Recovery from peripartum cardiomyopathy after treatment with bromocriptine
Abstract
Peripartum cardiomyopathy (PPCM) is a potentially devastating cause of heart failure that affects women late in pregnancy or in early puerperium. Recent findings showed that a 16 kDa fragment of prolactin may induce myocardial damage, and this offered a new option of treating PPCM by blocking prolactin with bromocriptine. We report on a 35-year-old woman with a twin gravidity who gave birth to two healthy boys at day 36/6 and developed a potentially fatal PPCM. Within 3 days since delivery she suffered from severe symptoms of heart failure (orthopnoea, pleural and pericardial effusion, reduced systolic function LVEF 15%). Bromocriptine 2.5 mg bid was added to standard heart failure therapy at day 6 after delivery, and within a week the patient recovered to NYHA functional class II. 2 months later she presented in a good state, NYHA class I, and MRI confirmed an LVEF of 60%. Balancing the potential side effects of bromocriptine against the very poor prognosis in severe PPCM our case supports the use of bromocriptine as a specific novel therapy.
1. Introduction
Peripartum cardiomyopathy (PPCM) is a rare but potentially devastating cause of heart failure that affects women late in pregnancy or in early puerperium. It has an incidence of approximately 1 in 4000 deliveries 1 and occurs more often in twin births 2. The origins of this disease were unknown until recently, and thus treatment was empirical and restricted to standard heart failure therapy with ACE-inhibitors, beta-blockers, diuretics, aldosterone antagonists and, in most severe cases, biventricular assist systems or heart transplantation. The prognosis of PPCM is controversial, but a left ventricular ejection fraction (LVEF) below 27% is associated with a mortality of between 9 and 30% 2,3. According to a new pathophysiological explanation reported recently by Hilfiker-Kleiner et al., a 16 kDa form of the post-partally upregulated hormone prolactin initiates cell apoptosis and disrupts capillaries, thus inducing myocardial damage and heart failure 4. This concept offers a new and causal option of treating PPCM by blocking prolactin with bromocriptine 5. In this letter we report on a patient with potentially fatal PPCM after a twin birth and her recovery after treatment with bromocriptine.
2. Case report
A 35-year-old woman (first and twin gravidity, BMI 21.8 kg/m2) was admitted to our department of obstetrics at the 35th week of gestation (35 weeks+1 day) due to imminent premature birth. She was free of cardiac symptoms before and had no family history of cardiomyopathy. On admission, she had proteinuria (3583 mg/d), slightly elevated blood pressure (138/90 mmHg) and leg oedema, which led to the diagnosis of preeclampsia. No cerebral symptoms were present. Liver enzymes, serum creatinine and platelets were within reference values. Bed rest was recommended and at day 36/6 she gave birth spontaneously to two healthy boys with birth weights of 2380 g and 2000 g. Over the following 3 days she developed severe symptoms of heart failure with dyspnoea and tachypnoea at rest (30/min), tachycardia (111/min), leg oedema and weight gain of 5 kg since delivery, and was transferred to our ICU. ECG showed sinus tachycardia but was otherwise normal. Blood pressure had normalized and proteinuria was less severe (2471 mg/d), hence a diagnosis of PPCM was probable and was confirmed by echocardiography. It showed left ventricular dilatation (LVEDd 60 mm), severe reduced systolic function (LVEF 25%), moderate mitral regurgitation 1-2° (out of a scale of 4°) and moderate to severe pulmonary hypertension (right ventricular pressure calculated via tricuspidal regurgitation 2° of 52 mmHg, not including right atrial pressure). Laboratory findings at ICU admission were (normal range in brackets): NT-proBNP 2661 pg/mL (<125 pg/mL), Troponin T 0.33 μg/L (<0.03 μg/L), creatine kinase 161 U/L (<145 U/L), ALAT 72 U/L (<34 U/L), ASAT 78 U/L (<35 U/L), Leukocytes 13.7/nL (4.5-11.0/nL), C-reactive protein 1.0 mg/dL (<0.5 mg/dL), S-creatinine 0.72 mg/dL (<1.0 mg/dL). Heart failure therapy was initiated with torasemide 5 mg, ramipril 2.5 mg, spironolactone 25 mg, bisoprolol 2.5 mg and stepwise increased. The patient was still nursing, but after the first dose of these medications the mother's milk was discarded due to the risk of potential drug transmission. Over the next 3 days, the patient had a slight weight loss (−2 kg) and a decrease in pulmonary hypertension, but her clinical status worsened with dyspnoea in upright position, bilateral pleural effusions and a circular pericardial effusion. MR-imaging showed an LVEF of 15%. Remarkably, there was no delayed enhancement in T1-weighted images after injection of Gd-DTPA, which made myocardial necrosis or fibrosis unlikely (Fig. 1, left side). This continuous deterioration stimulated a change in treatment and according to the theory of the damaging prolactin derivative mentioned previously we started treatment with bromocriptine 2.5 mg bid. Dyspnoea and tachycardia eased rapidly, by the 5th day of bromocriptine use the patient was able to stand upright and was transferred to a normal ward, and on day 12 she was discharged home in a stable condition and in NYHA class II-III. Bromocriptine was continued for a total of 6 weeks. At an outpatient visit 2 months later she presented in a good state, NYHA class I, with normalized laboratory parameters and without oedema. Echocardiography showed a still dilated (LVEDd 56 mm) but near normally contracting LV, mild residual regurgitation of aortic, mitral and tricuspid valves (1°), absence of pleural or pericardial effusion and normalized right ventricular pressure. MRI confirmed these findings with an LVEF of 60%, and additionally, myocardial oedema was excluded by T2 weighted image and fibrosis by T1 weighted image after contrast media (Fig. 1, right side).
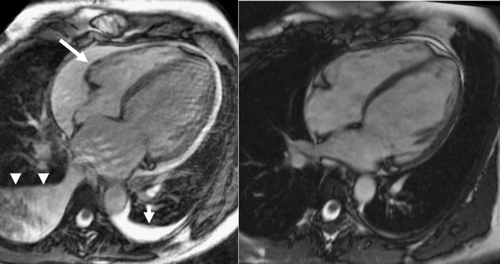
3. Discussion
The rapid recovery of our patient after bromocriptine treatment was all the more impressive because her condition deteriorated despite adequate heart failure medication and a poor prognosis was expected. Duran et al. 3 very recently published a report suggesting that persistent left ventricular dysfunction is probable in PPCM patients with LVEDd>55 mm and LVEF≤27%. Both of these criteria were applicable to our patient. Due to the novelty of the 16 kDa prolactin derivative theory 4, systematic clinical studies on bromocriptine are still lacking. Nevertheless, the MRI data presented here support the assumption of a PPCM aetiology beyond myocardial inflammation because no late enhancing structures were detected. Despite this, myocardial damage was obvious with increased troponin and creatine kinase levels and could be explained better by toxicity rather than by immune response. Balancing the potential side effects of bromocriptine against the very poor prognosis in severe PPCM our case supports the use of bromocriptine as a specific novel therapy 5. In patients with mild to moderate PPCM the immediate termination of nursing/breast feeding may be sufficient.




