients are classified as having CIED endocarditis if they have echocardiographic evidence of vegetations and two or more positive blood cultures for typical skin organisms (coagulase-negative Staphylococci, Corynebacterium species, Propionobacterium species, or one positive blood cultures for all other microorganisms.
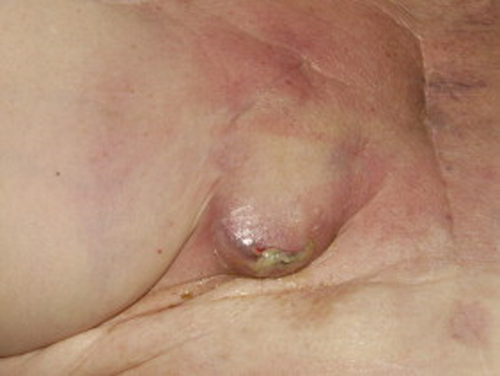
CIED pocket infection in a patient who presented 5 weeks after implant.
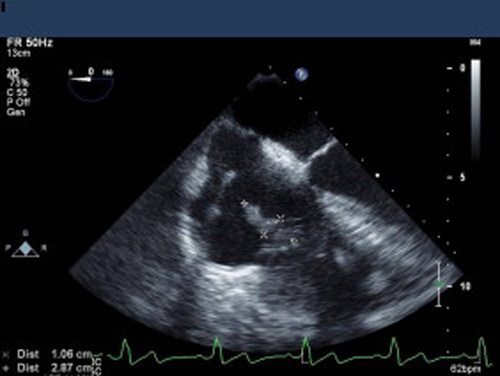
CIED endocarditis with bacteremia and TEE showing evidence of vegetations on the lead across the tricuspid valve.
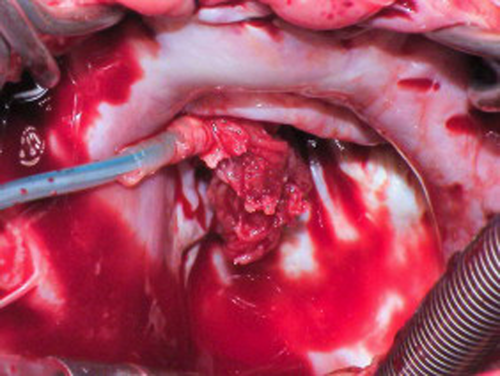
Surgical view of the same patient in Fig. 2 showing large vegetation attached to the ventricular lead and the tricuspid valve.
All patients with suspected CIED infection should have two sets of blood cultures drawn prior to initiation of any antibiotic therapy. Percutaneous aspiration of the generator pocket should not be performed as part of the diagnostic evaluation and is considered contraindicated [2]. Transthoracic echo (TTE) is of great value and if negative transesophageal echo (TEE) should be performed especially among patients with positive blood cultures or suspected CIED endocarditis as it is more sensitive in detecting vegetations. In our experience, we found that many patients presenting with pocket infection could have evidence of vegetations on TTE or TEE [7]. This has lowered our threshold in obtaining TEE for the majority of patients presenting with CIED infection. One however should be careful in interpreting echo findings. It is not unusual to detect echo-densities adherent to the leads. These often represent thrombus or fibrous tissue rather than true vegetation. Making the distinction visually could be challenging and often impossible. Therefore, the interpretation of the echo findings should take into account the clinical scenario. Incidental finding of a small mass adherent to the leads in the absence of any findings to suggest infection is probably thrombus or fibrous tissue and does not require any intervention. On the other hand, the lack of echo findings does not rule out the diagnosis of CIED infection. In patients with bacteremia without any echocardiographic findings, relapsing infections after completion of antibiotic course or persistent bacteremia despite antibiotic therapy support the diagnosis of CIED infection that requires its removal [2].
Despite these recommendations, diagnosing CIED infection could be challenging. Early after implantation procedure it is important to differentiate CIED pocket infection from incisional erythema or superficial wound infection or stitch abscess. These cases usually resolve with local measures and sometimes short course of oral antibiotics directed against staph species. Close follow up is important to avoid missing the diagnosis of CIED pocket infection. Patients with CIED pocket infections can present acutely or subacutely after device implant or replacement procedure, but sometimes it could be months or even years before the infection becomes manifest [7]. CIED endocarditis patients can have atypical presentations which could delay the diagnosis compared to patients with native or prosthetic valve endocarditis [18].
5 Management
The clinical presentation of patients with CIED pocket infection and those with endovascular infection can vary, but once the diagnosis is made, the management is similar and involves removal of all hardware along with antibiotic therapy [2], [7], [8], [15]. Conservative managements with antibiotics therapy only carries high failure rate and is associated with increased mortality [9]. Removal of the pulse generator and capping the leads should not be performed as the relapse rate of the retained leads is high [2]. CIED removal could be done percutaneously or surgically. Percutaneous extraction has evolved significantly over the years and currently is the method of choice for CIED removal. These procedures are usually safe but could be associated with significant risks including vascular or cardiac perforation leading to major bleeding or tamponade, hemothorax, pulmonary embolism and death [7]. Therefore they should be performed in centers that can provide the clinical skills, expertise, the appropriate equipment, and readily available surgical back up in case of complications. Surgical extraction is usually reserved for patients who already failed percutaneous extraction, or with retained leads not amenable to percutaneous extraction, patients with evidence of valve involvement with endocarditis that requires surgical intervention, presence of infected epicardial leads, or evidence of large vegetations over 2 cm in size. Recent data suggest that percutaneous extraction could be done safely even in the presence of vegetations larger than 2 cm, however, these cases should be evaluated individually on a case-by-case basis. Many factors other than the vegetation size play a role in the decision-making process including the general condition of the patient, comorbidities, involvement of the tricuspid valve, and the need and timing for reimplanting the CIED. For example, the patient who is pacemaker-dependent with evidence of endovascular infection and intracardiac vegetations represent a clinical dilemma as there will not be a period of “hardware free” to allow the infection to resolve prior to reimplant. These patients could be managed surgically with CIED removal and debridement of the valve and temporary epicardial lead placement to provide pacing followed by endovascular reimplant of a new device, or they can undergo percutaneous removal of CIED and implantation of endovascular temporary pacing lead, followed by surgical implantation of permanent epicardial leads. Extraction strategy should always take into account reimplant strategy and often requires team approach that includes the electrophysiologist, the infectious disease specialist, and the surgeon. While surgical approach could be successful in removing the leads, often times this could not be accomplished due to extensive fibrosis. Cutting both sides of the lead and leaving a remnant of an infected lead should be avoided. These remnants could cause recurrent infection and their extraction can represent a major technical challenge. Therefore these cases require collaboration. If the leads are cut distally at time of surgery, it is important to maintain the proximal portion of the lead in order to perform percutaneous removal of the remnant. Patients with Biventricular pacemakers or defibrillators represent an added challenge when they develop an infection. Upon the removal of the infected device, their hemodynamics can be affected negatively and therefore the operator should anticipate these negative hemodynamic effects and be ready to manage them. These patients require close observation sometimes in intensive care units and often require hemodynamic support medically or using intra-aortic balloon pumps if needed. Whether performed percutaneously or surgically, complete removal of all hardware is critical to effectively treat the infection. Complete removal and debridement of the fibrous scar and necrotic tissue in the device pocket should be performed with minimal disruption of the healthy fat, skin or muscle tissue. Samples of the pocket tissue and lead tips should be sent for gram stain and cultures.
Antimicrobial therapy plays an important role in the management of patients with CIED infections. After appropriate cultures are sent, antibiotics could be started. Since the majority of infections are due to staphylococcal species and significant portion of these are methicillin resistant, we usually use vancomycin as an initial empiric therapy and then we adjust the therapy based on the culture data. Empiric Gram negative coverage is usually reserved for ill patients in whom delaying therapy could be harmful. Initiating antibiotics could be postponed until the device is removed and cultures are sent form the device pocket and the lead in cases of infection limited to the pocket or device erosion in the absence of any other systemic signs or symptoms or infection. The data about the duration of antibiotic therapy are limited but in general, patients with CIED pocket infection are treated for 2 weeks and patients with endovascular infection are usually treated for 4–6 weeks.
6 Reimplant
The management plan for treating CIED infection should take into consideration the need for reimplant as this might affect our approach in CIED removal as discussed earlier. Reimplant should not be an automatic decision, but rather CIED removal should be an opportunity to reassess the need for the device. In our experience, almost one third of the patients undergoing CIED removal for infection did not require immediate reimplant [7]. Patients who had an ICD implanted for primary prevention for impaired left ventricular function might not need it if they have never had ICD therapies and the ventricular function has now recovered with medical therapy. Patients who underwent pacemaker implant for possible sinus node dysfunction might not need it (at least not immediately) if the data from the prior device showed minimal need for pacing. The emergence of new and advanced medical conditions that would affect survival or quality of life like would make us avoid reimplanting an ICD for primary prevention.
When reimplant is needed, it should be performed on the opposite side of the chest. Other alternative approaches include abdominal device with transiliac lead implantation or epicardial lead surgical implantation. There are no randomized trials to guide the timing of reimplant, but this usually depends on several factors including the type of infection, the presence of positive blood cultures, and the pathogen involved. Patients with positive blood cultures and no evidence of endocarditis (no valve vegetation) can be reimplanted if the repeat blood cultures after CIED removal remain negative for 72 h.
Patients with CIED endocarditis should not be reimplanted until at least 14 days after the first negative blood cultures after CIED removal. This is feasible in patients who are not dependent on the device. Wearable external defibrillator can provide patients with time to recover before a new ICD is reimplanted especially for patients who had ICD implanted for primary prevention and have no pacing indication. For patients who are pacemaker dependent, surgical epicardial pacemaker system implantation provides an alternative approach.
7 Outcomes and economic burden
CIED infections carry significant morbidity and mortality [7], [15]. In-hospital mortality among patients admitted with CIED infection ranges between 4% and 10% [7], [10], [17]-[19] and 1-year mortality ranges between 15% and 20% [7], [17], [18], [20]. Mortality is higher among patients with endovascular infection (especially with endocarditis) compared to patients with pocket infection [7], [18]. Contrary to common belief, only a small proportion of the in-hospital mortality is directly related to the extraction procedure itself, [7] reflecting the serious significance of CIED infection itself and the comorbidities among these patients.
The high morbidity and mortality, along with prolonged hospital stay, antimicrobial therapy, added to the cost of extraction and potential reimplantation of a new device, all result in significant financial burden that has been increasing over the last two decades [5], [21], [22].
8 Prevention
The best strategy to address CIED infection is to prevent its occurrence. This process starts with the decision of implanting a CIED. Thorough evaluation of the recipient of the device is invaluable and the following questions should always be addressed. Is the device indicated? Is the timing appropriate? Is the patient ready? CIED should not be implanted if there is any documented fever or concern for an active infection. Temporary pacemaker should be avoided if possible prior to implanting a new device. Patients who are found to be colonized with methicillin resistant Staphylococcus aureus can benefit from decolonization using nasal application of Bactroban ointment [23]. All efforts should be made to minimize the risk of bleeding and hematoma including holding the use of anticoagulants if possible. In our experience, if anticoagulation cannot be interrupted, we find that performing the implantation on coumadin (with INR equal or less than 2.5) carries less risk of hematoma than bridging with heparin. Recommendations for CIED implantation while using newer oral anticoagulants require further studies. The use of low molecular weight heparin should be avoided around the time of implantation. Strict surgical techniques should be applied at time of implantation, these include antiseptic skin preparation, washing the pocket with saline or antibiotic solution and perioperative antibiotic use [24]-[26]. Many centers use first generation cephalosporin, however, the increasing proportion of methicillin resistance among Staph species have led many centers, including our own institution, to use vancomycin for perioperative prophylaxis [2], [7]. Future advancement in minimizing CIED infection include the use or antibacterial envelope [27]. Rechargeable batteries in the future would hopefully minimize the need for CIED change procedures which carry a high risk for infection.
9 Conclusions
CIED infections represent a major complication after CIED and carries significant mortality and morbidity. Management of this complication usually involves device and lead removal along with antibiotic therapy. Future advancement should focus on measures to prevent CIED infections.
10 Disclosures
KGT: none.
BLW: medical advisory boards for Medtronic, St Jude Medical and Spectranetics.




