Cigarette smoke-induced left ventricular remodelling is associated with activation of mitogen-activated protein kinases
Abstract
Aim:
To determine the effects of cigarette smoke (CS) exposure on the expression/activation of mitogen-activated protein kinases (MAPKs) (extracellular signal-regulated kinase [ERK1/2], p38-kinase [p38] and c-Jun NH2-terminal protein kinase [JNK]), norepinephrine (NE) and myocardial structure and function.
Methods:
Rats were randomised to two groups: CS-exposed (n = 12) or room air (CON) (n = 10). After 5 weeks, the animals underwent echocardiography with pulse-wave Doppler flow measurements. Hearts were removed for microscopy and Western blot analysis.
Results:
CS exposure was associated with significant increases in NE urinary levels and larger ventricular dimensions (mm) (CON = left ventricular end diastolic dimension [LVEDD] 7.99±0.10, LV end systolic dimension [LVESD] 4.55±0.20, CS=LVEDD 8.3±0.10, LVESD 5.3±0.09, p=0.026, p=0.003). There was also evidence of systolic dysfunction in the CS-exposed group compared to the CON (fractional shortening %, CON=43±2, CS=36±.09, p=0.010). In CS-exposed hearts, significant increases in phosphorylated p38/total (0.975±0.05) and phosphorylated ERK1/2/totalERK1/2 (1.919±0.050) were found compared to CON hearts (0.464±0.008, 0.459±0.050, respectively). No significant differences were found in JNK levels between the groups.
Conclusions:
Increased NE levels and MAPK activation are associated with CS-related left ventricular remodelling. Published by Elsevier B.V. on behalf of European Society of Cardiology.
1. Introduction
Heart disease that results from primary as well as environmental cigarette smoke (CS) exposure more than likely develops as a result of a complex interaction among the many constituents in cigarette smoke. CS contains over 4000 different constituents, one of which is nicotine 1. Many of the adverse cardiovascular effects of CS have been attributed to nicotine. Nicotine administration is associated with changes in cardiac output and heart rate; however, the effect of nicotine on the initiation and progression of CS-mediated cardiovascular events remains controversial 1 2 3. Furthermore, small clinical trials of nicotine replacement therapies have not shown an increased cardiovascular risk, even in patients with cardiovascular disease, suggesting the need to consider CS as a whole rather than nicotine alone 4,5. Using nicotine alone may lead to inaccurate information regarding the pathophysiological effects of CS.
Others have examined the effects of 4-5 weeks of CS exposure on echocardiographic-derived parameters and found increased left ventricular end diastolic and systolic dimensions, indicating some degree of left ventricular (LV) remodelling 6,7. To our knowledge, no reports have examined the effects of CS on both myocardial structure and signal transduction pathways such as mitogen-activated protein kinases (MAPKs), which govern cell survival and growth in the myocardium. There is evidence from others (in humans and in animal models) that CS is associated with increased levels of norepinephrine (NE) and tumour necrosis factor α 8,9. These latter extracellular signals activate MAPKs, such as extracellular-regulated kinase (ERK1/2), p38 kinase (p38) and c-Jun NH2-terminal protein kinase (JNK). In the myocardium, activation of MAPKs such as ERK1/2, p38 and JNK plays a key role in the pathogenesis of many processes, such as hypertrophy, heart failure and reperfusion injury 10. Therefore, the primary objectives of this investigation were to determine in adult male rats the expression/activation of MAPKs that are involved in the regulation of cell growth, cell differentiation and cell death (ERK1/2, p38, JNK) and determine if increased expression of MAPKs parallels CS-induced histological and structural changes in the myocardium (as assessed via electron microscopy and echocardiography, respectively). Furthermore, we measured urinary levels of NE, since CS exposure in humans is associated with increased NE levels and NE is associated with the activation of MAPKs. It was hypothesized that CS exposure would significantly alter left ventricular structure and be associated with systolic and diastolic dysfunction as well as activation of MAPKs.
2. Methods
2.1. Groups and treatment
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee. After arrival and a brief acclimation period, age-matched male Sprague-Dawley rats (beginning body weights 210-260 g) were randomly divided into the following groups: control (CON) animals (n=10) not exposed to cigarette smoke, and CS-exposed animals (n=12) exposed to 45 min of CS twice a day for 5 weeks.
Similar to other investigators, we used a smoke exposure system (University of Kentucky Tobacco and Health Research Institute smoking machine) which exposes rats to mainstream smoke (nose-only exposure) from a cigarette 11,12. This system has been extensively used to study smoking-related diseases, and it allows for exposure to CS in conscious, restrained rats. As noted above, rats in the CS group received CS twice daily for 5 weeks. The University of Kentucky reference (2R1) cigarettes were used in all experiments. The 2R1 cigarette has a tar yield of 34.3 mg and nicotine yield of 2.16 mg. As a point of reference, the average yield of a cigarette marketed in the U.S. in 1994 was 12.1 mg tar and between 1.72 and 1.89 mg nicotine. Cigarettes were placed into the cigarette puffer, and a peristaltic pump was used to generate puffs at a frequency of 1/min, durations of 2.4 s and puff volumes of 38 ml 11. Animals received two 45- to 60-min CS sessions per day. Urinary cotinine levels were measured at the end of the 5-week protocol and were quantified using an enzyme-linked radioimmunoassay (ELISA) (Cozart Bioscience, Oxfordshire, UK). A 24-hour urine creatinine measure was also taken, and cotinine levels were normalized to urinary creatinine levels. In the CON group, the cotinine level was 15±5 (ng/mmol/creatinine), and it was 1102±306 (ng/mmol/creatinine) in the CS group. These cotinine levels were in the range of those reported in human active smokers (i.e., those who smoked>10 cigarettes/day) 13.
2.2. Urinary norepinephrine
Norepinephrine (NE) levels can be influenced by physical stress, such as handling and anaesthesia. Therefore, 2 days prior to sacrifice, both groups of animals were placed into metabolic cages, and urine was collected for a 24-hour period. Urine was collected into tubes containing 6 M hydrochloric acid. The total 24-hour volume was measured, and 1-ml aliquots were stored at −20 °C until assay. NE was measured using an ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO). All samples were run in duplicate.
2.3. Echocardiography
All echocardiograms were performed as previously described by our laboratory and according to the American Society of Echocardiography guidelines 14,15. Echocardiograms were performed by the same experienced sonographer using the Sequoia C256 Echocardiography System (Acuson Corporation, Mountain View, CA) and a 15.0 MHz transducer. Before the procedure, animals were anaesthetized with an initial dose of methoxyflurane, and sedation was maintained thereafter via intubation with 1% isoflurane, using a Harvard small-animal ventilator (respiratory rate 80 breaths per minute, respiratory volume 2.5 ml). Body temperature was monitored using a rectal thermistor and maintained at 37 °C using a warming plate perfused with a water circulating bath. The transducer was placed on the left thorax, and M-mode and 2-dimensional echocardiography images were obtained in the parasternal long- and short-axis views by directing the ultrasound beam at the mid-papillary muscle level. The measurements listed below were obtained after well-defined, continuous interface of the anterior and posterior walls were visualized. All parameters were measured with electronic calipers, and mean calculations were obtained from three or more consecutive cardiac cycles. For all animals, three to four beats were recorded using the same transducer position. Fractional shortening (FS) and relative wall thickness (RWT) were calculated as previously described. Using pulse-wave Doppler echocardiography, we also measured signals from the left ventricular inflow and outflow track and obtained indices of diastolic function such as: isovolumic relaxation time (IVRT) (time between the closure of the aortic valve [S2] and opening of the mitral value), deceleration time (DT), and E/A ratio (the E wave represents early rapid filling of the ventricle; the A wave represents late filling of the ventricle). Mean values were used for statistical analysis.
2.4. Tissue collection
After sacrifice, the myocardium was removed and rinsed in normal saline. A small (<1-mm) section was removed and fixed in 2.5% glutaraldehyde-buffered (pH 7.4) solution. Two more small sections were cut, and one was flash-frozen and stored at −80 °C for Western blot analysis.
2.5. Electron microscopy
One-mm sections were subsequently postfixed in osmium, dehydrated in acetone and flat-embedded in epoxy resin. Ultrathin sections were cut and then examined with a Phillips EM-400 electron microscope (Phillips Electronic Instruments, Mount Vernon, NY). A pathologist blinded to the experiment groups examined micrographs from at least 2 blocks (10 random electron micrographs) for ultrastructural changes.
2.6. MAPK Western blot analysis
Using methods previously described by our laboratory, immunoblots were performed using 40-μg total protein homogenates 16. Briefly, protein homogenates were prepared from the left ventricle, and total protein concentrations were determined by Lowry (Bio-Rad) assay. Equal amounts of protein (40 μg) were loaded onto a 10% SDS-PAGE, subjected to gel electrophoresis, and electroblotted onto nitrocellulose membranes that were blocked with milk, washed and then incubated with primary antibody. Primary antibodies employed included: rabbit polyclonal anti-phospho-p38 MAPK, anti-phospho-ERK1/2, anti-phospho-JNK anti-P38, anti-ERK1/2 and anti-JNK (Cell Signaling Technology, Inc., Boston, MA). Membranes were extensively washed and then incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA). Bands were visualized with the ECL system (Amersham, Little Chalfont, UK) and quantified with Chemi Doc XRS (Bio-Rad) applying Quantity One software.
2.7. Statistical analysis
All data are expressed as the mean±SEM. Data were compared using a one-way analysis of variance (ANOVA). When a significant F ratio (p<0.05) was found, group comparisons were made using a Fisher's post-hoc procedure for multiple comparisons (Sigmastat v 3.5, SYSTAT Software, Richmond, CA).
3. Results
No differences were found between groups in the ending body weight (BW) or heart weight (HW)-to-tibia ratio (Table 1). The HW-to-BW ratio, however, was significantly greater in the CS group than in the CON group (Table 1). Urinary NE levels were also significantly increased in CS-exposed animals compared to CON animals (p=0.004).
| BW (gm) | HW-to-BW (g/g) | HW-to-Tibia (g/cm) | Norepinephrine (pg/100 μl) | |
|---|---|---|---|---|
| CON | 347±5.8 | 3.00±.001 | 0.28±.004 | 10±5 |
| CS | 365±5.7 | 3.24±.06* | 0.31±.023 | 1270±33* |
- a Values are mean±SEM, BW—body weight, HW-to-BW ratio—heart weight to BW ratio.
- * Indicates significantly greater than CON (p=0.01).
Fig. 1 shows representative electron micrographs of the ultrastructure of the LV muscle from CON and CS-exposed rats. A pathologist blinded to group assignment examined and scored sections for the general appearance of the tissue, changes in intracellular organelles (such as the mitochondria and sarcoplasmic reticulum), and evidence of cell loss or myofibril disarray. No significant ultrastructural changes were found in either group.
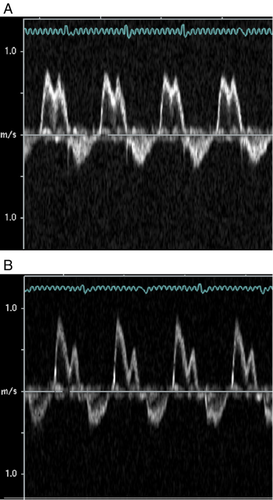
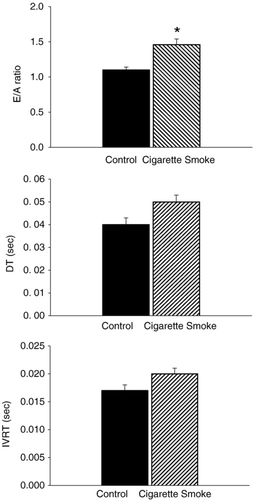
Significant increases in LVEDD and LVESD were found in the CS group compared to the CON group (Table 2). Left ventricular posterior wall thickness in diastole (LVPWD), left ventricular posterior wall thickness at systole (LVPWS) and RWT were not significantly different between the groups; however, FS was significantly lower in the CS group than in the CON group (Table 2). Using pulse-wave Doppler imaging, we found well-defined signals in 94% of the animals (representative images are shown in Fig. 2). We found a significant increase in the E/A ratio in the CS group compared to the CON group (Fig. 3). IVRT and DT were not significantly different between groups (Fig. 3).
| Control | CS | p | |
|---|---|---|---|
| Heart rate (bpm) | 341±11 | 316±6 | 0.083 |
| LVEDD (mm) | 7.99±0.1 | 8.3±0.10 | 0.026 |
| LVESD (mm) | 4.55±0.2 | 5.3±0.09 | 0.003 |
| LVPWD (mm) | 1.40±0.06 | 1.40±0.07 | 0.990 |
| LVPWS (mm) | 2.40±0.04 | 2.18±0.14 | 0.200 |
| FS (%) | 43±2.1 | 36±0.9 | 0.010 |
| LV mass (g) | 0.67±.03 | 0.72±.04 | 0.320 |
| RWT | 0.35±.01 | 0.33±.01 | 0.430 |
- a Abbreviations used: FS—fractional shortening, left ventricle (LV) end diastolic dimension (LVEDD), LV end systolic dimension (LVESD), LV posterior wall thickness in diastole (LVPWD) and LV posterior wall thickness in systole (LVPWS), relative wall thickness (RWT=2×LVPWD/EDD). Data are expressed as mean±SEM.
3.1. Western blotting of MAPKs
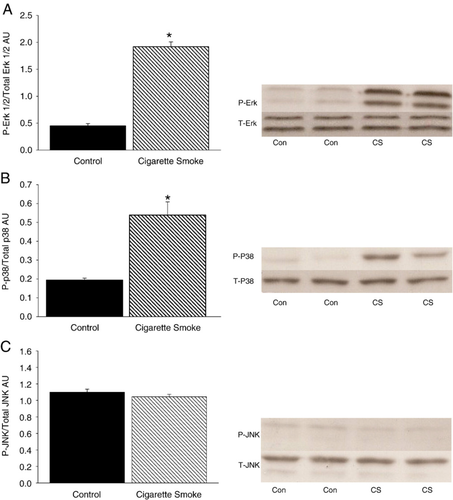
Protein levels of total p38, ERK1/2, JNK, P-p38, P-ERK1/2 and P-JNK were determined using Western blotting. Levels were expressed as the ratio of phosphorylated (P)-to-total protein levels. The levels of P-p38 and P-ERK1/2 were significantly greater in the CS-exposed hearts compared to the CON hearts (Fig. 4, Panels A and B) (p<0.01). No significant differences were found in JNK levels between the groups (Fig. 4, Panel C).
4. Discussion
This is the first study to demonstrate that CS-induced ventricular remodelling is linked to activation of MAPKs. Our data also suggest that CS smoke exposure is associated with systolic dysfunction and increased adrenergic drive. Collectively, these data support the involvement of MAPKs in CS-induced remodelling and cardiac dysfunction.
To date, there have been few studies examining the effects of CS on the myocardium, and to our knowledge no investigations have examined cell mechanisms that are linked to the toxic effects of CS on the myocardium. Although CS affects the entire cardiovascular system, it has been generally accepted that the most adverse effects were on the vasculature and included endothelial dysfunction and accelerated atherosclerosis 17. However, a landmark prospective clinical study by Hartz and colleagues in 1984 established in men younger than 55 years of age (without evidence of coronary artery disease or history of myocardial infarction) that CS exposure was associated with diffuse ventricular hypokinesis 18. The associations between CS and reduced left ventricular function and greater left ventricular mass have been re-affirmed in more recent large population-based epidemiological studies using magnetic resonance imaging to evaluate myocardial structure and function 19,20. These data are further substantiated by our findings and those of others, which demonstrate that CS exposure is associated with ventricular remodelling 6,7.
Our findings of an increase in EDD and ESD and a decrease in FS in CS-exposed animals are similar to others, who have shown that 4 months of CS exposure is associated with cardiac remodelling and systolic dysfunction 6,7. Changes in diastolic function after CS exposure have been equivocal in animal models and in human beings. Doppler-derived echocardiography parameters that reflect diastolic dysfunction include an increase in the E/A ratio and prolonged DT and IVRT values. In the present study, we found a significant increase in the E/A ratio in the CS group compared to CON group; however, even though DT and IVRT were increased in the CS group compared to CON, these differences were not significant. Following 4 months of CS exposure, Castardeli et al. found no change in the E/A ratio between CON and CS-exposed rats 6,7. In human subjects, investigators have examined the acute one-time effect of CS exposure on diastolic function 21. Alam et al. found evidence of diastolic dysfunction in healthy subjects immediately after smoking one cigarette 21. In a similar study, 30 min after subjects smoked one cigarette, Karakaya et al. found evidence of diastolic dysfunction in subjects with coronary artery disease, while no significant changes were found in healthy subjects 22. To our knowledge, there are no studies examining longer durations of CS exposure on diastolic function in human subjects. It would be interesting to determine if CS exposure alters intracellular organelles (e.g., sarcoplasmic reticulum) or molecular events (sarcoplasmic reticulum uptake of calcium) that govern diastolic function.
In our study, 5 weeks of CS exposure was associated with increased urinary NE levels. CS is associated with increased sympathetic activation and NE levels according to Cryer et al. 8 and Narkiewicz et al. 23. Via activation of different adrenergic receptor (AR) subtypes, NE can be associated with either myocyte hypertrophy (α1A AR) or apoptosis (β1 AR) 24. Activation of the α1A AR is also associated with increased ERK1/2 activation 25. We did not examine cardiac tissue for evidence of apoptosis or myocyte hypertrophy (e.g., increased cell size or protein synthesis).
Our data clearly indicate that CS activates MAPKs such as p38 and ERK1/2. Our findings concur with previous studies, which have established a role for activation of MAPK signalling in the pathogenesis of CS-induced lung injury (emphysema) and inflammation 26,27. Furthermore, others have shown that CS-induced phosphorylation of ERK1/2 precedes the activation of protooncogenes that belong to the AP-1 transcription family (e.g., fos and jun). Importantly, genes that encode for growth factors (e.g., insulin-like growth factor) and extracellular matrix metalloproteinases (MMPs) have an AP-1 site in their promoter or enhancer region 28. MMPs are proteinases that can degrade almost all of the extracellular matrix proteins. In terms of the myocardium, increased MMP activity is associated with breakdown of the supporting fibrillar collagen network. Loss of the supporting fibrillar collagen network, along with loss of the collagen tethers, leads to myocyte slippage, ventricular wall thinning, and enlargement 29. Mercer et al. 26 and Ning et al. 30 have demonstrated a strong causal role for ERK1/2 signalling and the activation of MMP in pulmonary epithelial cells and the consequent development of emphysema, a disease characterized by loss of alveolar attachments and parenchymal destruction.
We also found that 5 weeks of CS exposure was associated with increased p38 phosphorylation. p38 has also been referred to as a cytokine-suppressive anti-inflammatory drug-binding protein 10. p38 is strongly activated by cytokines, such as TNFα, and other cellular stressors, such as oxidative stress and hyperosmolarity. Using an animal model, Liu et al. 31 and Li et al. 32 have shown that inhibition of p38 (in particular the α isoform) protects the heart against remodelling following myocardial infarction. In addition, Li et al. demonstrated that there were fewer infiltrating macrophages and apoptotic cells after animals were treated with the p38 inhibitor SD-282. Collectively, the latter data suggest that activation of the p38 pathway is a key mechanism in ischaemia-induced myocardial dysfunction. Considering CS exposure is associated with increased TNFα and oxidative stress 9,33, it is not surprising that we found increases in p38 phosphorylation. Using human pulmonary artery endothelial cells in culture, Low et al. 34 demonstrated that p38 mediates CS-induced endothelial permeability. It will be important to conduct future experiments to determine if there is evidence of apoptosis and inflammation and if a longer duration of CS exposure is associated with more severe changes, as well as activation of JNK.
The way in which CS activates both the p38 and ERK1/2 MAPK cascades remains unknown and could potentially occur at multiple levels: via stimulation/release hormones or by activation of upstream kinases, such as MAPK kinase kinase (MEKK) or MAPK kinases (MEK). As noted above, NE is associated with activation of the MAPK cascade (e.g., ERK1/2). CS exposure is also associated with increased cytokine levels, such as TNFα, which in turn is associated with activation of p38 MAPKs. However, it is well established that CS contains oxidants and is a potent stimulator of oxidative stress. Therefore, it is also possible that CS may (via the generation of oxidants) directly activate upstream kinases, such as MEKK or MEK 35,36. We did not find an increase in JNK after 5 weeks of CS exposure. Perhaps the preferential activation of p38 and ERK1/2 are related to either the duration of CS exposure or overall level of oxidative stress.
We did not find any significant changes in the appearance of the sarcomeres, myofilaments, or mitochondria between groups upon ultrastructural analysis. This is in contrast to Zornoff et al. 37, who reported that 30 days of environmental tobacco smoke exposure in male Wistar rats was associated with disorganization of the myofibrils and mitochondrial swelling. In the Zornoff et al. study, animals were exposed to 10 cigarettes in the morning and then again in the afternoon. In our protocol, animals were exposed to 45 min of mainstream smoke (approximately 2-3 cigarettes) twice a day. The earlier investigators did not report cotinine levels; therefore, it is difficult to compare our two studies in terms of the amount of smoke exposure. However, the animals in the Zornoff protocol more than likely received more smoke exposure, perhaps explaining their morphological tissue findings. In addition, the type of cigarette (2R1) we used has a greater tar and nicotine content compared to the type of cigarette used by others 37. Also, as noted in the methods section, the tar and nicotine content of the R21 is greater than conventional cigarettes available to the public. However, using this type of cigarette, as well as an intermittent, short-term (5-week) exposure protocol in a rodent model, our urinary cotinine levels (1102±306 ng/mmol/creatinine) approximate those reported in human active smokers (i.e., those who smoked >10 cigarettes/day) 13.
4.1. Study limitations
There are several limitations in this study. CS induces the secretion of different types of circulating neurohumoral stimuli, such as NE, that are linked to MAPK activation. In our intermittent rodent CS model, we found large increases in urinary NE. However, we did not assay for increases in other stimuli, such as TNFα, which others have found to be increased in smoking human beings 38 and animal models exposed to CS 39. Similar to NE, TNFα is a potent activator of MAPKs; therefore, in future studies, it would be important to determine if TNFα serum and/or myocardial tissues levels are increased after CS. Second, because we measured MAPK activation and structural and functional parameters of LV remodelling at the same time point and did not attempt to inhibit MAPK activation, we cannot conclude that CS-induced activation of MAPK leads to cardiac remodelling. Using cultured human pulmonary artery endothelial cells, Low et al. found that the addition of a p38 MPK-selective inhibitor (SB203580) prevented CS-induced p38 activation and the development of endothelial damage (i.e., increased endothelial cell permeability) 34. p38 MAPK inhibitors are available and have been associated with positive and protective effects in animal models of inflammation 40 and myocardial injury 32. Findings of the present study could be confirmed in future studies incorporating the use of MAPK inhibitors.
5. Conclusion
In conclusion, this study identified that 5 weeks of mainstream CS exposure is associated with LV remodelling, systolic dysfunction and activation of p38 and ERK1/2 MAPKs. MAPKs govern cell survival and growth in the myocardium, and others have shown that activation of MAPKs plays a key role in the pathogenesis of hypertrophy, heart failure and ischaemia/reperfusion injury. Moreover, in other tissues, CS activation of the MAPK signalling cascade is considered to be a key mechanistic event leading to cell injury, altered cell differentiation and inflammation 27. Therefore, activation of MAPKs may represent an important event in CS-induced cardiotoxicity.
Acknowledgements
The authors thank Dr. John Van Fleet (Purdue University) for his excellent morphological analysis and Amy Tarr for her excellent laboratory assistance. This study was supported by National Institutes of Health grant AA015578. The authors thank Kevin Grandfield for editorial assistance.




