Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure
Abstract
Background:
An adequate energy-protein intake (EPI) when combined with amino acid supplementation may have a positive impact nutritional and metabolic status in patients with chronic heart failure (CHF).
Methods and results:
Thirty eight stable CHF patients (27 males, 73.5±4 years; BMI 22.5±1.4 kg/m2), with severe depletion of muscle mass and were randomised to oral supplements of essential amino acids 8 g/day (EAA group; n=21) or no supplements (controls; n=17). patients had adequate EPI (energy ≥ 30 kcal/kg; proteins >1.1 g/kg). At baseline and 2-months after randomisation, the patients underwent metabolic (plasma lactate, pyruvate concentration; serum insulin level; estimate of insulin resistance by HOMA index), nutritional (measure of nitrogen balance), and functional (exercise test, walking test) evaluations.
Body weight increased by >1 kg in 80% of supplemented patients (mean 2.96 kg) and in 30% of controls (mean 2.3 kg) (interaction <0.05). Changes in arm muscle area, nitrogen balance, and HOMA index were similar between the two treatment groups.
Plasma lactate and pyruvate levels increased in controls (p<0.01 for both) but decreased in the supplemented group (p<0.01 and 0.02 respectively). EAA supplemented patients but not controls improved both exercise output and peak oxygen consumption and walking test.
Conclusions:
Adequate EPI when combined with essential amino acid supplementation may improve nutritional and metabolic status in most muscle-depleted CHF patients.
1. Introduction
The prognostic importance of increasing body weight (BW) in patients with chronic heart failure (CHF) has been confirmed by recent studies showing that patients with a body mass index (BMI) of 30-34.9 kg/m2 have a lower mortality rate than those with a BMI<30 kg/m2 1 2 3 4.
Nutrition is obviously the natural way of increasing BW. However, we suspect that even if adequate, nutrition as energy-protein intake (EPI) alone might not be enough to increase BW in malnourished CHF patients, unless it can also limit possible metabolic derangements in the skeletal muscles 5,6.
We base our hypothesis on clinical evidence that severe muscle protein malnutrition and normal BW may co-exist (39% of clinically stable CHF patients) 7 and on documented evidence in CHF of skeletal muscle energy shortage 8, which is essential for protein synthesis 9. In addition, we also asked whether simple nutritional substrates such as essential amino acids (EAAs) could enhance the ability of EPI to increase BW, while also attenuating metabolic abnormalities in skeletal muscles. The rationale behind using EAAs derives from the documented ability of amino acids to regulate protein turnover by acting on protein metabolism 10, increasing aerobic metabolism 11 and increasing the biological activities of anabolic hormones 12,13 such as insulin and insulin-like growth factor-1 (IGF-1).
We therefore wanted to identify certain metabolic aspects of muscle-depleted normal weight patients with CHF and to investigate whether EAA supplements could improve both BW/muscle mass and metabolic derangements in muscles over time.
2. Methods
2.1. Population
Forty-four clinically stable CHF outpatients were enrolled in the study. Patients were selected from two clinical databases according to the following criteria:
- normal BW (= a body mass index (BMI) >20<25 kg/m2 which had been stable over the past year) and high depletion of skeletal muscle mass (arm muscle area, AMA; <10° percentile of normal values for age and sex) 14,
- stable daily EPI over the past year, providing energy ≥30 kcal/kg and proteins >1.1 g/kg 7. This was assumed to be adequate as it was associated with a positive nitrogen balance (NB), that is >+1 g/24 h, indicating a predominance of anabolic processes 7,
- adequate daily physical activity.
According to our clinical practice, anthropometric variables, nutritional intake, and nitrogen balance were checked every 4 months.
Patients with diabetes mellitus treated with hypoglycaemic drugs or insulin therapy, with liver and renal insufficiency were excluded from the study. Clinical stability of CHF was assessed according to the absence of fluid retention (peripheral or pulmonary), normal jugular venous pressure and no changes in medication over the past three months.
2.2. Study procedures
At 8 am, after a 12-h overnight fast, plasma concentrations of lactate 15, pyruvate 16 (μmol/ml) and serum insulin (μU/ml) 7 were measured from peripheral venous blood samples in all patients. The plasma lactate/pyruvate ratio (L/P) was also calculated. These specific acids were assessed because elevated levels in the plasma may be caused by alterations in the regulation of the glucose metabolic pathway 17 and not just by hypoxia which was not present in our patients.
Insulin resistance was estimated using the Homeostasis Model Assessment (HOMA) 18 as follows:
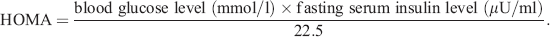 (1)
(1)The normal value of HOMA in healthy elderly patients aged 61 to 81 years is <2.4 19.
All patients had their NB calculated as described previously 10. Urinary nitrogen analysis to calculate NB was made using a urease/glutamate dehydrogenase coupled enzymatic technique (BUN method, Urea Nitrogen Flex® reagent cartridge, Dade Behring).
Nutritional intake was measured using a 7-day food diary 7. Each patient was asked to keep a food diary at home for 7 consecutive days, before and after meals, to record the type and weight of cooked or uncooked food which they consumed. We converted this data to raw equivalents using appropriate tables when necessary 7,20.
Patients became accustomed to keeping a nutritional diary as an essential part of their clinical control over time. Moreover, we regularly reminded the patients of the importance of keeping an accurate diary for this study. The principal investigator was responsible for keeping the patients informed, for checking the food diaries and for calculating nutritional intakes (using a computer program set up by our group) 21. Energy (kcal) and macronutrient intake (carbohydrates, proteins, and lipids expressed in g) were also related to the patients' body weight (kg). Energy was also expressed as a percentage of basal metabolic rate (BMR) 21.
After completing the baseline evaluations, the patients underwent a 6-min walking test 22 and bicycle exercise test. All exercise tests were performed in the morning after an overnight fast of between 12-16 h. We adopted a RAMP protocol in which the work load increase was 10 W/min with pedalling at 40 to 50 rpm. The patients were connected to a system for respiratory gas analysis, as described elsewhere 23. Patients were asked to exercise until exhausted. Among cardio respiratory variables, we measured oxygen peak (VO2 peak) and respiratory exchange ratio (VCO2/VO2).
Patients were then assigned to treatment according to a randomised allocation procedure. A randomisation list was generated using SAS statistical software, A and B being the identifiers of the blinded treatment. The list was made available to both the cardiologists (C.O., A.G., A.T., and E.V.) and the hospital pharmacist. The cardiologists allocated patients sequentially to treatment A or B according to the randomisation list. The first author of the article (A.R.) who interpreted all the results was blinded to the treatment allocation. Supplemented patients were informed by the cardiologists that the amino acid supplement served to implement their dietary intake; the patients did not receive any information about the reason for giving the supplement.
Twenty two patients (EEA group) received an oral nutritional mixture supplement, which provided 8 g of essential amino acids (EAAs) per day; (Aminotrofic, Professional Dietetics, Milan, Italy; see Table 1) (4 g in the morning +4 g in the afternoon diluted in half a glass of water). The remaining 22 patients received no supplementation (control group). The amount of nitrogen in the EAA supplement (1.28 g) was added to the nitrogen intake from the diet, in order to calculate the total nitrogen intake for supplemented patients.
| kcal | 35.3 |
| kj | 149.9 |
| Total amino acids of which | 4 g |
| l-Leucine | 1250 mg |
| l-Lysine | 650 mg |
| l-Isoleucine | 625 mg |
| l-Valine | 625 mg |
| l-Threonine | 350 mg |
| l-Cysteine | 150 mg |
| l-Histidine | 150 mg |
| l-Phenylalanine | 100 mg |
| l-Methionine | 50 mg |
| l-Tyrosine | 30 mg |
| l-Tryptophan | 20 mg |
After 2 months, patients underwent the same clinical, functional biochemical and nutritional (7-day food diary) investigations as at baseline. Moreover, BW was recorded and AMA was calculated 14. Patients were weighed with underwear only, between 9 am and 10 am. The baseline and 2 month anthropometric measures were both assessed by the same investigator. The coefficient of variability of triceps skin fold thickness and mid-arm muscle circumference, necessary to calculate AMA, was 1%.
Given that the normal daily variation of BW is 0.5±0.2 kg 24, we considered an increase in body weight greater than 1 kg (BW>1 kg) over 2-months, as significant. We assumed that an increase in skeletal muscle mass (AMA) was significant over 2 months when it was associated with a BW increase of >1 kg.
This study was approved by the Technical Ethics Committee of Foundation “S. Maugeri”, Pavia, Italy and written informed consent was obtained from all participants after the nature of the study had been explained.
2.3. Evaluation of patient compliance with EAAs
Patient compliance was estimated by counting the number of amino acid packets remaining 2 months after the start of the protocol (each patient was given 60 packets of EAA supplement). This allowed us to estimate patient compliance over the 2-month study period. In order to judge patient reliability (at 2-months), we also took venous blood samples for measurement of fasting plasma leucine concentration (the main amino acid in the packet). Importantly, the blood samples were drawn 60 min after the patient should have taken the morning packet of EAAs. The patients were not informed of their plasma leucine measurement.
Patient reliability was judged based on plasma leucine concentrations. In our laboratory, for healthy elderly subjects, the increase in baseline plasma leucine 60 min after 4 g EAA ingestion was +145%. The same procedure was also applied to the control patients.
2.4. Statistical analysis
The χ2 test was used to compare the distribution of demographic, clinical, functional and treatment characteristics as well as of a BW increase >1 kg between the two patient groups. Comparisons between groups at baseline and after two months from the start of the protocol were performed using ANOVA. Results are shown as a mean value±SD. Differences were considered statistically significant at p<0.05.
3. Results
Fig. 1 is a flow chart of patient participation in the EAA supplementation study. There were five dropouts in the control group and one in the intervention groups. Thus, the analysis included 38 patients (17 controls +21 supplemented). The demographic, clinical functional and treatment characteristics of the patients at baseline are summarised in Table 2.
| PATIENTS n=38 | |||
|---|---|---|---|
| Controls | EAAs | p value | |
| Number (n) | 17 | 21 | ns |
| Age (yr) | 73.1±2.8 | 74.5±5 | ns |
| Men/women | 14/3 | 13/8 | ns |
| Aetiology | |||
| Coronary artery disease | 7 (41.2%) | 12 (57.1%) | ns |
| Valvular heart disease | 2 (11.7%) | 3 (14.3%) | ns |
| Idiopathic | 8 (47%) | 6 (28.6%) | ns |
| Duration of disease (months) | |||
| <18 | 7 | 4 | ns |
| ≥18 | 10 | 17 | ns |
| NYHA class | |||
| I | 0 | 0 | ns |
| II | 12 (70.6%) | 15 (71.4%) | ns |
| III | 5 (29.4%) | 6 (28.6%) | ns |
| IV | 0 | 0 | ns |
| Left ventricular ejection fraction | 29.7±9.2% | 29.4±17.4% | ns |
| Medication | |||
| ACE inhibition | 17pts (88.2%) | 21pts (100%) | ns |
| β blockers | 15pts (88.2%) | 20pts (95.2%) | ns |
| Digoxin | 7pts (41%) | 8pts (38%) | ns |
| Diuretics | 17pts (100%) | 21pts (100%) | ns |
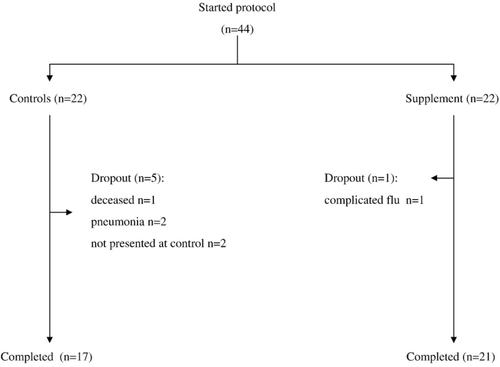
Table 3 shows the nutritional intake of patients in both treatment groups. Table 4 shows the nutritional status, nitrogen balance, plasma levels of glucose, lactate, and pyruvate, serum insulin concentrations, and insulin resistance from HOMA in both patient groups at baseline and at 2-months.
| Controls | EAA supplemented | |||
|---|---|---|---|---|
| Baseline | 2-mo | Baseline | 2-mo | |
| Energy | ||||
| kcal | 1884±205 | 1950±279 | 1890±250 | 1950±300 |
| kcal/kg | 31±2.9 | 32.3±3.2 | 33.7±3 | 33.5±2.7 |
| % BMR | 132% | - | 136% | - |
| Carbohydrates | ||||
| g | 222±36 | 239±21.5 | 263±90 | 303±115 |
| g/kg | 3.65±0.6 | 3.9±0.9 | 4.7±1.8 | 5.2±2.1 |
| Proteins | ||||
| g | 68±9.6 | 67±9.1 | 78±11 | 76±27 |
| g/kg | 1.12±0.16 | 1.1±0.15 | 1.4±0.2 | 1.3±0.5 |
| Lipids | ||||
| g | 73±12 | 79.6±24 | 64.3±27 | 76±40 |
| g/kg | 1.2±0.2 | 1.3±0.4 | 1.15±0.5 | 1.3±0.7 |
| Fatty acids (% energy) | ||||
| Saturated | 11.5±3 | 13±4 | 12±2 | 11.8±4 |
| Monounsaturated | 18.5±2.5 | 16±3.9 | 14±3 | 13±5 |
| Polyunsaturated | 5±2 | 7.7±3 | 4.5±2 | 10.3±3 |
| Alcohol (g) | 19±5 | 17±7 | 16±4 | 20±6 |
| Fibre (g) | 15.4±5 | 17±8 | 22±9 | 20±5 |
| Calcium (mg) | 1001±350 | 17±8 | 22±9 | 20±5 |
| Phosphorus (mg) | 1226±420 | 1021±500 | 850±324 | 1456±645 |
| Potassium (mg) | 2590±185 | 2894±350 | 2330±155 | 2950±284 |
| Sodium (mg) | 1401±155 | 1404±107 | 1258±270 | 1200±159 |
| Iron (mg) | 10.5±4 | 9.2±3 | 12±5 | 11.5±4 |
| Zinc (mg) | 10.2±3 | 9.5±4 | 9±4 | 9.8±3.5 |
| Thiamine (mg) | 0.5±0.1 | 1.2±0.3 | 1.02±0.15 | 1.07±0.22 |
| Riboflavin (mg) | 1.3±0.2 | 1.1±0.3 | 1.15±0.2 | 1.35±0.4 |
| Niacin (mg) | 15.5±4.8 | 17.7±5.2 | 17.9±3.2 | 16±4.1 |
| Vit A (μg) | 650±135 | 705±250 | 594±101 | 674±189 |
| Vit C (mg) | 66±32 | 75±28 | 79±15 | 85±25 |
| Tocoferol (mg) | 6.5±3 | 8.2±4 | 7.1±3.5 | 7.9±4.1 |
| Colecalciferol (μg) | 1.95±0.8 | 2.1±0.9 | 2.1±1 | 2.4±1.1 |
| Water (ml)a | 1113±155 | 990±250 | 1005±175 | 1120±148 |
- a Data are expressed as mean±standard deviation (SD).
- b BMR = basal metabolic rate.
- c Statistical analysis (Anova test and Fisher's PLSD test): No differences in macro-micronutrients were found between the two subgroups and respectively within each subgroup neither at baseline nor at 2 months.
- a Water content in food.
| Controls | EAA supplemented | |||
|---|---|---|---|---|
| Baseline | 2-mo | Baseline | 2-mo | |
| Body weight kg | 60.8±7 | 61.2±6.3 | 55.9±17 | 58.2±7.2^ |
| Body mass index kg/m2 | 23.2±1.4 | 23.6±1.5 | 22.5±2.1 | 23.4±1.9^ |
| Triceps skin fold thickness mm | 11.9±3.7 (6)a | 11.4±3.7 | 10.4±4.4 | 10.3±3.9 |
| Arm muscle mass area cm2 | 34.2±5 (44.7)a | 37.1±4^^ | 31.2±9.9 | 34.9±10^^ |
| Nitrogen balance g/24 h | 3±2.8 | 2.8±2.5 | 3.9±3.3 | 3.6±3.3 |
| Plasma glucose mg/dl | 90±13 | 96±8 | 95±26 | 97±21 |
| Serum insulin μU/ml | 21.4±6.6 (nv 10±3.8) | 21.3±6.7 | 18±15 | 16±12 |
| HOMA index | 4.77±1.8 (nv 2.1±0.2) | 5.08±2 | 4.31±3.2 | 3.7±2.5^^^ |
| Plasma glyconeogenic substrates | ||||
| Lactate (μmol/ml) | 1.1±0.4 (nv 0.2±0.15) | 1.27±0.4^ | 1.2±0.3 | 0.9±0.35^°° |
| Pyruvate (μmol/ml) | 0.018±0.009 (nv 0.015±0.008) | 0.027±0.012^ | 0.02±0.01 | 0.015±0.008^^°° |
| Plasma lactate/pyruvate ratio | 61±44 (nv 14±10) | 47±31 | 60±30 | 60±25 |
- a Data are expressed as main±standard deviation (SD).
- b nv: normal value of laboratory.
- c Statistical analysis (Anova test and Fisher's PLSD test):
- d Intra groups differences (2 mo vs baseline):
- ^ p < 0.01
- ^^ p<0.02
- ^^^ p<0.06.
- h Control group 2 mo vs EAA supplemented group 2 mo:
- °° p <0.001.
- a Values corresponding to 10° percentile.
3.1. Baseline characteristics of the study population
According to our selection criteria, the two groups of patients had similar body mass index (BMI) and reduction in skeletal mass (AMA). Subcutaneous fat tissue (TST) was within normal values for age and sex and was similar for both groups. Moreover, daily nutritional intakes were not significantly different between the two groups. Both groups had similar positive nitrogen balance (NB=>+1 g/day) indicating that the patients' body metabolism was oriented towards the anabolic state.
Both groups had a similar state of insulin resistance (HOMA>2.4). Plasma lactate and pyruvate concentrations as well as L/P ratio were far higher in both groups than previously determined in our laboratory for healthy elderly subjects. There were no differences in respiratory variables or physical capacities between the two groups (Table 5).
| Controls | EAAs supplemented | |||
|---|---|---|---|---|
| Baseline | 2-mo | Baseline | 2-mo | |
| A) Cycle ergometer testing | ||||
| Power output (W) | 85±24 | 88±22 | 80±28 | 95±25**^ |
| Respiratory variables | ||||
| 1) VO2 (ml O2/kg/min) | ||||
| Rest | 4.2±0.6 | 4.4±0.8 | 4.5±0.8 | 4.2±1 |
| Peak | 12.9±2.7 | 13±3.5 | 13.5±1.7 | 14.9±1.9*^^ |
| 2) Respiratory exchange ratio | ||||
| Rest | 0.88±0.01 | 0.95±0.1 | 0.87±0.08 | 0.96±0.06 |
| Peak | 1.1±0.09 | 1.12±0.1 | 1.1±0.2 | 1.15±0.2 |
| B) 6-min walking test (m) | 298±142 | 310±155 | 331±124 | 405±130***^ |
- a Inter group differences in time courses of:
- * p<0.05 oxygen consumption.
- ** p<0.01 work output.
- *** p<0.001 walking ability.
- e Intra group differences (2 mo vs baseline):
- ^ p<0.02
- ^^ p<0.05.
3.2. Patient compliance to EAA supplementation
The results showed that the intervention group very probably complied with the EAA supplementation. Indeed, none of the patients returned any packets at the end of the 2 month study period. In addition, the increase in plasma leucine concentration at 2 months was 279% of the baseline value (128±28 μmol/L at baseline vs 478±104 μmol/L at 2 months, p<0.001), which suggests patient reliability. In the control patients, no significant changes were observed in plasma leucine concentration between baseline and 2-months (115±22 μmol/L at baseline to 121±31 μmol/L).
3.3. Patient evaluation after two months of study treatment
No significant changes in New York Heart Association (NYHA) functional class, clinical, nutritional intake, or functional characteristics were found between the two groups (data not shown).
A BW increase of more than 1 kg was found in 80% of the supplemented group (from 58±4 kg to 61±3 kg; +2.96±1.56 kg, p<0.01 compared with baseline) and in 30% of the non-supplemented group (from 59.9±3.5 kg to 62.2±3.2 kg;+2.3±0.8 kg, p<0.05 vs baseline). This difference in distribution between the two treatment groups was significant (p<0.05). Since there was a different male:female ratio between the two treatment groups, we calculated the sex related differences in BW improvements within each group and found that they were similar. Indeed, the BW gain in the EAA group was +3.3±2.4 kg in males and +2.7±0.6 in females (ns). In the control group, males gained +2±0.7 kg and females +2.6±0.9 kg (ns).
No patient, regardless of treatment group, had a reduced BW at baseline. Baseline skeletal muscle mass (AMA) increased significantly and similarly in both groups. No patient, regardless of group had a reduced AMA over time. Baseline subcutaneous fat tissue (TST) in both groups remained virtually unchanged.
Insulin resistance (HOMA) tended to deteriorate in the non-supplemented CHF patients (from 4.7±1.8 to 5.1±2; +15%; p=0.06) while it improved in the EAA group (from 4±3.2 to 3.7±2.6 −15%; p=0.07). However, this difference was not significant.
The effect on plasma lactate and pyruvate was different in the two treatment groups. Indeed, lactate increased in all control patients (from 1.1±0.4 μmol/ml to 1.27±0.4 μmol/ml; p<0.01) but decreased in all supplemented ones (from 1.2±0.3 μmol/ml to 0.9±0.35 μmol/ml; p<0.01). The difference in effect between the two groups was highly significant (p<0.001).
Like lactate, plasma pyruvate concentration increased in all control CHF patients (from 0.0018±0.009 to 0.027±0.012 μmol/ml; p<0.01) but decreased in all EEA CHF patients (from 0.02±0.01 to 0.015±0.008 μmol/ml; p<0.02). Plasma L/P ratio tended to decrease in the controls but remained unchanged in the EAA group.
After 2 months, patients in the EAA group significantly improved their bicycle exercise capacity (+15±7 W, p<0.02 vs basal value), peak oxygen consumption (VO2 peak +1.1±0.3 ml/kg/min, p<0.05 vs basal value) and walking capacity (+74±25 m, p<0.02 vs basal value) (Table 5).There was no improvement in the control group.
In summary, the results of this study show that adequate EPI alone was associated over time with: a) a BW increase of more than 1 kg in 30% of CHF patients; b) a tendency to worsening insulin resistance; c) increased plasma lactate and pyruvate levels; and d) unchanged exercise and walking capacities. The combination of adequate EPI +8 g EEAs was associated with a) a BW increase of more than 1 kg in 80% of CHF patients; b) a trend towards an improvement in insulin resistance; c) decreased plasma lactate and pyruvate levels; and d) improved exercise and walking capacities.
4. Discussion
This study shows that in muscle-depleted, normal weight, clinically stable patients with CHF, alterations in muscle metabolism may co-exist with an anabolic body state. The results show that adequate EPI increases BW by more than 1 kg in less than one third of patients and moreover, it may not limit metabolic disorders in the muscle, but may indeed exacerbate the problem over time. Conversely, if enriched with EAAs, adequate EPI can increase BW by more than 1 kg in up to 80% of patients and improve metabolic disorders in the muscle.
4.1. Baseline muscle metabolic disorders and anabolic state
This study shows that in clinically stable muscle-depleted CHF, an adequate EPI was associated with an anabolic state, but did not correct alterations in muscle glucose oxidation.
Elevated plasma lactate and pyruvate levels suggest reduced muscle capacity to oxidize pyruvate (glucose) rather than an excess of glucose anaerobic breakdown due to the rate of oxygen use 25. A number of conditions associated with CHF including hormone imbalance 7,26, insulin resistance 27 28 29 and cytokine overproduction 25,28 can reduce pyruvate oxidation by inactivating the pyruvate dehydrogenase complex (PDH), the rate-limiting step for lactate and pyruvate entry into the citric acid cycle for oxidation 30,31.
In particular, cytokine overproduction may play a major role in perturbing muscle aerobic glucose metabolism. For instance, chronic intravenous infusion of interleukin 1 (IL-1) has been found to cause a 40% reduction in active form-PDH in the skeletal muscle of healthy rats 31. This metabolic effect has been shown to induce elevated plasma lactate concentrations similar to those observed in patients and animals with sepsis 32 33 34 35.
Moreover, modulation of the tumour necrosis factor response abrogates muscle lactate production and hyperlactatemia during hypermetabolic sepsis 36,37.
The reduced pyruvate oxidation leading to accumulation of glycolytic intermediates, cellular acidosis and reduced energy reserves, are all metabolic disturbances that can potentially have an impact on nutritional status. Indeed, cellular acidosis can induce both somatic and myofribillar proteolysis 38 and cell energy shortage may induce reduced protein synthesis 9.
The mechanisms leading to increased lactate formation and reduced pyruvate oxidation could also explain the co-existence of muscle depletion and fat tissue/body weight conservation observed in our study patients. For instance, at an adipose tissue level the lypolitic activity of high cortisol levels 7,39 can be counteracted by increased serum insulin concentration, however in muscles the proteolytic effect of cortisol contributes to cellular metabolic derangements. Again, while the conservation of fat tissue may be favoured by beta-blockers 2,3, a reduction of protein synthesis might also be due to growth hormone resistance 26 and insulin-like growth factor-1 (IGF-1) reduction 40.
As the patients in our study had optimal nutritional intake and clinical conditions, it is conceivable that any disturbances in their current anabolic state and/or a further worsening disorders of muscle metabolism, for instance from large reductions in nutritional intake and/or metabolic stress (infection, haemodynamic instability) may bring about a global loss of body weight 41.
4.2. Two months after randomisation
This study clearly shows the nutritional and metabolic benefits for patients of EAAs supplements and suggests that the improvement in nutritional status may be a consequence of the well documented metabolic activities exerted by EAAs. Indeed, EAAs can influence both protein turnover and aerobic metabolism in muscle (Fig. 2).
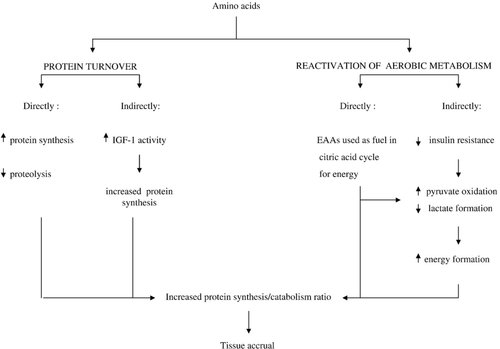
Concerning protein turnover, EAAs act on protein metabolism by both direct and indirect mechanisms. Directly, EAAs stimulate protein synthesis 42 and inhibit proteolysis 43 in several tissues including skeletal muscle, heart, liver, and pancreatic β-cells. It is worth noting that EAAs can promote protein synthesis independently of insulin 10 which is relevant for insulin resistant CHF. Indirectly, EAAs may stimulate protein synthesis by increasing biological activity of insulin-like growth factor 1 (IGF-1). Levels of IGF-1, which have been shown to be low in both cachectic 26 and non-cachectic 40 CHF patients, are positively associated with muscle protein synthesis 44 and negatively associated with protein breakdown 45.
The stimulation of aerobic metabolism in muscle 9,11 (Fig. 2) is the other mechanism by which EAAs can improve both metabolic derangements and at the same time nutritional status. Here again, EAAs may act by both direct and indirect mechanisms.
EAAs directly influence the aerobic pathway, as they can enter it at various levels and can be used as an alternative fuel for energy production 9. The reactivation of aerobic pathway activity makes the lactate ⇆ pyruvate reaction shift to the right 9. In this way, lactate formation diminishes and more pyruvate is processed in the aerobic pathway. This may explain the reduction in plasma lactate and pyruvate levels observed in CHF patients after 2-months of EAA supplementation.
EAAs could improve glucose oxidation indirectly by reducing insulin resistance 12,46. Thus, EAAs can re-modulate the muscle cell glucose breakdown/use ratio. The improvement in both nutritional state and metabolic abnormality may explain increased exercise output and oxidative metabolism as well as walking capacity in supplemented patients. The increased respiratory capacity and walking test confirm the findings of both a previous 47 and a very recent investigation 23 that used the same substrates (EAAs), at the same dose (8 g/d) in a similar elderly population.
It is conceivable that in our study, EAAs may have favoured patient tissue accrual by harmonizing protein synthesis processes and cell energy production essential for protein synthesis. In this way, EAAs seems to act as a bridge between nutritional intake and muscle metabolism.
The fact that an adequate EPI alone cannot reduce metabolic disturbances in muscle, suggests that amino acids from food proteins are not sufficient and/or capable of attenuating these metabolic muscle defects. This might limit protein synthesis and tissue accrual in most non-supplemented patients.
In contrast, since adequate EPI alone is associated over time with an increase in muscle disturbances we suspect that attempts to excessively force nutrition in CHF in order to increase body weight could induce/increase insulin resistance and worsen the metabolic state of patients.
5. Clinical implications
In light of a very recent investigation showing the importance of maintaining BMI between 30 and 34.9 kg/m2 in order to reduce mortality in patients with CHF 4, our results, particularly if corroborated by a larger clinical trial, may offer one way of improving BMI. According to a recent report by Anker et al., our patients could be defined as “sarcopenics” 41. In sarcopenic patients, the combination of adequate nutrition and EAAs might prevent the transition from a sarcopenic condition to a global loss of body weight 41.
The determination of plasma lactate and pyruvate levels both at baseline and over time offers the physician a rapid understanding of both muscle metabolism and its time course after pharmacological and nutritional interventions. Increased plasma lactate and pyruvate concentrations should not be related to cellular hypoxia alone, but may also be considered as products of altered cell substrate use. In this case, an otherwise adequate EPI should not be increased but rather amino acids supplementation should be considered.
6. Limitations
For technical reasons we did not use dual X-ray absorptiometry (DXA) methods to measure body composition. This would have strengthened our discussion. Another method we could have used to assess body composition is bioelectrical impedance analysis (BIA) and more importantly, bioimpedance spectroscopy (BIS) which provides an estimate of body cell mass. However, we preferred not to use these methods because they can be problematic for individual assessment in the clinic, particularly in CHF patients with abnormal fluid distribution or body geometry 48.
The male:female ratio was not equally represented between the two groups of patients. However this did not limit the interpretation of the data as the weight changes in patients achieving a >1 kg increase in BW at 2-months were similar for males and females in each group. Moreover there was a similar BW gain when males and females were compared between the two groups.
At first glance, the selection of patients based on their specific nutritional intakes (energy ≥30 kcal/kg+proteins>1.1 g/kg) might seem to be a limitation of this study. However, we believe that this is a strength, since choosing patients with stable nutritional intake over time allows a better balance between nutrition and metabolism and hence, a more reliable link between them.
Plasma lactate and pyruvate levels might not automatically reflect their relative tissue concentrations. However, in the absence of peripheral oedema, particularly in the arms, from where the blood samples were taken, it is reasonable to assume that the passage of EAAs from the cells to the blood stream occurred without any difficulty.
Finally, plasma cytokine levels were not determined. This is a major issue as a recent study showed that inflammatory cytokines, together with weight loss and reduced food intake, are involved in inducing cachexia and poor functional status and prognosis 49.
7. Conclusions
This study shows that it is possible to improve nutritional status and muscle metabolic derangements in muscle-depleted normal weight patients with CHF, by combining an adequate energy-protein intake with essential amino acid supplementation.
Acknowledgement
We would like to thank Prof. Robert Coates (Centro Linguistico, Bocconi University, Milano, Italy), medical writer, for his linguistic revision.




